Abstract
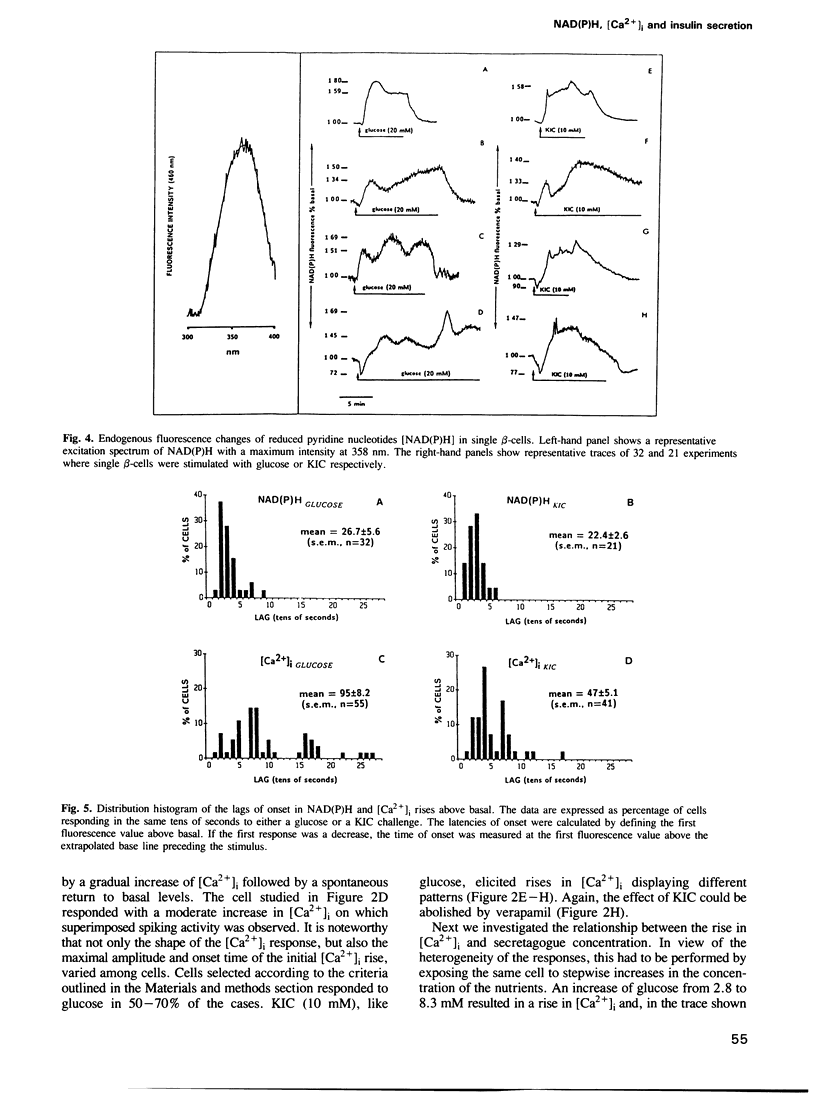
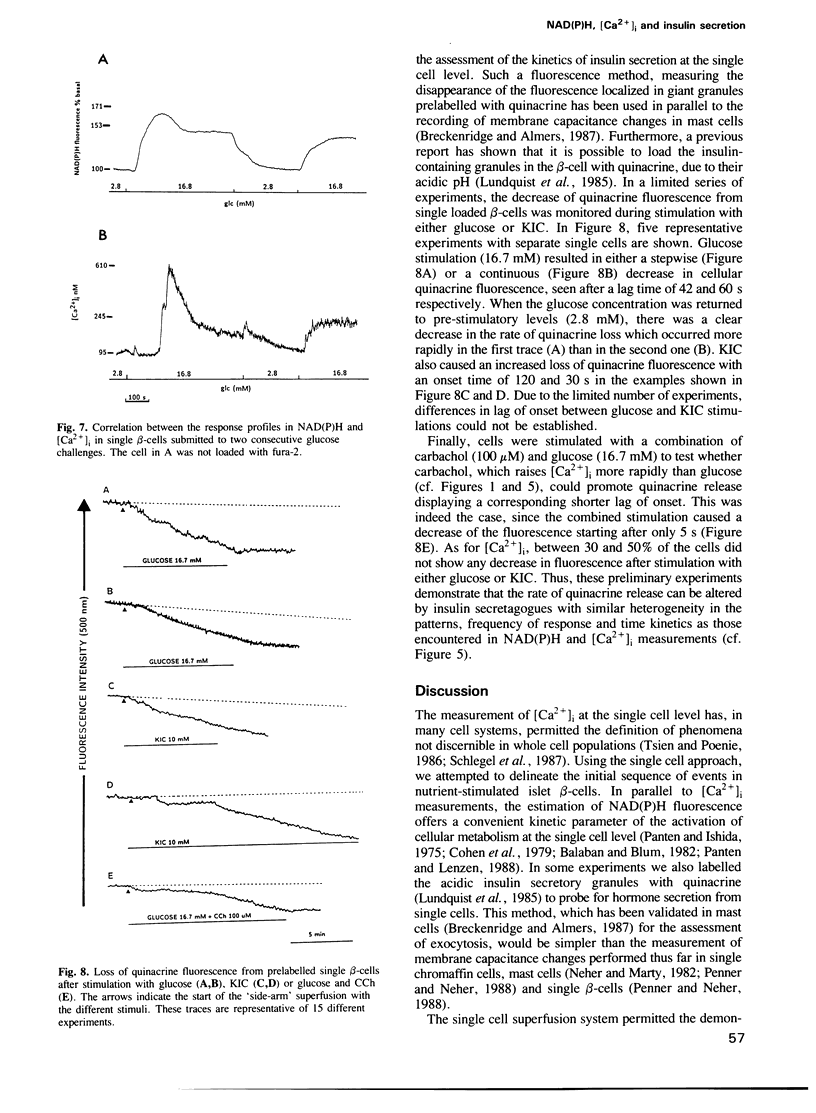
It is generally believed that the initiation of insulin secretion by nutrient stimuli necessitates the generation of metabolic coupling factors, leading to membrane depolarization and the gating of voltage-sensitive Ca2+ channels. To establish this sequence of events, the kinetics of endogenous fluorescence of reduced pyridine nucleotides [NAD(P)H], reflecting nutrient metabolism, were compared to those of cytosolic calcium ([Ca2+]i) rises in single cultured rat islet beta-cells. In preliminary experiments, the loss of quinacrine fluorescence from prelabelled cells was used as an indicator of secretion. This dye is concentrated in the acidic insulin-containing secretory granules. Both glucose and 2-ketoisocaproate (KIC) raised [Ca2+]i in a dose-dependent manner. There was marked cellular heterogeneity in the [Ca2+]i response patterns. The two nutrient stimuli also increased NAD(P)H fluorescence, again showing cell-to-cell variations. In combined experiments, where the two parameters were measured in the same cell, the elevation of the NAD(P)H fluorescence preceded the rise in [Ca2+]i, confirming the statistical evaluation performed on separate cells. The application of two consecutive glucose challenges revealed coordinated changes in [Ca2+]i and NAD(P)H fluorescence. Finally, quinacrine secretion was stimulated by two nutrients with onset times similar to those recorded for [Ca2+]i elevations. These results clearly demonstrate that increased metabolism occurs during the lag period preceding Ca2+ influx via voltage-sensitive Ca2+ channels, a prerequisite for the triggering of insulin secretion by nutrient stimuli.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. P., Bumiller G., Düppenbecker H., Heinze E., Lutz S., Verspohl E. J. Pentose phosphate shunt, pyridine nucleotides, glutathione, and insulin secretion of fetal islets. Am J Physiol. 1983 Apr;244(4):E354–E360. doi: 10.1152/ajpendo.1983.244.4.E354. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J Physiol. 1987 Apr;385:517–529. doi: 10.1113/jphysiol.1987.sp016505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Blum J. J. Hormone-induced changes in NADH fluorescence and O2 consumption of rat hepatocytes. Am J Physiol. 1982 Mar;242(3):C172–C177. doi: 10.1152/ajpcell.1982.242.3.C172. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Meda P. The gap junction: a channel for multiple functions? Eur J Clin Invest. 1988 Oct;18(5):444–453. doi: 10.1111/j.1365-2362.1988.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Pancreatic islets synthesize phospholipids de novo from glucose via acyl-dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1985 Oct 30;132(2):467–473. doi: 10.1016/0006-291x(85)91157-x. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H. Effects of pyridine nucleotides on the gating of ATP-sensitive potassium channels in insulin-secreting cells. J Membr Biol. 1988 Jun;102(3):205–216. doi: 10.1007/BF01925714. [DOI] [PubMed] [Google Scholar]
- Grapengiesser E., Gylfe E., Hellman B. Three types of cytoplasmic Ca2+ oscillations in stimulated pancreatic beta-cells. Arch Biochem Biophys. 1989 Jan;268(1):404–407. doi: 10.1016/0003-9861(89)90602-4. [DOI] [PubMed] [Google Scholar]
- Grill V., Adamson U., Cerasi E. Immediate and time-dependent effects of glucose on insulin release from rat pancreatic tissue. Evidence for different mechanisms of action. J Clin Invest. 1978 Apr;61(4):1034–1043. doi: 10.1172/JCI109002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E. Nutrient secretagogues induce bimodal early changes in cytoplasmic calcium of insulin-releasing ob/ob mouse beta-cells. J Biol Chem. 1988 Sep 25;263(27):13750–13754. [PubMed] [Google Scholar]
- Hedeskov C. J., Capito K., Thams P. Cytosolic ratios of free [NADPH]/[NADP+] and [NADH]/[NAD+] in mouse pancreatic islets, and nutrient-induced insulin secretion. Biochem J. 1987 Jan 1;241(1):161–167. doi: 10.1042/bj2410161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Effects of amino acids on membrane potential and 86Rb+ fluxes in pancreatic beta-cells. Am J Physiol. 1981 Mar;240(3):E245–E252. doi: 10.1152/ajpendo.1981.240.3.E245. [DOI] [PubMed] [Google Scholar]
- Hoenig M., Sharp G. W. Glucose induces insulin release and a rise in cytosolic calcium concentration in a transplantable rat insulinoma. Endocrinology. 1986 Dec;119(6):2502–2507. doi: 10.1210/endo-119-6-2502. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Malaisse W. J. Dynamics of O2 consumption in rat pancreatic islets. Diabetologia. 1980 May;18(5):395–405. doi: 10.1007/BF00276821. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Sener A., Herchuelz A., Atwater I., Kawazu S., Boschero A. C., Somers G., Devis G., Malaisse W. J. Similarities in the stimulus-secretion coupling mechanisms of glucose- and 2-keto acid-induced insulin release. Endocrinology. 1980 Jan;106(1):203–219. doi: 10.1210/endo-106-1-203. [DOI] [PubMed] [Google Scholar]
- Kohen E., Kohen C., Thorell B., Mintz D. H., Rabinovitch A. Intercellular communication in pancreatic islet monolayer cultures: a microfluorometric study. Science. 1979 May 25;204(4395):862–865. doi: 10.1126/science.35828. [DOI] [PubMed] [Google Scholar]
- Lundquist I., Ahrén B., Håkanson R., Sundler F. Quinacrine accumulation in pancreatic islet cells of rat and mouse: relationship to functional activity and effects on basal and stimulated insulin secretion. Diabetologia. 1985 Mar;28(3):161–166. doi: 10.1007/BF00273865. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A. Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim Biophys Acta. 1987 Feb 18;927(2):190–195. doi: 10.1016/0167-4889(87)90134-0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Stimulus-secretion coupling in the pancreatic B-cell: introductory remarks. Experientia. 1984 Oct 15;40(10):1025–1026. [PubMed] [Google Scholar]
- Matschinsky F. M., Ghosh A. K., Meglasson M. D., Prentki M., June V., von Allman D. Metabolic concomitants in pure, pancreatic beta cells during glucose-stimulated insulin secretion. J Biol Chem. 1986 Oct 25;261(30):14057–14061. [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Berggren P. O. Dual effect of glucose on cytoplasmic free Ca2+ concentration and insulin release reflects the beta-cell being deprived of fuel. Biochem Biophys Res Commun. 1988 Jun 30;153(3):984–991. doi: 10.1016/s0006-291x(88)81325-1. [DOI] [PubMed] [Google Scholar]
- Panten U., Ishida H. Fluorescence of oxidized flavoproteins from perifused isolated pancreatic islets. Diabetologia. 1975 Dec;11(6):569–573. doi: 10.1007/BF01222108. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Peter-Riesch B., Fathi M., Schlegel W., Wollheim C. B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988 Apr;81(4):1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., in't Veld P. A., Van de Winkel M., Maes E., Schuit F. C., Gepts W. A new in vitro model for the study of pancreatic A and B cells. Endocrinology. 1985 Sep;117(3):806–816. doi: 10.1210/endo-117-3-806. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Ashcroft F. M., Trube G. Single Ca channel currents in mouse pancreatic B-cells. Pflugers Arch. 1988 Oct;412(6):597–603. doi: 10.1007/BF00583760. [DOI] [PubMed] [Google Scholar]
- Salomon D., Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res. 1986 Feb;162(2):507–520. doi: 10.1016/0014-4827(86)90354-x. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Wuarin F., Zahnd G. R., Wollheim C. B. Monitoring receptor mediated regulation of cytosolic calcium in single pituitary cells by dual excitation microfluorimetry. J Recept Res. 1988;8(1-4):493–507. doi: 10.3109/10799898809049007. [DOI] [PubMed] [Google Scholar]
- Schuit F. C., In't Veld P. A., Pipeleers D. G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Malaisse-Lagae F., Dufrane S. P., Malaisse W. J. The coupling of metabolic to secretory events in pancreatic islets. The cytosolic redox state. Biochem J. 1984 Jun 1;220(2):433–440. doi: 10.1042/bj2200433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan Y., Meda P., Neufeld M., Orci L. Stimulation of insulin secretion reveals heterogeneity of pancreatic B cells in vivo. J Clin Invest. 1987 Jul;80(1):175–183. doi: 10.1172/JCI113045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R., Peters M., McShane P., Gray D. W., Morris P. J. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986 Dec;42(6):689–691. doi: 10.1097/00007890-198612000-00022. [DOI] [PubMed] [Google Scholar]
- Vara E., Fernández-Martín O., García C., Tamarit-Rodríguez J. Palmitate dependence of insulin secretion, "de novo" phospholipid synthesis and 45Ca2+-turnover in glucose stimulated rat islets. Diabetologia. 1988 Sep;31(9):687–693. doi: 10.1007/BF00278753. [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Petersen O. H. The effect of a cell-permeable diacylglycerol analogue on single Ca2+ (Ba2+) channel currents in the insulin-secreting cell line RINm5F. Q J Exp Physiol. 1989 May;74(3):367–370. doi: 10.1113/expphysiol.1989.sp003280. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Wollheim C. B., Dunne M. J., Peter-Riesch B., Bruzzone R., Pozzan T., Petersen O. H. Activators of protein kinase C depolarize insulin-secreting cells by closing K+ channels. EMBO J. 1988 Aug;7(8):2443–2449. doi: 10.1002/j.1460-2075.1988.tb03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Ullrich S., Pozzan T. Glyceraldehyde, but not cyclic AMP-stimulated insulin release is preceded by a rise in cytosolic free Ca2+. FEBS Lett. 1984 Nov 5;177(1):17–22. doi: 10.1016/0014-5793(84)80972-2. [DOI] [PubMed] [Google Scholar]


