Abstract
Asthma manifests as airway hyperresponsiveness and inflammation, including coughing, wheezing, and shortness of breath. Immune cells and airway structural cells orchestrate asthma pathophysiology, leading to mucus secretion, airway narrowing, and obstruction. Phosphoinositide 3-kinase, a lipid kinase, plays a crucial role in many of the cellular and molecular mechanisms driving asthma pathophysiology and represents an attractive therapeutic target. Here, we summarize the diverse roles of phosphoinositide 3-kinase in the pathogenesis of asthma and discuss novel therapeutic approaches to treatment.
Keywords: phosphoinositide 3-kinase, asthma, bronchodilator, inflammation
Clinical Relevance
Ground-breaking studies that demonstrate the potential of phosphoinositide 3-kinase (PI3K) inhibitors for asthma treatment have recently emerged, namely, that PI3K inhibitors can bronchodilate. With the recent U.S. Food and Drug Administration approval of PI3K inhibitors for certain malignancies, the potential for repositioning PI3K inhibitors for asthma is more compelling than ever. This review illuminates the multifaceted roles of PI3K in asthma pathogenesis and the clinical potential of isoform-selective inhibitors in asthma.
Asthma, characterized by airway hyperresponsiveness (AHR) and inflammation, manifests as wheezing, coughing, and shortness of breath (1, 2). Exposure to allergen, in part, drives the release of inflammatory mediators from immune cells, inducing mucus secretion, airway narrowing, and obstruction (3). These pathological responses are mediated by airway structural cells, including airway epithelial cells and airway smooth muscle (ASM) cells (4–6). Phosphoinositide 3-kinase (PI3K), a multifunctional lipid kinase, is central to the development of AHR and inflammation, and plays crucial roles in nearly all aspects of asthma pathogenesis. The centrality of PI3K to asthma pathogenesis makes PI3K an attractive therapeutic target. In this article, we review the diverse roles of PI3K in the pathogenesis of asthma, and discuss novel therapeutic approaches to treatment.
PI3K Activation and Function
Initially discovered in studies of carcinogenesis, PI3K mediates cell functions, including proliferation, metabolism, and motility—all of which are important to cancer progression (7). Other studies determined that PI3K plays a role in a myriad of diseases.
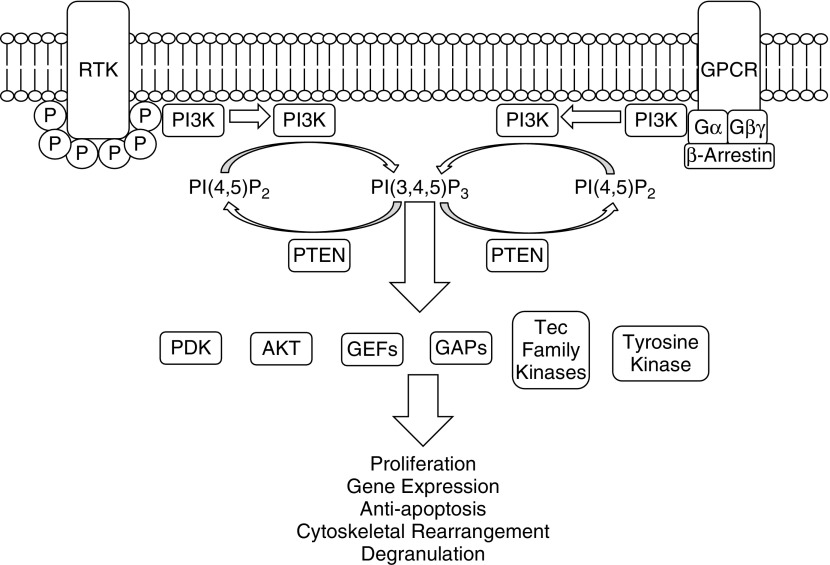
PI3K phosphorylates the D-3 position of the plasma membrane lipid phosphatidylinositol-4-5-bisphosphate, generating phosphatidylinositol-3-4-5-trisphosphate (PI[3,4,5]P3) (8, 9). Cytosolic signaling proteins with pleckstrin homology domains accumulate at sites of PI3K activation by direct binding to PI(3,4,5)P3. Proteins with pleckstrin homology domains include protein kinases, scaffolding proteins, and guanine exchange factors (GEFs), all of which will become activated and initiate signaling cascades (10–13). Notable effectors of PI3K signaling include protein serine/threonine kinases, Akt and phosphoinositide-dependent kinase (PDK) 1, as shown in Figure 1. Binding of Akt and PDK1 to PI(3,4,5)P3 brings these proteins into proximity, inducing phosphorylation of Akt by PDK1. Activation of Akt by PDK1 activates mammalian target of rapamycin (mTOR) complex 1 and a host of other proteins, affecting cell growth and proliferation (14).
Figure 1.
Class I phosphoinositide 3-kinase (PI3K) signaling. Class I PI3Ks are activated upon agonist binding to receptor tyrosine kinases (RTK) or G protein–coupled receptors (GPCRs). RTKs will recruit PI3Ks to phosphorylated tyrosine residues, resulting in activation of PI3Ks. GPCRs can activate PI3Ks via G proteins, such as Gα or Gβγ, and through β-arrestins. PI3K phosphorylates the D-3 position of the plasma membrane lipid phosphatidylinositol-4-5-bisphosphate (PI[4,5]P2), generating PI(3,4,5)P3. PI(3,4,5)P3 will recruit proteins with pleckstrin homology domains, which include phosphoinositide-dependent kinase (PDK) 1, AKT kinase (AKT), guanine exchange factors (GEFs), GTPase accelerating proteins (GAPs), Tec family kinases, and nonreceptor tyrosine kinases. These signaling proteins will subsequently affect cellular functions, including proliferation, gene expression, antiapoptosis, cytoskeletal rearrangement, and degranulation. PTEN, phosphatase and tensin homolog.
Signaling of the PI3K pathway terminates by dephosphorylation of PI(3,4,5)P3 by the enzyme, phosphatase and tensin homolog (PTEN), the main endogenous PI3K inhibitor (15). PTEN is a tumor suppressor and is important in many cancers. The PI3K pathway is also inactivated by Src-homology domain 2–containing phosphatases SH2 domain-containing inositol phosphatase 1 and 2, found primarily in blood cells (16).
PI3Ks consist of heterodimers composed of a p110 catalytic subunit and a regulatory subunit. The PI3K family is divided into three classes (classes I, II, and III) based on structural and functional characteristics, as shown in Figure 1. Further divided into subclasses based on their p110 catalytic subunit, class IA PI3Ks are composed of p110α, p110β, and p110δ isoforms, whereas class IB contains solely the p110γ isoform. Class IA PI3Ks contain p85, p55, or p50 phosphotyrosine-binding regulatory subunits. Class IB PI3Ks contain p101 or p84/p87 regulatory subunits, which allow activation by the βγ subunits of G protein–coupled receptors (GPCRs) (17). Class II PI3Ks consist of three isoforms (C2α, C2β, and C2γ) that are ubiquitously expressed (18, 19). Class III PI3K includes only one member, vacuolar protein-sorting mutant 34, that regulates endocytosis, Toll-like receptor (TLR) signaling, and vesicular trafficking (20). Classes II and III are not well studied, due to the lack of highly selective pharmacological inhibitors. Class I is the most thoroughly studied PI3K family, and is the focus of this review.
Functions of Class I PI3K
Due to the limitations of selective inhibition strategies, the study of class I PI3K family members remains challenging (21). Unlike the p110δ and p110γ subunits, the p110α and p110β subunits are required for cell proliferation and embryonic development (22). Consequently, in vivo experiments requiring genetic knockdown of p110α and p110β pose obstacles.
The p110δ and p110γ isoforms are predominantly, but not exclusively, expressed in leukocytes, and play important roles in innate and adaptive immune responses (23). Activated TLRs in leukocytes recruit p110δ and p110γ and initiate signaling (24). In T cells, p110δ and p110γ negatively regulate TLR-induced IL-12 and IFN-γ production, facilitating Th1 responses and diminishing Th2 responses (25). p110δ knockout mice and mice expressing inactive p110δ catalytic subunit demonstrate impaired CD28-costimulated clonal expansion and differentiation, highlighting the necessity of p110δ in T cell activation (26, 27). Interestingly, patients with activated PI3K Δ syndrome manifest increased susceptibility to airway infections, bronchiectasis, and lymphoproliferation (28). p110γ-deficient mice display reduced thymocyte survival and T cell maturation, with a significant decrease in CD4+ cells (29). PI3K also mediates B cell function, as p110δ knockout mice display impaired B cell receptor–mediated antigen presentation (30). In neutrophils, PI3K regulates nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidase and is important for reactive oxygen species generation. Stimulation of numerous neutrophil receptors, including GPCRs, cytokine receptors, integrin receptors, and Fc receptors, activates GEFs. GEFs, such as phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein and cytohesin4, mediate chemotaxis, vesicle trafficking, degranulation, and NADPH oxidase activation (31).
PI3K serves to promote immune cell survival by modulating antiapoptosis signaling. The PI3K/AKT pathway inhibits proapoptotic proteins, including B cell lymphoma 2 and associated proteins. PI3K signaling also facilitates activation of prosurvival proteins, including B-cell lymphoma-extra large, induced myeloid leukemia cell differentiation protein, and NF-κB (32).
Collectively, these studies demonstrate that p110δ and p110γ are integral to the orchestration of both the innate and adaptive immune responses, including leukocyte migration, activation, B cell and T cell maturation, neutrophil NADPH oxidase activation, and antigen response.
PI3K and Asthma
Atopic asthma manifests when T cells mature into a Th2 subtype upon allergen exposure and release mediators that activate other immune cells, such as mast cells, granulocytes, and B cells. Activated immune cells then elicit responses from structural cells, such as ASM and airway epithelial cells, which culminate in AHR, inflammation, and remodeling. PI3Ks play important roles in the responses of airway immune cells and structural cells that mediate these pathophysiological processes.
The importance of PI3K in asthma is demonstrated by in vivo experiments that show that PI3K inhibitors prevent pathogenesis of allergen-induced AHR and inflammation (25, 33). IC87114, a p110δ-selective inhibitor, attenuated allergic airway inflammation and AHR in a murine model (34). p110δ also mediates lung inflammation induced by Aspergillus fumigatus via a mechanism involving endoplasmic reticulum stress (35). In addition, allergen-induced AHR does not develop in p110γ-deficient mice (36). Taken together, these experiments suggest that PI3K is necessary for the development of asthma.
PI3K and Asthma: Structural Cells
Structural cells, including ASM and epithelial cells, are the main effector cells of inflammatory mediators released during asthma. ASM cells proliferate and shorten upon exposure to inflammatory mediators, inducing airway remodeling and obstruction (6). Epithelial cells recruit eosinophils by releasing eotaxin. Eosinophils subsequently release major basic protein, inducing epithelial damage (4). PI3Ks play an important role in mediating both ASM and epithelial cell responses.
ASM, the pivotal cell type mediating AHR, is the primary target for bronchodilation, a major therapeutic strategy. In asthma, ASM maintains airway tone, secretes inflammatory mediators, and undergoes hypertrophy and hyperplasia. ASM shortening occurs upon agonist binding to a GPCR, resulting in an elevation of intracellular calcium, myosin light-chain (MLC) phosphorylation, and actin–myosin cross-bridge cycling, via the canonical inositol trisphosphate and calmodulin–mediated pathway. In parallel, inhibition of MLC phosphatase by Rho kinase sustains MLC phosphorylation and maintains ASM tone.
PI3K activation is necessary for the modulation of ASM contraction and the accumulation of contractile proteins (37, 38). Importantly, PI3K contributes to airway tone via its regulation of Rho kinase. In human ex vivo small airways contracted to agonist, PI3K inhibitors evoke bronchodilation (39). p110δ and p110γ subunits are required for the development of AHR in mice (34, 40, 41). Furthermore, cytokine-mediated induction of CD38, a calcium signaling protein important to the development of AHR, was impaired after treatment with PI3K inhibitors (42). P110γ activity was found to be elevated in ASM derived from subjects with asthma, and was also important for β-2 adrenergic receptor resensitization (43). These studies highlight the importance of PI3K in ASM contraction and AHR development.
ASM also secretes chemokines and cytokines, such as IL-6, vascular endothelial growth factor (VEGF), and CXCL-8, which contribute to the recruitment of immune cells in asthma. PI3K is necessary for IL-6 secretion induced by transforming growth factor-β, a cytokine important to airway remodeling and AHR development (44). Under mechanical strain, ASM signals through PI3K/Akt/mTOR and extracellular signal-regulated kinase pathways, inducing hypoxia-inducible factor, a transcription factor required for VEGF expression (45). VEGF release by mechanical strain of human airway smooth muscle may account for the angiogenesis seen after repeated asthma exacerbations (45). In addition, PI3K and mitogen-activated protein kinase pathways regulate the synergy of IL-17 and IL-1β to enhance CXCL-8 expression (46). Taken together, these studies highlight the importance of PI3K to ASM chemokine and cytokine secretion.
In addition to ASM contraction and mediator secretion, PI3K plays an important role in airway remodeling. Airway remodeling refers to the structural changes that occur during asthma. ASM undergoes hyperplasia and hypertrophy to increase ASM mass. Th2 cytokines modulate airway contraction by secreting matrix metallopeptidase-1 from the ASM cells via PI3K activation (47). PI3K is also required for growth factor–induced cell migration (48–51). Furthermore, activation of class IA PI3K is sufficient to stimulate DNA synthesis and growth, which promote airway remodeling (52).
ASM derived from subjects with asthma display increased proliferation, responsiveness to contractile agonist, and mediator release, suggesting an epigenetic alteration. Small RNAs are essential to the establishment of an epigenetic signature, and profiling of small RNAs suggests that the PI3K pathway is enhanced in bronchial smooth muscle cells from patients with asthma (53).
The airway epithelium, considered an essential modulator of inflammation, lies at the interface between the host and the environment. The epithelium represents the first line of defense against microorganisms, toxicants, and allergens, and expresses many pattern recognition receptors to rapidly detect and respond to pathogen-associated molecular patterns found in microbes or to damage-associated molecular patterns released upon tissue damage, cell death, or cellular stress. Activation of epithelial pattern recognition receptors releases cytokines, chemokines, and antimicrobial peptides, which attracts and activates innate and adaptive immune cells. Studies show that endothelial cell activation is a key triggering event in the recognition of inhaled allergens that activates the local network of dendritic cells that coordinate the subsequent immune response. PI3K mediates epithelial responses to environmental stimuli. PI3K is important during viral-induced asthma exacerbations, due to its importance in virus internalization (54, 55). In an allergen-induced model of asthma, PI3K activation is increased and PTEN activation is decreased in the airway epithelium. PTEN protein expression and PTEN activity were also decreased in epithelial cells of mice exposed to allergen. Immunoreactive PTEN localized in epithelial layers around the bronchioles of control mice; PTEN rapidly disappeared in allergen-exposed lungs of mice, suggesting that the PI3K/PTEN pathway modulates epithelial cell function in asthma (56).
PI3K also facilitates mediator release from epithelial cells (57, 58). PI3K inhibition prevents expression of IFN-γ–induced protein 10, a mediator released during virus-induced asthma exacerbations (59). PI3K also modulates inducible nitric oxide synthase and nitric oxide signaling in the airway epithelium, promoting the development of airway inflammation (59, 60). P110δ inhibition attenuates antigen-induced airway inflammation and hyperresponsiveness through the modulation VEGF-induced vascular leakage (61). PI3K inhibition also reduces the mucus hypersecretory phenotype and goblet cell metaplasia induced by IL-13, an important cytokine that is associated with asthma (62).
PI3K and Asthma: Immune Cells
Allergen exposure induces Th2 differentiation of T cells that, in turn, secrete cytokines that promote allergic inflammation. Th2 cytokines stimulate B cells to produce IgE and other antibodies. These cytokines include IL-4, which stimulates the production of IgE, IL-5, which activates locally recruited eosinophils, and IL-13, which stimulates mucus secretion from bronchial submucosal glands and promotes IgE production by B cells. As in other hypersensitivity reactions, IgE coats submucosal mast cells, and repeated exposure to the allergen triggers the mast cells to release granule contents and produce cytokines and other mediators, which collectively induce an asthma phenotype.
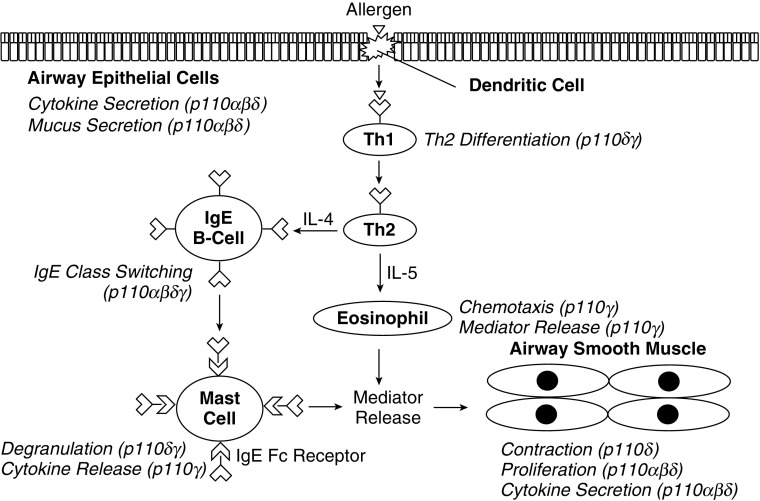
T cells, in part, promote the development of asthma, secreting cytokines that are sufficient to induce AHR and B cell class switching to IgE. Class I PI3Ks play roles in T cell activation, differentiation, and proliferation, with p110δ and p110γ being the main contributors, as shown in Figure 2. Upon activation by antigen-presenting cells, naive CD4+ cells will proliferate and differentiate to various T helper effector subsets, including the Th2 subtype. Class I PI3Ks have important roles in T cell functions. T cell receptor engagement by antigen activates p110δ through tyrosine kinase signaling cascades (63). p110γ is recruited by G protein activation through GPCRs, which include chemokine receptors (64). Mice lacking both p110δ and p110γ are markedly impaired in T cell dFB CELLSevelopment, and are unable to rearrange the T cell receptor α and β chains (65). In naive CD4+ T cells, evidence suggests that class I PI3Ks are activated upon engagement by antigen-presenting cells (66). PI3Ks are required for the differentiation of Th effector subsets, with Th2 differentiation being necessary for the development of atopic asthma (27).
Figure 2.
Functions of class I PI3K isoforms in cells mediating asthma pathophysiology. A schematic representation of the roles of class I PI3K isoforms in various cell types involved in asthma pathophysiology. Th1, T-helper cell type 1; Th2, T-helper cell type 2.
B cells secrete IgE into circulation, which will then bind to high-affinity high-affinity IgE receptors on the surface of mast cells. PI3K negatively regulates IgE expression and IgE cell surface receptor expression levels. Blockade of PI3K signaling markedly enhanced B cell IgE class switching and increased IgE levels in vivo, despite reduced type 2 cytokine production (67, 68).
Mast cells, the central effector cell in allergic diseases, are present in increased numbers in the airways of patients with asthma. Binding of allergen to IgE on the cell surface induces a signal transduction cascade that releases mediators, such as histamine and prostaglandin D2. The release of histamine and prostaglandin D2 evokes bronchoconstriction. IgE-mediated mast cell degranulation is augmented by PI3K (69). In addition, PI3K was found to mediate VEGF release in murine models, which, in turn, promotes vascular leakage in asthma (70).
Increased numbers of eosinophils are also present in the airways of some patients with asthma. The recruitment, growth, and survival of eosinophils are promoted by factors released from airway epithelial cells, Th2 cells, and mast cells. Eosinophils express a variety of proinflammatory cytokines, Th2 cytokines, and chemokines that can activate mast cells and stimulate the epithelium. Eosinophils can also present antigen to T cells, and release growth factors, such as transforming growth factor-β, promoting inflammation in asthma. Inhibition of PI3K blunts eosinophil chemotaxis, a function important in asthma (71, 72). In addition, PI3K induces mediator release in allergic asthma, irrespective of allergen challenge model (73). Eosinophils derived from subjects with atopic asthma have elevated PI3K activation, suggesting that PI3K may be an important mediator of eosinophil function in asthma (74).
Neutrophils are present in some, but not all, patients with asthma. Neutrophilic migration and activation releases mediators that contribute to asthma exacerbations. PI3K is necessary for neutrophil migration and degranulation in asthma (73, 75).
PI3K Inhibition as a Potential Therapeutic Strategy for Asthma
PI3K has gathered much attention as a potential therapeutic target in asthma, as outlined in Table 1 (76). Pan inhibitors of class I PI3K (wortmannin and LY294002) have toxicity profiles and unfavorable pharmacokinetic characteristics that preclude their clinical use (77). The focus, therefore, has shifted to isoform-selective PI3K inhibitors. PI3Kα inhibitors have been studied in the treatment of solid tumors, but have not been approved for clinical use, as shown in Table 2 (78). PI3Kδ and PI3Kγ, as well as dual inhibitors, are promising candidates. PI3Kγ inhibitors exhibit anti-inflammatory properties, but none have been approved for clinical use. Idelalisib, a PI3Kδ inhibitor, has been approved for the treatment of multiple hematologic malignancies.
Table 1.
Characteristics of Phosphoinositide 3-Kinase in Asthma
| Cell/Animal | Class I PI3K Subunits | Role in Asthma | References |
|---|---|---|---|
| Mice | p110δ | AHR | 25, 33 |
| p110δ | Inflammation | 35 | |
| p110γ | Inflammation | 36 | |
| p110γ | Remodeling | 36 | |
| Airway smooth muscle | p110δ, p110γ | Contraction | 37–42 |
| p110α, p110β, p110δ | Proliferation | 18 | |
| p110α, p110δ | Gene Expression | 42 | |
| p110γ | β-2 Adrenergic Receptor resensitization | 43 | |
| p110δ | IL-6 secretion | 44 | |
| p110α, p110β, p110δ | VEGF expression | 45 | |
| p110α, p110β, p110δ | CXCL-8 expression | 46 | |
| p110α, p110β, p110δ | MMP-1 secretion | 47 | |
| p110α, p110β, p110δ | Proliferation | 48–52 | |
| Airway epithelial cells | p110α, p110β, p110δ | IP-10 secretion | 59 |
| p110α, p110β, p110δ | Nitric oxide signaling | 59, 60 | |
| p110α, p110β, p110δ | Mucus secretion | 62 | |
| T cells | p110δ, p110γ | Th2 differentiation | 27, 63–66 |
| B cells | p110α, p110β, p110δ, p110γ | IgE class switching | 67, 68 |
| Mast cells | p110δ | Degranulation | 69 |
| p110γ | Cytokine release | 70 | |
| Eosinophils | p110γ | Chemotaxis | 71, 72 |
| p110γ | Mediator release | 73 | |
| Neutrophils | p110δ, p110γ | Migration | 75 |
| p110α, p110β, p110δ, p110γ | Degranulation | 73 |
Definition of abbreviations: AHR, airway hyperresponsiveness; CXCL-8, C-X-C motif chemokine ligand 8; IP-10, IFN-γ–induced protein 10; MMP-1, matrix metallopeptidase 1; P110α, phosphatidylinositol 3-kinase catalytic subunit α isoform; P110β, phosphatidylinositol 3-kinase catalytic subunit β isoform; P110δ, phosphatidylinositol 3-kinase catalytic subunit δ isoform; P110γ, phosphatidylinositol 3-kinase catalytic subunit γ isoform; PI3K, phosphoinositide 3-kinase; Th2, T-helper cell type 2; VEGF, vascular endothelial growth factor.
Table 2.
Phosphoinositide 3-Kinase Inhibitors in Clinical Trials for Asthma and Chronic Obstructive Pulmonary Disease
| Compound | Target | Indication | Clinical Trial Identifier |
|---|---|---|---|
| Idelalisib (CAL-101) | p110δ | Chronic lymphocytic leukemia | FDA and EMA approved, 2014 |
| GSK2269557 | p110δ | Asthma | NCT01462617, Phase 1 |
| IPI-145 | p110δ/p110γ | Asthma | NCT01653756, Phase 2 |
| RV-1729 | p110δ/p110γ | Asthma/COPD | NCT01813084, Phase 1 |
| AQX-1125 | SHIP1 activator | COPD | NCT01954628, Phase 2 |
Definition of abbreviations: COPD, chronic obstructive pulmonary disease; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; P110δ, phosphatidylinositol 3-kinase catalytic subunit δ isoform; P110γ, phosphatidylinositol 3-kinase catalytic subunit γ isoform; SHIP, SH2 domain-containing inositol phosphatase.
Side effects, such as leukopenia, colitis, and skin rashes, have occurred upon treatment with idelalisib (79). Given the anti-inflammatory and bronchodilator effects, PI3Kδ inhibition may be a promising strategy for clinical development, especially with inhaled formulations to minimize side effects. Dual PI3Kδ/PI3Kγ inhibitors have also been effective in mitigating inflammatory disorders in animal models, and may be an alternate approach (77). IPI-145 (duvelisib) and RV-1729 are PI3Kδ/PI3Kγ combination inhibitors that are being developed for clinical use (75, 80). GSK2269557, an inhaled PI3Kδ inhibitor, is being developed for severe asthma (80). TG100-115, another PI3Kδ/PI3Kγ combination inhibitor, has been effective in allergen-induced asthma models (81).
AKT and mTOR complex 1 are activated downstream of PI3K, and oral inhibitors, including miltefosine and rapamycin, have been developed for clinical use. None of these drugs have been tested for use in airway diseases. Rapamycin has shown promise in inhibiting mTOR activation in chronic obstructive pulmonary disease (82), and inhibits allergic inflammation in allergen-challenged mice, while inhibiting eosinophil differentiation (83, 84).
Although promising, PI3K inhibition as a therapeutic strategy is not without challenges. Problems include lack of specificity, adverse effects, and loss of efficacy. PI3K inhibitors, like most kinase inhibitors, target the ATP-binding pocket. Adequate inhibitor potency must be achieved to compete with ATP for binding. The structural similarity of the ATP-binding pocket in all kinases makes specific targeting difficult. Having an improved understanding of the ATP-binding pocket structure may target the development of inhibitors with improved specificity and potency. Adverse effects occur due to the myriad important functions of PI3K in other tissues. Inhaled delivery systems can promote topical deposition, while minimizing system effects. Development of inhaled PI3K inhibitors with adequate potency has been difficult, but the many preclinical studies and early clinical trials provide hope for future success of PI3K inhibition in airway diseases.
Conclusions
Asthma represents a syndrome that manifests as immune cell activation, inflammatory mediator release, and development of airway obstruction. PI3K, a crucial signaling molecule, plays a role in nearly all aspects of asthma pathophysiology. Inhibition of PI3K blunts mucus production, prevents mast cell degranulation, deters immune cell recruitment, and facilitates bronchodilation, all of which are therapeutically beneficial. Accordingly, PI3K represents an attractive target for the treatment of asthma.
Footnotes
This work was supported by National Institutes of Health grants 1F31HL134264-01 and P01-HL114471-03.
Author Contributions: E.J.Y. reviewed literature, created the tables and figures, and wrote the manuscript; C.A.O. reviewed literature and edited the manuscript; K.S. reviewed literature and contributed to figure and table creation; R.A.P. supervised the literature-reviewing process, contributed to figure and table creation, and edited the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0308TR on December 15, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Postma DS, Kerstjens HAM. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:S187–S192. doi: 10.1164/ajrccm.158.supplement_2.13tac170. [DOI] [PubMed] [Google Scholar]
- 2.Lemanske RF, Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2) suppl 2:S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690:24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 5.Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc. 2008;5:89–96. doi: 10.1513/pats.200705-063VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black JL, Panettieri RA, Jr, Banerjee A, Berger P. Airway smooth muscle in asthma: just a target for bronchodilation? Clin Chest Med. 2012;33:543–558. doi: 10.1016/j.ccm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 8.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 9.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 10.Welch HCE, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 12.Parry RV, Riley JL, Ward SG. Signalling to suit function: tailoring phosphoinositide 3-kinase during T-cell activation. Trends Immunol. 2007;28:161–168. doi: 10.1016/j.it.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 15.Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10:RA235–RA241. [PubMed] [Google Scholar]
- 16.Cui B, Chen L, Zhang S, Mraz M, Fecteau J-F, Yu J, Ghia EM, Zhang L, Bao L, Rassenti LZ, et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124:546–554. doi: 10.1182/blood-2014-03-559690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domin J, Pages F, Volinia S, Rittenhouse SE, Zvelebil MJ, Stein RC, Waterfield MD.Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin Biochem J 1997326pt 1):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domin J, Gaidarov I, Smith MEK, Keen JH, Waterfield MD. The class II phosphoinositide 3-kinase PI3K-C2α is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J Biol Chem. 2000;275:11943–11950. doi: 10.1074/jbc.275.16.11943. [DOI] [PubMed] [Google Scholar]
- 20.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finan PM, Thomas MJ. PI 3-kinase inhibition: a therapeutic target for respiratory disease. Biochem Soc Trans. 2004;32:378–382. doi: 10.1042/bst0320378. [DOI] [PubMed] [Google Scholar]
- 22.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 23.Chantry D, Vojtek A, Kashishian A, Holtzman DA, Wood C, Gray PW, Cooper JA, Hoekstra MF. p110δ, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272:19236–19241. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CC, Lin WT, Liang CM, Liang SM. Class I and III phosphatidylinositol 3′-kinase play distinct roles in TLR signaling pathway. J Immunol. 2006;176:5943–5949. doi: 10.4049/jimmunol.176.10.5943. [DOI] [PubMed] [Google Scholar]
- 25.Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110δ in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416–424. doi: 10.1002/eji.200636401. [DOI] [PubMed] [Google Scholar]
- 26.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 27.Okkenhaug K, Patton DT, Bilancio A, Garçon F, Rowan WC, Vanhaesebroeck B. The p110δ isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 28.Angulo I, Vadas O, Garçon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 30.Al-Alwan MM, Okkenhaug K, Vanhaesebroeck B, Hayflick JS, Marshall AJ. Requirement for phosphoinositide 3-kinase p110δ signaling in B cell antigen receptor–mediated antigen presentation. J Immunol. 2007;178:2328–2335. doi: 10.4049/jimmunol.178.4.2328. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins PT, Stephens LR, Suire S, Wilson M. PI3K signaling in neutrophils. Curr Top Microbiol Immunol. 2010;346:183–202. doi: 10.1007/82_2010_40. [DOI] [PubMed] [Google Scholar]
- 32.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 33.Tsang F, Fred Wong WS. Inhibitors of tyrosine kinase signaling cascade attenuated antigen challenge of guinea-pig airways in vitro. Am J Respir Crit Care Med. 2000;162:126–133. doi: 10.1164/ajrccm.162.1.9908105. [DOI] [PubMed] [Google Scholar]
- 34.Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase δ attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006;20:455–465. doi: 10.1096/fj.05-5045com. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Jeong JS, Kim SR, Cho SH, Kolliputi N, Ko YH, Lee KB, Park SC, Park HJ, Lee YC. Phosphoinositide 3-kinase-δ regulates fungus-induced allergic lung inflammation through endoplasmic reticulum stress. Thorax. 2016;71:52–63. doi: 10.1136/thoraxjnl-2015-207096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim DH, Cho JY, Song DJ, Lee SY, Miller M, Broide DH. PI3Kγ deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2009;296:L210–L219. doi: 10.1152/ajplung.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge Q, Moir LM, Trian T, Niimi K, Poniris M, Shepherd PR, Black JL, Oliver BG, Burgess JK. The phosphoinositide 3′-kinase p110δ modulates contractile protein production and IL-6 release in human airway smooth muscle. J Cell Physiol. 2012;227:3044–3052. doi: 10.1002/jcp.23046. [DOI] [PubMed] [Google Scholar]
- 38.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol. 2004;31:266–275. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 39.Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, Andrews A, Himes B, Damoiseaux R, Liggett S, et al. Inhibition of phosphoinositide 3-kinase (PI3K) promotes bronchodilation of human small airways in a Rho kinase–dependent manner. Br J Pharmacol. 2016 doi: 10.1111/bph.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Abel PW, Toews ML, Deng C, Casale TB, Xie Y, Tu Y. Phosphoinositide 3-kinase γ regulates airway smooth muscle contraction by modulating calcium oscillations. J Pharmacol Exp Ther. 2010;334:703–709. doi: 10.1124/jpet.110.168518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farghaly HSM, Blagbrough IS, Medina-Tato DA, Watson ML. Interleukin 13 increases contractility of murine tracheal smooth muscle by a phosphoinositide 3-kinase p110δ–dependent mechanism. Mol Pharmacol. 2008;73:1530–1537. doi: 10.1124/mol.108.045419. [DOI] [PubMed] [Google Scholar]
- 42.Jude JA, Tirumurugaan KG, Kang BN, Panettieri RA, Walseth TF, Kannan MS. Regulation of CD38 expression in human airway smooth muscle cells: role of class I phosphatidylinositol 3 kinases. Am J Respir Cell Mol Biol. 2012;47:427–435. doi: 10.1165/rcmb.2012-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta MK, Asosingh K, Aronica M, Comhair S, Cao G, Erzurum S, Panettieri RA, Jr, Naga Prasad SV. Defective Resensitization in human airway smooth muscle cells evokes β-adrenergic receptor dysfunction in severe asthma. PLoS One. 2015;10:e0125803. doi: 10.1371/journal.pone.0125803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamed JS, Boriek AM. Stretch augments TGF-β1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L413–L424. doi: 10.1152/ajplung.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasaneen NA, Zucker S, Lin RZ, Vaday GG, Panettieri RA, Foda HD. Angiogenesis is induced by airway smooth muscle strain. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1059–L1068. doi: 10.1152/ajplung.00480.2006. [DOI] [PubMed] [Google Scholar]
- 46.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1β–mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1023–L1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 47.Ohta Y, Hayashi M, Kanemaru T, Abe K, Ito Y, Oike M. Dual modulation of airway smooth muscle contraction by Th2 cytokines via matrix metalloproteinase-1 production. J Immunol. 2008;180:4191–4199. doi: 10.4049/jimmunol.180.6.4191. [DOI] [PubMed] [Google Scholar]
- 48.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L354–L363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- 49.Krymskaya VP, Hoffman R, Eszterhas A, Kane S, Ciocca V, Panettieri RA., Jr EGF activates ErbB-2 and stimulates phosphatidylinositol 3-kinase in human airway smooth muscle cells. Am J Physiol. 1999;276:L246–L255. doi: 10.1152/ajplung.1999.276.2.L246. [DOI] [PubMed] [Google Scholar]
- 50.Krymskaya VP, Hoffman R, Eszterhas A, Ciocca V, Panettieri RA., Jr TGF-β1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am J Physiol. 1997;273:L1220–L1227. doi: 10.1152/ajplung.1997.273.6.L1220. [DOI] [PubMed] [Google Scholar]
- 51.Irani C, Goncharova EA, Hunter DS, Walker CL, Panettieri RA, Krymskaya VP. Phosphatidylinositol 3-kinase but not tuberin is required for PDGF-induced cell migration. Am J Physiol Lung Cell Mol Physiol. 2002;282:L854–L862. doi: 10.1152/ajplung.00291.2001. [DOI] [PubMed] [Google Scholar]
- 52.Krymskaya VP, Ammit AJ, Hoffman RK, Eszterhas AJ, Panettieri RA., Jr Activation of class IA PI3K stimulates DNA synthesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1009–L1018. doi: 10.1152/ajplung.2001.280.5.L1009. [DOI] [PubMed] [Google Scholar]
- 53.Alexandrova E, Miglino N, Hashim A, Nassa G, Stellato C, Tamm M, Baty F, Brutsche M, Weisz A, Borger P. Small RNA profiling reveals deregulated phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/Akt pathway in bronchial smooth muscle cells from asthmatic patients. J Allergy Clin Immunol. 2016;137:58–67. doi: 10.1016/j.jaci.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 54.Lau C, Wang X, Song L, North M, Wiehler S, Proud D, Chow CW. Syk associates with clathrin and mediates phosphatidylinositol 3-kinase activation during human rhinovirus internalization. J Immunol. 2008;180:870–880. doi: 10.4049/jimmunol.180.2.870. [DOI] [PubMed] [Google Scholar]
- 55.Morton PE, Hicks A, Ortiz-Zapater E, Raghavan S, Pike R, Noble A, Woodfin A, Jenkins G, Rayner E, Santis G, et al. TNFα promotes CAR-dependent migration of leukocytes across epithelial monolayers. Sci Rep. 2016;6:26321. doi: 10.1038/srep26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, Lee YC. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–1092. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SR, Lee KS, Park HS, Park SJ, Min KH, Moon H, Puri KD, Lee YC. HIF-1α inhibition ameliorates an allergic airway disease via VEGF suppression in bronchial epithelium. Eur J Immunol. 2010;40:2858–2869. doi: 10.1002/eji.200939948. [DOI] [PubMed] [Google Scholar]
- 58.Matsuzaki S, Ishizuka T, Hisada T, Aoki H, Komachi M, Ichimonji I, Utsugi M, Ono A, Koga Y, Dobashi K, et al. Lysophosphatidic acid inhibits CC chemokine ligand 5/RANTES production by blocking IRF-1–mediated gene transcription in human bronchial epithelial cells. J Immunol. 2010;185:4863–4872. doi: 10.4049/jimmunol.1000904. [DOI] [PubMed] [Google Scholar]
- 59.Cakebread JA, Haitchi HM, Xu Y, Holgate ST, Roberts G, Davies DE. Rhinovirus-16 induced release of IP-10 and IL-8 is augmented by Th2 cytokines in a pediatric bronchial epithelial cell model. PLoS One. 2014;9:e94010. doi: 10.1371/journal.pone.0094010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia X, Hu X, Xu H, Wu L, Dai Y, Yang L, Xu Z. Phosphatidylinositol 3-kinase inhibitor suppresses inducible nitric oxide synthase expression in bronchiole epithelial cells in asthmatic rats. Mol Cell Biochem. 2012;359:293–299. doi: 10.1007/s11010-011-1023-y. [DOI] [PubMed] [Google Scholar]
- 61.Lee KS, Park SJ, Kim SR, Min KH, Lee KY, Choe YH, Hong SH, Lee YR, Kim JS, Hong SJ, et al. Inhibition of VEGF blocks TGF-β1 production through a PI3K/Akt signalling pathway. Eur Respir J. 2008;31:523–531. doi: 10.1183/09031936.00125007. [DOI] [PubMed] [Google Scholar]
- 62.Atherton HC, Jones G, Danahay H. IL-13–induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003:L730–L739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 63.Jarmin SJ, David R, Ma L, Chai JG, Dewchand H, Takesono A, Ridley AJ, Okkenhaug K, Marelli-Berg FM. T cell receptor–induced phosphoinositide-3-kinase p110δ activity is required for T cell localization to antigenic tissue in mice. J Clin Invest. 2008;118:1154–1164. doi: 10.1172/JCI33267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110γ isoform of phosphatidylinositol 3-kinase. J Immunol. 2008;180:2081–2088. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 65.Webb LMC, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110γ and p110δ catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 66.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 67.Doi T, Obayashi K, Kadowaki T, Fujii H, Koyasu S. PI3K is a negative regulator of IgE production. Int Immunol. 2008;20:499–508. doi: 10.1093/intimm/dxn009. [DOI] [PubMed] [Google Scholar]
- 68.Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, Vanhaesebroeck B, Marshall AJ. Genetic or pharmaceutical blockade of p110δ phosphoinositide 3-kinase enhances IgE production. J Allergy Clin Immunol. 2008:811–819.e2. doi: 10.1016/j.jaci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Xu H, Gu L-N, Yang Q-Y, Zhao D-Y, Liu F. MiR-221 promotes IgE-mediated activation of mast cells degranulation by PI3K/Akt/PLCγ/Ca(2+) pathway. J Bioenerg Biomembr. 2016;48:293–299. doi: 10.1007/s10863-016-9659-7. [DOI] [PubMed] [Google Scholar]
- 70.Lee KS, Kim SR, Park SJ, Min KH, Lee KY, Choe YH, Park SY, Chai OH, Zhang X, Song CH, et al. Mast cells can mediate vascular permeability through regulation of the PI3K–HIF-1α–VEGF axis. Am J Respir Crit Care Med. 2008;178:787–797. doi: 10.1164/rccm.200801-008OC. [DOI] [PubMed] [Google Scholar]
- 71.Mishra RK, Scaife JE, Harb Z, Gray BC, Djukanovic R, Dent G. Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K) Allergy. 2005;60:1204–1207. doi: 10.1111/j.1398-9995.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 72.Kang BN, Ha SG, Ge XN, Reza Hosseinkhani M, Bahaie NS, Greenberg Y, Blumenthal MN, Puri KD, Rao SP, Sriramarao P. The p110δ subunit of PI3K regulates bone marrow–derived eosinophil trafficking and airway eosinophilia in allergen-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1179–L1191. doi: 10.1152/ajplung.00005.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kämpe M, Lampinen M, Stolt I, Janson C, Stålenheim G, Carlson M. PI3-kinase regulates eosinophil and neutrophil degranulation in patients with allergic rhinitis and allergic asthma irrespective of allergen challenge model. Inflammation. 2012;35:230–239. doi: 10.1007/s10753-011-9309-5. [DOI] [PubMed] [Google Scholar]
- 74. Bracke M, van de Graaf E, Lammers JW, Coffer PJ, Koenderman L. In vivo priming of FcαR functioning on eosinophils of allergic asthmatics. J Leukoc Biol 2000;68:655–661. [PubMed] [Google Scholar]
- 75.Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, Pink MM, Proctor JL, Lussier J, Martin CM, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20:1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Ward S, Sotsios Y, Dowden J, Bruce I, Finan P. Therapeutic potential of phosphoinositide 3-kinase inhibitors. Chem Biol. 2003;10:207–213. doi: 10.1016/s1074-5521(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 77.Barnes PJ. Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2016;68:788–815. doi: 10.1124/pr.116.012518. [DOI] [PubMed] [Google Scholar]
- 78.Rückle T, Schwarz MK, Rommel C. PI3Kγ inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 79.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stark A-K, Sriskantharajah S, Hessel EM, Okkenhaug K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol. 2015;23:82–91. doi: 10.1016/j.coph.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doukas J, Eide L, Stebbins K, Racanelli-Layton A, Dellamary L, Martin M, Dneprovskaia E, Noronha G, Soll R, Wrasidlo W, et al. Aerosolized phosphoinositide 3-kinase γ/δ inhibitor TG100-115 [3-[2,4-diamino-6-(3-hydroxyphenyl)pteridin-7-yl]phenol] as a therapeutic candidate for asthma and chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2009;328:758–765. doi: 10.1124/jpet.108.144311. [DOI] [PubMed] [Google Scholar]
- 82.Mitani A, Ito K, Vuppusetty C, Barnes PJ, Mercado N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am J Respir Crit Care Med. 2016;193:143–153. doi: 10.1164/rccm.201503-0593OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mushaben EM, Brandt EB, Hershey GKK, Le Cras TD. Differential effects of rapamycin and dexamethasone in mouse models of established allergic asthma. PLoS One. 2013;8:e54426. doi: 10.1371/journal.pone.0054426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hua W, Liu H, Xia LX, Tian BP, Huang HQ, Chen ZY, Ju ZY, Li W, Chen ZH, Shen HH. Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice. Respirology. 2015;20:1055–1065. doi: 10.1111/resp.12554. [DOI] [PubMed] [Google Scholar]