Abstract
Bitter taste receptors (TAS2Rs) are expressed on human airway smooth muscle (HASM) and evoke marked relaxation. Agonist interaction with TAS2Rs activates phospholipase C and increases compartmentalized intracellular Ca2+ ([Ca2+]i) via inositol 1,4,5 triphosphate. In taste cells, the G protein gustducin couples TAS2R to phospholipase C; however, we find very low levels of Gαgust mRNA or protein in HASM. We hypothesized that another G protein in HASM transmits TAS2R function. TAS2R signaling to [Ca2+]i, extracellular signal–regulated kinase (ERK) 1/2, and physiologic relaxation was sensitive to pertussis toxin, confirming a role for a member of the Gi family. α subunit expression in HASM was Gαi2 > Gαi1 = Gαi3 > Gαtrans1 ≈ Gαtrans2, with Gαgust and Gαo at the limits of detection (>100-fold lower than Gαi2). Small interfering RNA knockdowns in HASM showed losses of [Ca2+]i and ERK1/2 signaling when Gαi1, Gαi2, or Gαi3 were reduced. Gαtrans1 and Gαtrans2 knockdowns had no effect on [Ca2+]i and a minimal, transient effect on ERK1/2 phosphorylation. Furthermore, Gαgust and Gαo knockdowns did not affect any TAS2R signaling. In overexpression experiments in human embryonic kidney-293T cells, we confirmed an agonist-dependent physical interaction between TAS2R14 and Gαi2. ASM cells from transgenic mice expressing a peptide inhibitor of Gαi2 had attenuated relaxation to TAS2R agonist. These data indicate that, unlike in taste cells, TAS2Rs couple to the prevalent G proteins, Gαi1, Gαi2, and Gαi3, with no evidence for functional coupling to Gαgust. This absence of function for the “canonical” TAS2R G protein in HASM may be due to the very low expression of Gαgust, indicating that TAS2Rs can optionally couple to several G proteins in a cell type–dependent manner contingent upon G protein expression.
Keywords: bitter taste receptors, G proteins, airway smooth muscle, asthma, chronic obstructive pulmonary disease
Clinical Relevance
Unexpectedly, bitter taste receptors have been localized on airway smooth muscle. These G protein–coupled receptors are activated by a variety of natural and synthetic compounds, resulting in smooth muscle relaxation, thus pointing toward a new class of direct bronchodilators for asthma and chronic obstructive pulmonary disease. A puzzle in the signal transduction cascade for these extraoral receptors is what G protein they use to transmit agonist binding to effector activation. We show here that in airway smooth muscle they do not functionally use the canonical bitter taste receptor G protein Gαgust but instead use Gαi1, Gαi2, and Gαi3. These findings now provide for a methodical approach for drug discovery and consideration of whether pathologic processes that modify these Gαi proteins could alter therapeutic effectiveness.
Bitter taste receptors (TAS2Rs) have been traditionally thought to be expressed on taste cells of the tongue, signaling to the brain as part of a system to avoid ingestion of toxic plants (1, 2). These receptors are members of the superfamily of cell surface G protein–coupled receptors (GPCRs). Over the last few years, we and others have demonstrated expression of certain TAS2R subtypes on airway smooth muscle (ASM) of human (3), monkey (4), mouse (3), and guinea pig (5). Activation of TAS2Rs on ASM results in profound relaxation and bronchodilation by a non-cAMP mechanism (3, 6). This action represents a new pathway for development of direct bronchodilators for the treatment of obstructive lung disease. The three highest expressing TAS2R subtypes in human ASM (HASM) are TAS2R10, -14, and -31. Like other GPCRs, TAS2Rs carry out signaling by coupling to G proteins with subsequent activation of an effector enzyme. The TAS2Rs of taste cells (1) have been shown to couple to the G protein, gustducin; agonist activation and coupling results in the dissociation of the α subunit (Gαgust) and the βγ subunits, with βγ activating phospholipase C (PLC), generating inositol 1,4,5 triphosphate (IP3). IP3 binding to its receptor on the endoplasmic reticulum releases intracellular Ca2+ ([Ca2+]i), which subsequently activates a transient receptor potential channel, increasing Na+ influx. This influx depolarizes the cell membrane, causing release of neurotransmitter, which stimulates the adjacent type III taste cell to communicate to the central nervous system.
The pathway diverges in ASM at the IP3 and/or [Ca2+]i release steps (3). In HASM cells, membrane potential hyperpolarizes, whereas it depolarizes in taste cells. Indeed, agonists of other GPCRs that increase [Ca2+]i in HASM, such as acetylcholine, histamine, and leukotrienes, lead to membrane depolarization and smooth muscle contraction, as opposed to the increase in [Ca2+]i evoked by TAS2R agonists that causes hyperpolarization and relaxation. Studies to date have indicated that the [Ca2+]i released from TAS2Rs resides in a specialized compartment, which differs from the more global increase in [Ca2+]i observed with contractile GPCRs (3). Recent data also indicate that IP3 production is more restricted than previously thought (7), providing additional signaling plasticity by different receptors. Multiwell plate–based fluorescence readers, such as used for this and most TAS2R signaling studies, accurately quantitate [Ca2+]i even though it may be somewhat localized within the cell.
TAS2Rs have now also been reported to be expressed in a number of other extraoral tissues, including stomach, colon, thyroid, brain, and white blood cells, where pharmacologic activation results in multiple biologic actions (8). The presumption has been with ASM, as well as these other tissues, that TAS2R function occurs by receptor coupling to gustducin. However, the expression of this taste receptor G protein is very low, and indeed difficult to detect at the protein or mRNA levels in HASM and some of these other tissues, compared with taste cells, where it is abundant. Gustducin is a member of the “Gi family” of G protein α subunits, which includes Gαi1, Gαi2, Gαi3, Gαo, and Gαz, and the transducins (the visual G proteins), Gαtrans1 and Gαtrans2. Some of these G proteins within this family share a high degree of sequence identity, and, given the very low levels of expression of Gαgust in HASM, we have undertaken the task of ascertaining which of the Gi family of G proteins carries out signal transduction of TAS2Rs in HASM. We hypothesized that one or more other members of this family that are abundantly expressed in these cells are responsible for TAS2R functional coupling in HASM.
Materials and Methods
Cell Culture and Transfections
Human embryonic kidney-293T (HEK-293T), primary HASM (passages 3–8; Lonza, Basel, Switzerland), and the D9 human telomerase reverse transcriptase immortalized HASM cells (D9 hTERT) (9) were grown as previously described (9, 10). In some experiments, cells were treated with pertussis toxin (PTX) at the indicated concentrations in media for 24 hours. For Gα subunit knockdowns, HASM cells were transfected with 100 μM of small interfering RNAs (siRNAs) 24 hours and again 48 hours after plating using lipofectamine 2000 (Invitrogene, Carlsbad, CA). The siRNAs are shown in Table E1 in the online supplement. Transfections with cDNAs encoding TAS2R14 and Gαi2 were performed in HEK-293T (10).
Western Blots and Quantitative RT-PCR
To detect Gi α subunit protein expression, SDS-PAGE and Western blots were performed using solubilized whole-cell lysates exactly as previously described (10) using primary antibodies and titers listed in Table E2 and subsequent secondary antibody at a titer of 1:10,000 for chemoluminescence (Thermo Scientific, Waltham, CA) with detection using the ChemiDoc MP (Bio-Rad, Hercules, CA). Bands were quantitated using the provided software or Image-J (National Institutes of Health, Bethesda, MD). Quantitative RT-PCR was performed on HASM RNA using methods exactly as previously described (3). The primers were from Applied Biosystems (Foster City, CA; Table E3). Comparisons were made using the 2−ΔCT method where ΔCT is the difference in CT values between the transcript of interest and that of glyceraldehyde 3-phosphate dehydrogenase.
[Ca2+]i, Extracellular Signal–Regulated Kinase 1/2 Measurements
[Ca2+]i was measured in response to the TAS2R14 agonist, diphenhydramine (DPD), in cells plated in 96-well plates and loaded with the green fluorescent calcium indicator Fluo-4 (Life Technologies, Carlsbad, CA) as previously described (10). Results are shown as either real-time measurements or as graphs showing peak [Ca2+]i responses from multiple experiments. For extracellular signal–regulated kinase (ERK) 1/2 activation studies, cells were serum starved for 18 hours, treated with vehicle or agonist, and SDS-PAGE and Western blots were performed using antibodies (Table E1) for total ERK1/2 and phospho-ERK1/2 with previously described methods (10, 11).
Receptor-Gα Interaction
cDNA constructs encoding TAS2R14 and Gαi2 were cotransfected transiently into HEK-293 cells using lipofectamine 2000. After 48 hours, cells were exposed to 500 μM DPD for the indicated time followed by treatment with a membrane-permeant cross-linking reagent (dithiobis (succinimidyl propionate); 1 mM) for 30 minutes in non–amine-containing buffer. Excess cross-linker was quenched in 25 mM Tris (pH 7.4) for 15 minutes at room temperature, and cells were lysed in 0.1% CHAPS buffer, followed by coimmunoprecipitation, as described previously (10).
Magnetic Twisting Cytometry
These studies were performed as previously described (12, 13). Briefly, Ary-Gly-Asp–coated ferrimagnetic beads were bound to cell surface integrin receptors on ASM cells. The beads are magnetized horizontally and twisted in a vertically aligned magnetic field. Bead displacement measures the increase or decrease in the twist in individual cells, corresponding to contraction and relaxation, respectively.
Genomic and Statistical Analysis
Human Gi family α subunits were aligned using the alignment program COBALT and the identity matrix values were calculated using blastp within BLAST (all programs are from the National Institutes of Health). Results from biochemical studies are presented as mean (±SE) of the indicated number of experiments. Comparisons were performed by paired or unpaired two-sided t tests, with significance imparted at P less than 0.05. Magnetic twisting cytometry results were analyzed by a nested ANOVA (14).
Results
TAS2R Signaling to [Ca2+]i, ERK1/2, and Relaxation in HASM Is Sensitive to PTX
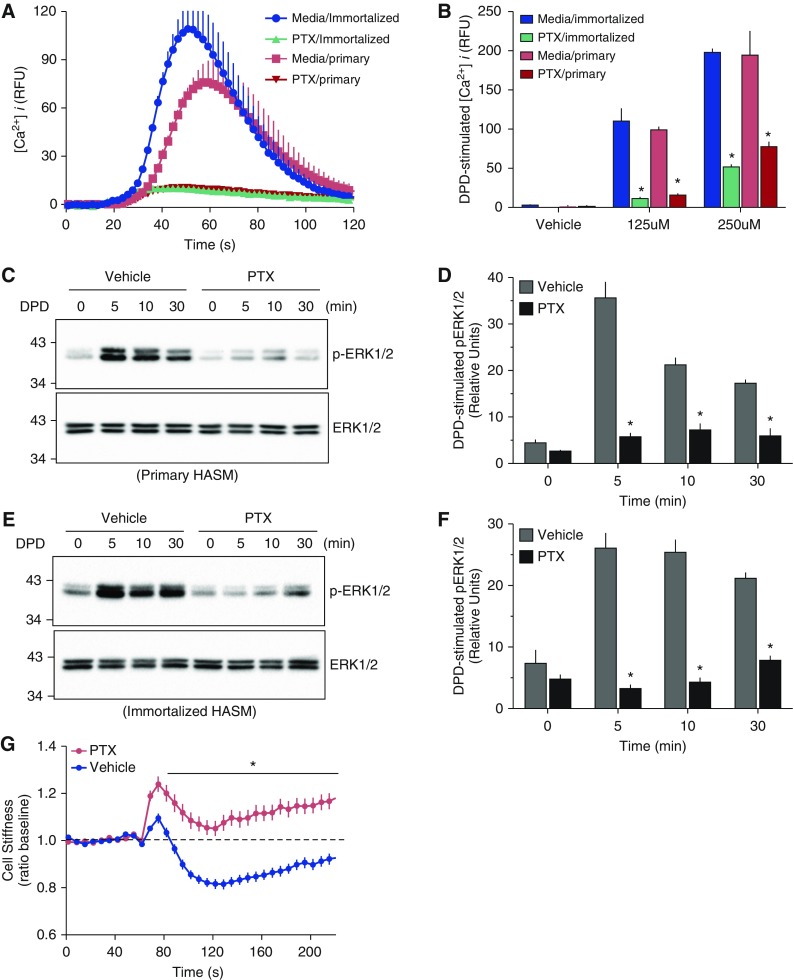
We first pursued a verification that the intracellular signaling and physiological effects that occur in HASM in response to TAS2R agonist are mediated through the members of the Gi family of G proteins, which (except for Gαz) are inactivated by PTX. Primary and immortalized HASM were exposed to vehicle or PTX for 24 hours, washed, and loaded with Fluo-4. The [Ca2+]i response to the TAS2R14 agonist DPD is shown in Figures 1A and 1B. This response in primary HASM is clearly sensitive to PTX treatment, with greater than 90% of the [Ca2+]i signal inhibited by the toxin. Studies using the immortalized HASM cell line designated D9 hTERT, showed virtually identical results (Figures 1A and 1B). Additional studies were also performed using phosphorylation of ERK1/2 as the signal readout. The early increase in phospho-ERK1/2 from GPCR activation (∼5–10 min of agonist exposure) is due to receptor G protein interaction. Responses at the 30-minute time point are β-arrestin dependent and relatively independent of G protein interaction (15, 16). We thus expected that the 5- and 10-minute signals would be blocked by PTX pretreatment if coupling was by one or more Gαi subunits. As shown in Figures 1C and 1D, DPD exposure resulted in marked phosphorylation of ERK1/2 in the primary HASM cells, which was inhibited approximately 85% by PTX pretreatment. Similar results were observed in the immortalized HASM (Figures 1E and 1F). Finally, we examined a physiologic response of HASM, using magnetic twisting cytometry. As previously described, TAS2R agonists cause a decrease in twisting force (“relaxation”) in HASM (3, 13). Figure 1G shows that the DPD-promoted decrease in twisting force was markedly attenuated by PTX pretreatment. Taken together, these data confirm that the biochemical and physiologic responses to agonist by TAS2Rs in HASM cells are transduced via one or more members of the PTX-sensitive G proteins of the Gi family.
Figure 1.
Bitter taste receptor (TAS2R) function in human airway smooth muscle (HASM) is inhibited by pertussis toxin (PTX). Primary HASM cells or D9 immortalized HASM cells were treated with media alone or media with 0.5 μg PTX for 24 hours, and the intracellular Ca2+ ([Ca2+]i) response to 250 μM of the TAS2R14 agonist diphenhydramine (DPD) or vehicle was determined. (A) Representative tracings in which DPD or vehicle was added at the 19-second time point. (B) The mean (±SE) results of the peak [Ca2+]i from five experiments, *P < 0.01 PTX versus media alone treatment. (C and E) Representative Western blots of phospho–extracellular signal–regulated kinase (ERK) 1/2 and total ERK1/2 in response to 500 μM DPD from 0 to 30 minutes. (D and F) Mean (±SE) of the phospho-ERK1/2 responses from four experiments, *P < 0.01 PTX versus media. (G) Physiologic response of primary HASM to 250 μM DPD in the absence or presence of PTX pretreatment, as determined by magnetic twisting cytometry. Results are mean (±SE) from 445 to 466 measurements from cells from 4 different culture wells. *P < 0.001 PTX versus vehicle. RFU, relative fluorescent units.
Expression of the Gi Family of α Subunits in HASM
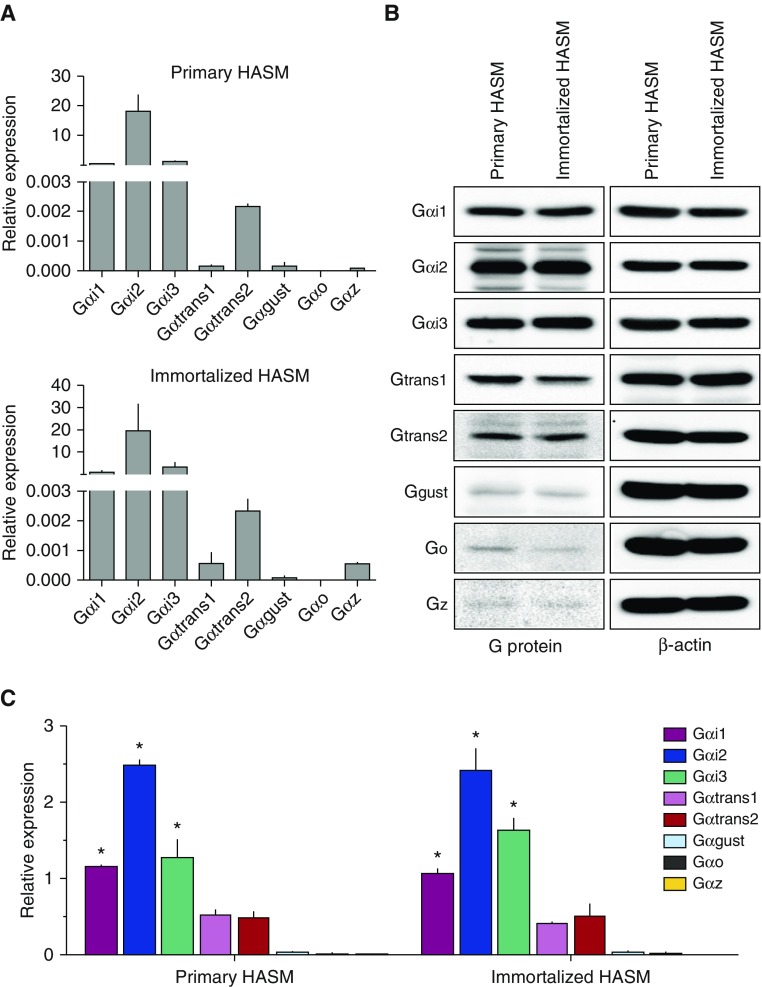
We ascertained both mRNA and protein expression of the eight α subunits. Figure 2A shows the mRNA expression in HASM using quantitative RT-PCR and is expressed as 2−ΔCt, where ΔCt is defined as the difference between Ct values of the given G protein α subunit and glyceraldehyde 3-phosphate dehydrogenase. By far the most abundant mRNAs of this class in HASM are Gαi1, Gαi2, and Gαi3, being more than 5,000-fold greater than Gαgust. Indeed, Gαgust mRNA expression was quite low (CT values ∼30), Gαo was typically below the limits of detection, and Gαz transcripts were not detected or were very low. Gαtrans2 mRNA levels were reliably detected, but were still several hundred fold less than any of the Gαi transcripts, whereas Gαtrans1 transcripts were minimally detected (Figure 2A). Overall, the pattern of the mRNA levels of these Gαi subunits was the same between primary HASM cells and the D9 hTERT immortalized HASM line (Figure 2A). For comparison of the protein expression levels, we encountered low expression of some of the α subunits. Thus, in Figure 2B, we show the long exposure times from the images for representative Western blots for Gαgust, Gαo, and Gαz. As indicated, Gαi1, Gαi2, and Gαi3 were the most abundantly expressed at the protein level, whereas expression levels of Gαgust, Gαo, and Gαz were very low. Gαtrans1 and Gαtrans2 were expressed at intermediate levels compared with the others in the group. Figure 2C shows results from multiple experiments, normalized to β-actin, and provides a semiquantitative assessment of Gαi family α subunit protein expression in HASM cells. The mRNA and protein data are congruent except for Gαtrans1, where we observe protein expression, but little mRNA expression in either cell line. Taken together, though, the results of these expression studies indicate that Gαi1, Gαi2, and Gαi3 are the dominant Gi protein α subunits in HASM, with intermediate levels of Gαtrans1 and Gαtrans2 protein expression. Gαgust expression was very low, whereas Gαo and Gαz were inconsistently detected at the very low level.
Figure 2.
Expression of Gα subunits in HASM. (A) Mean mRNA expression as determined by quantitative RT-PCR (three experiments). (B) Representative Western blots for the eight Gα subunits and β-actin (the exposures for Gαgust, Gαo, and Gαz were greater than the other subunits). (C) Mean (±SE) of protein expression presented as the ratio of the α subunit to β-actin from three to four experiments (*P < 0.001 compared with all other subunits).
siRNA Knockdowns Identify the Primary Transducers of TAS2R Signaling in HASM
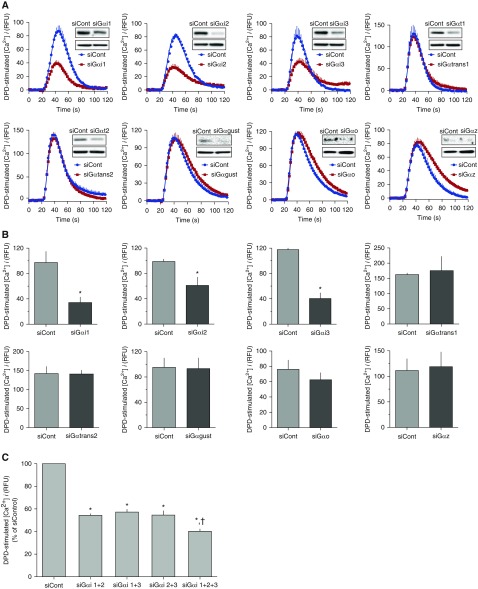
We next ascertained the effects of siRNA transfections that decrease specific Gα subunits on the two signaling pathways, [Ca2+]i and ERK1/2. For these studies, we used the immortalized HASM line (which has the same expression and function phenotypes as the primary HASM cells; see Figures 1 and 2), because the former was found to be more readily transfected. Figure 3A shows representative DPD-stimulated [Ca2+]i tracings from these cells under conditions of scrambled or Gα subunit siRNA transfections. Knockdown of Gαi1, Gαi2, and Gαi3 each resulted in an approximately 40–50% decrease in [Ca2+]i signaling. In contrast, transfections that yielded knockdowns of Gαtrans1, Gαtrans2, Gαgust, Gαo, and Gαz had no effect on [Ca2+]i signaling (Figures 3A and 3B). Transfections with siRNAs for all three Gαi subunits reduced [Ca2+]i signaling greater than knockdowns of any single Gαi subunit (Figure 3C), but the effect was not additive.
Figure 3.
Selective knockdowns of certain Gα subunits in HASM attenuate TAS2R signaling to [Ca2+]i. (A) Representative [Ca2+]i tracings in response to 250 μM DPD after transfection of immortalized HASM cells with control (scrambled) or α subunit–specific small interfering RNA (siRNA), with the inset showing a representative Western blot indicating the degree of α subunit knockdown. (B) Mean (±SE) of the peak [Ca2+]i response to 250 μM DPD from four experiments. *P < 0.01 versus control siRNA. (C) Peak [Ca2+]i DPD responses to various combinations of Gαi1, Gαi2, or Gαi3 knockdowns. *P < 0.01 versus control siRNA, †P < 0.05 versus all other combinations (n = 5–6).
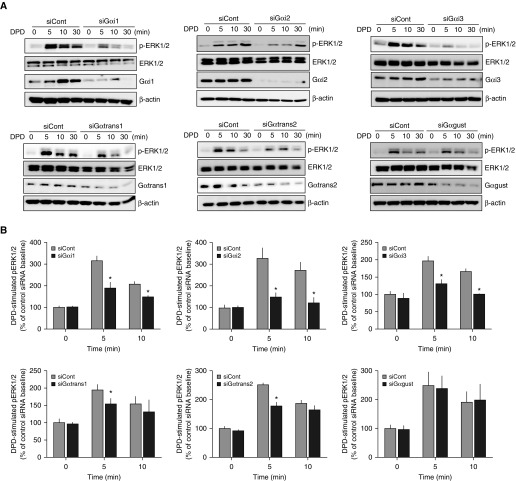
Representative ERK1/2 experiments are shown in Figure 4A. Due to low or nonexistent expressions, Gαo and Gαz knockdown experiments were not performed. As can be seen, knockdown of any of the Gαi subunits caused a decrease in DPD-stimulated phosphorylation of ERK1/2 at the 5- and 10-minute time points (Figure 4B). From these data, we cannot define, in absolute terms, which is the “preferred” Gαi subunit for TAS2R signal transduction, because the siRNAs reduced Gαi proteins to different levels (Figure 4A). However, it is nevertheless clear that each of these highly expressed subunits contributes to TAS2R coupling to this pathway. For the transducins, we observed a small decrease in phospho-ERK1/2 at the 5-minute, but not at the 10-minute, time point with knockdown of either Gαtrans1 or Gαtrans2 (Figures 4A and 4B). Knockdown of Gαgust had no effect on agonist-promoted phosphorylation of ERK1/2.
Figure 4.
Selective knockdowns of certain Gα subunits in HASM attenuates TAS2R signaling to ERK1/2 phosphorylation. (A) Representative Western blots from immortalized HASM cells showing phosphorylated ERK1/2 (pERK1/2), total ERK1/2, the Gα subunit expression, and β-actin, under control or siRNA knockdown conditions. Cells were treated with 500 μM DPD for the indicated times. (B) Mean (±SE) results of pERK1/2 expression normalized to the group mean baseline (t = 0) condition from four experiments. *P < 0.05 versus control siRNA.
Gαi2 Directly Couples to TAS2R14 in an Agonist-Dependent Manner
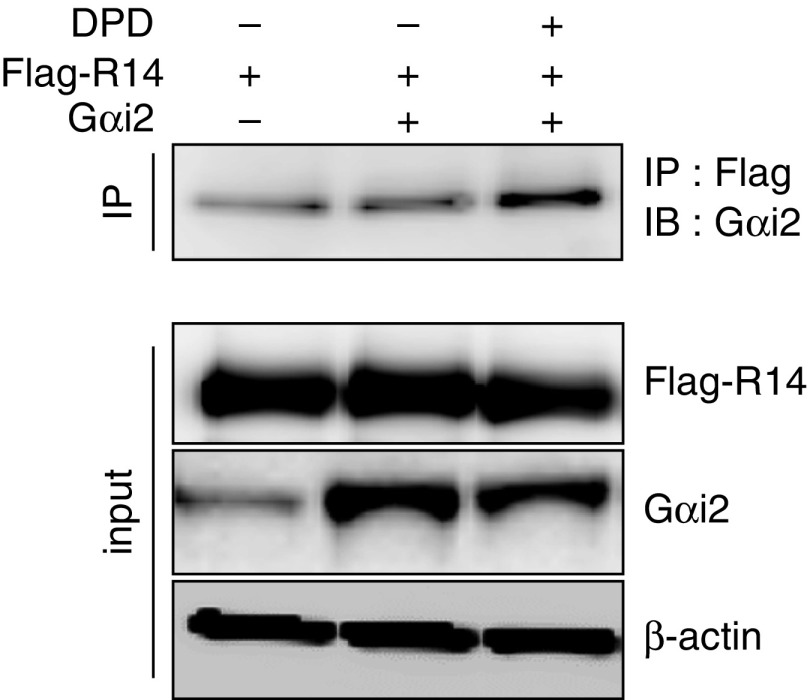
As indicated in the above studies, each of the Gαi subunits appears to be capable of transducing TAS2R signaling to [Ca2+]i or ERK1/2. Given that Gαi2 has roughly 68% amino acid identity with the canonical TAS2R G protein, gustducin (Table 1), we performed experiments to confirm that Gαi2 can indeed directly interact with TAS2R14 (the receptor for DPD) in an agonist-dependent manner. HEK-293T cells were transfected with Gαi2 and FLAG-tagged TAS2R14. After 48 hours, the cells were exposed to vehicle or the agonist, DPD, and, 3 minutes later, the cells were treated with the cross-linking agent, dithiobis (succinimidyl propionate). The cross-linking step was used to ensure that any physical coupling between G protein and receptor was not lost during the subsequent coimmunoprecepitation steps. The cells were then solubilized, immunoprecipitated with FLAG antibody, and the precipitate subjected to SDS-PAGE and Western blotting with anti-Gαi2 antibody. As shown in Figure 5, the coimmunoprecipitation signal was increased when cells were treated with the agonist, consistent with agonist-promoted physical coupling of the receptor to this G protein α subunit.
Table 1.
Homology Matrix of Amino Acid Identities Shown as Percent Identity for the Human Gαi Family of G Proteins
| Gαgust | Gαtrans1 | Gαtrans2 | Gαi1 | Gαi2 | Gαi3 | Gαo | Gαz | Accession Number | |
|---|---|---|---|---|---|---|---|---|---|
| Gαgust | 100 | 78 | 80 | 68 | 68 | 68 | 62 | 58 | A8MTJ3.2 |
| Gαtrans1 | — | 100 | 82 | 68 | 66 | 65 | 63 | 54 | P11488.5 |
| Gαtrans2 | — | — | 100 | 69 | 70 | 69 | 61 | 57 | NP_005263.1 |
| Gαi1 | — | — | — | 100 | 88 | 94 | 71 | 67 | NP_002060.4 |
| Gαi2 | — | — | — | — | 100 | 86 | 68 | 66 | CAG33064.1 |
| Gαi3 | — | — | — | — | — | 100 | 70 | 67 | AAM12621.1 |
| Gαo | — | — | — | — | — | — | 100 | 62 | AAH30027.2 |
| Gαz | — | — | — | — | — | — | — | 100 | CAG30381.1 |
Figure 5.
TAS2R14 physically couples to Gαi2. Human embryonic kidney-293T cells were transfected with cDNA constructs to express FLAG-TAS2R14 and Gαi2, exposed to the agonist DPD or vehicle for 3 minutes, and then the cells were treated with the cross-linking agent dithiobis (succinimidyl propionate). Solubilized lysates were immunoprecipitated with FLAG-antibody and the precipitates immunoblotted for Gαi2. An increase in the coimmunoprecipitation signal was observed with TAS2R14 agonist exposure. Results are representative of three experiments. IB, immunoblot; IP, immunoprecipitation.
Signaling and Physiologic Effects of Overexpressing Gαi2 and Its Peptide Inhibitor on TAS2R14 Function in ASM
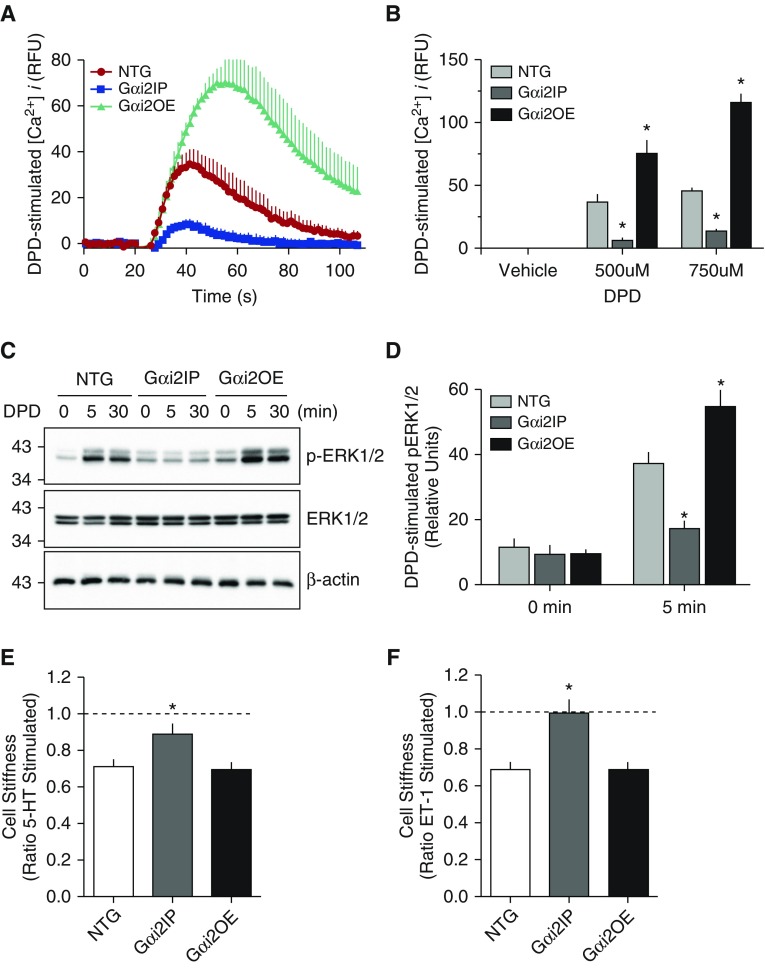
The above results lead us to explore TAS2R signaling with previously constructed transgenic mouse ASM cell lines overexpressing Gαi2 (Gαi2OE) or expressing a Gαi2 inhibitory peptide (Gαi2IP). ASM cells derived from nontransgenic (NTG) littermates were used as controls. In transgenic Gαi2IP ASM, DPD-mediated [Ca2+]i signaling was significantly attenuated by over 50% compared with NTG cells (Figures 6A and 6B). These results are consistent with the knockdown studies shown in Figure 3B. In the transgenic Gαi2OE ASM, DPD-mediated [Ca2+]i signaling was enhanced by roughly twofold. The results of TAS2R-mediated phosphorylation of ERK1/2 in these cells are shown in Figures 6C and 6D, and revealed a decrease in this signal in Gαi2IP cells and a modest increase in the Gαi2OE cells. To ascertain the physiologic consequences of this altered functional coupling to Gαi2, we used magnetic twisting cytometry (Figure 6E). Cells were twisted (contracted) with endothelin-1 or 5-hydroxytryptamine. NTG mouse ASM displayed approximately 35% decrease in cell stiffness (relaxation) upon TAS2R14 activation. In contrast, cells expressing the Gαi2IP showed little or no change in TAS2R-mediated stiffness, consistent with depressed coupling between the receptor and native Gαi. However, the Gαi2OE mouse ASM showed a decrease in stiffness with TAS2R14 activation that was not different than NTG.
Figure 6.
Biochemical and physiologic signaling in ASM cells from transgenic mice overexpressing Gαi2 (Gαi2OE) or a Gαi2 peptide inhibitor (Gαi2IP). (A) Representative [Ca2+]i tracings from mouse ASM Gαi2OE or Gαi2IP cells in response to 500 μM DPD. (B) Mean (±SE) results of peak [Ca2+]i response from four experiments. *P < 0.01 versus nontransgenic (NTG). (C) Representative results of the pERK1/2 response from the three cell lines. (D) Mean (±SE) of the pERK1/2 response from four experiments, *P < 0.05 versus NTG. (E and F) Physiologic consequences of inhibiting and overexpressing Gαi2, as determined using magnetic twisting cytometry. Cells were first contracted with 10 μM of endothelin-1 (ET-1) or 5-hydroxytryptamine (5-HT), as indicated. Results are from 178–293 measurements. *P < 0.005 versus NTG.
Discussion
The typical therapeutic agents for treating asthma and chronic obstructive pulmonary disease are corticosteroids to reduce inflammation, and direct and indirect bronchodilators for treating airway obstruction. Indirect bronchodilators (17, 18) are antagonists for receptors (such as the M3-muscarinic) that contract ASM, which are activated by local increases in bronchospastic mediators (such as acetylcholine). Their effectiveness is dependent on the relative activity of a given pathway. In contrast, direct bronchodilators relax ASM regardless of the constrictive signal. Currently, only one class of direct bronchodilators, β-agonists, acting at β2-adrenergic receptors (β2AR), are available for clinical use. β-agonists are used for acute relief of airway obstruction and as controller therapy for prevention. The treatment response to β-agonists, however, is associated with significant interindividual variability and a number of unfavorable outcomes (19–25).
We have undertaken a systematic approach to identify and characterize HASM G protein–coupled receptors to find other pathways that act to constrict or dilate airways, so as to consider additional receptors for therapeutic development (26). Unexpectedly, we found expression of multiple TAS2Rs on HASM, with 6 of the 25 human subtypes expressed at levels greater than the β2AR (3). Agonists for these TAS2Rs evoked marked relaxation of HASM in ex vivo studies, single-cell measurements, and an in vivo murine model of asthma (3). These findings have led to considering TAS2R agonists as therapy for obstructive lung diseases, either as primary agents or in addition to β-agonists (17, 27). Ongoing studies have used high-throughput screening and medicinal chemistry to identify agonists with high affinity and selectivity (2). Subsequently, TAS2Rs have been identified on cell types in other organs, indicating a previously unrecognized chemosensory system in the body that has a broad range of physiologic and pathologic implications, and also represents new avenues for drug development (8). Of concern in understanding TAS2R signaling in extraoral tissues is the very low expression of Gαgust found in most of these cell types. Although it is now recognized that GPCRs can couple to more than one G protein, this is typically in the setting of abundantly expressed G proteins (28, 29). In terms of non–taste cell TAS2Rs, such as those on HASM, the presumed canonical G protein is at the limit of detection by quantitative RT-PCR and Western blots, suggesting that the main transducer of TAS2R signaling in HASM is not Gαgust. Because of the degree of amino acid identity (Table 1), and the sensitivity of the biochemical and physiological responses of TAS2R activation in HASM to PTX, we hypothesized that one or more members of the Gi family, which are abundantly expressed in HASM, carries out signal transduction from receptor to effector in this cell type.
Here, we show that, in ASM, the Gαi1,2,3 subfamily clearly carries out TAS2R signal transduction, with no measurable contribution from Gαgust. This is most likely due to the fact that these Gαi subunits have the capacity to couple to TAS2Rs (as we show here), and that the low expression of Gαgust, which we also know couples to TAS2Rs, in essence minimizes any potential functional relevance of this G protein in this particular cell type. The transducins, which have even greater amino acid identity with gustducin than Gαi1,2,3 (Table 1), also appear to play a minor role, at least in signaling to ERK1/2. The lower expression levels of the transducins may also be the basis for its minor role in TAS2R signaling in HASM, as we also know that these G proteins can couple to TAS2Rs (30). The fact that the ERK1/2 pathway, but not the [Ca2+]i pathway, was affected by transducin knockdowns is a phenomenon observed in other multidimensional signaling systems. It may be related to different levels of signaling amplification that are present between two pathways, such that there are spare receptor G protein units for one pathway versus another (31). This also illustrates the advantages of the knockdown strategy we used here as compared with simply overexpressing a given α subunit, which could lead to either promiscuous coupling or saturation. Indeed, we did not observe an increase in ASM relaxation to TAS2R agonist in the mouse ASM Gαi2OE cells (Figure 6E), probably because of the abundance of this α subunit in ASM cells in the native state.
We have previously shown that TAS2R signaling is sensitive to gallein (which sequesters the Gβγ subunit) and PLC inhibitors (3). Taken together with the current data, it is clear that Gβγ released from a heterodimer composed of Gαi1, Gαi2, or Gαi3 α subunits carries out the majority of TAS2R signaling in HASM, and, indeed, multiple Gβγ subunit combinations found in Gi heterodimers have been shown to stimulate PLC (32, 33). It is recognized that G protein α subunits are dynamically regulated by multiple pathologic events, and we can now place the relevance of such changes into the context of TAS2R agonist treatment of obstructive airways disease. Gαi2 (and/or Gαi1 and Gαi3) is increased in various animal or cell models of bronchial hyperresponsiveness, airway inflammation, and asthma (11, 34–38). This increase has also been shown to decrease β-agonist bronchodilator responsiveness, because β2ARs also couple to Gαi, and thus attenuate receptor-activated adenylyl cyclase (11). However, this increase in Gαi would not be expected to affect TAS2R function, and may even enhance it, given that this subfamily is responsible for the ASM relaxation response to TAS2R activation. Chronic activation of some Gi-coupled receptors has been shown to down-regulate cellular levels of Gαi protein. For example, with the α2AAR, stably transfected and natively expressing cells display approximately 40% loss of Gαi2 after 24 hours of exposure to high (saturating) concentrations of receptor agonist (39, 40). There are many Gi-coupled receptors expressed on HASM (26), and it is conceivable that prolonged exposure to certain locally produced substances in pathologic states, such as asthma or chronic obstructive pulmonary disease, might result in Gαi down-regulation. Although we are not aware of such decreases, this particular scenario might lead to a reduction of the effectiveness of TAS2R agonists in relaxing ASM. Even a complete loss of one of these subunits, however, would not be expected to result in total loss of TAS2R function, because Gαi1, Gαi2, and Gαi3 all appear to be capable of transmitting TAS2R activation in HASM.
In the taste field, Gαgust has been considered the primary G protein for TAS2Rs; however, there is evidence within several studies that suggest alternative coupling. Gαgust knockout mice retain some sensitivity to bitter tastants, suggesting that one or more additional G proteins in taste cells may provide for coupling (41). Given the high degree of homology between gustducin and transducin (Table 1), a transgenic mouse was developed overexpressing Gαtrans1 on the Gαgust null background. Bitter taste responsiveness in this mouse was partially, but not fully, restored, which suggested that Gαtrans1 can substitute for Gαgust, but expression levels or heterogeneity of transgene expression may have limited rescue (30). In other studies, where single-cell recordings were undertaken, in some cells there was actually no difference in TAS2R signaling by some agonists between Gαgust(−) and Gαgust(+) cells, again pointing to alternative G proteins that can functionally couple to TAS2Rs (42). Taken together with the current work, where Gαgust expression in HASM is very low, it may be the case that any of several Gi family α subunits will couple to TAS2Rs, and that the principal TAS2R-activated G protein in a given cell type is based primarily on expression levels. As indicated in Table 1 and the amino acid sequence alignment in Figure E1, one can readily group these eight G proteins into several subfamilies. Gαtrans1 and Gαtrans2 show the greatest homology to Gαgust (78 and 80%, respectively). Gαi1, Gαi2, and Gαi3 are highly similar to each other, and are 68% identical to Gαgust. Given our results, and the locations of the differences in amino acid sequences between this group and Gαgust (43) (Figure E1), this degree of homology appears to be adequate for TAS2R coupling. Although Gαo has some degree of sequence identity to Gαi1, Gαi2, and Gαi3, the identity to Gαgust is 62%. A similar comparison is apparent for Gαz, with identity to Gαgust being 58%. It thus seems less likely that Gαo and Gαz participate in TAS2R signaling in HASM due to this lower amino acid similarity to Gαgust, the low protein expression of these subunits, and the PTX insensitivity, which excludes Gαz. Regardless, our goal for this study was not to determine the “affinities” of the eight α subunits for coupling to TAS2Rs, but rather to ascertain which of these α subunits are pertinent for TAS2R signaling in HASM. We show that, in this cell type, unlike that of the taste cell, Gαi1, Gαi2, and Gαi3 are the principal G proteins to which TAS2Rs couple to intracellular signaling and relaxation.
Acknowledgments
Acknowledgments
The authors thank Tara Rosin and Charmaine Disimile for manuscript preparation (University of South Florida Morsani College of Medicine).
Footnotes
This work was supported by National Institutes of Health grants HL045967, HL114471, and HL107261.
Author Contributions: Conception, design, and conduct of experiments, analysis of data, and interpretation of results—D.K., J.A.W., E.G., S.S.A., and S.B.L.; writing and preparation of the manuscript—D.K., J.A.W., S.S.A., and S.B.L.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0304OC on February 1, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol. 2005;154:37–72. doi: 10.1007/s10254-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol. 2011;45:1069–1074. doi: 10.1165/rcmb.2011-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulkkinen V, Manson ML, Säfholm J, Adner M, Dahlén SE. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol. 2012;303:L956–L966. doi: 10.1152/ajplung.00205.2012. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande DA, Robinett KS, Wang WC, Sham JS, An SS, Liggett SB. Bronchodilator activity of bitter tastants in human tissue. Nat Med. 2011;17:776–778. doi: 10.1038/nm0711-776b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson GD, Ellefsen KL, Dawson SP, Pearson JE, Parker I. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Sci Signal. 2016;9:ra108. doi: 10.1126/scisignal.aag1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AA, Liggett SB, Munger SD. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012;26:4827–4831. doi: 10.1096/fj.12-215087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L523–L534. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Pauer SH, Yong HM, An SS, Liggett SB. β2-adrenergic receptors chaperone trapped bitter taste receptor 14 to the cell surface as a heterodimer and exert unidirectional desensitization of taste receptor function. J Biol Chem. 2016;291:17616–17628. doi: 10.1074/jbc.M116.722736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGraw DW, Elwing JM, Fogel KM, Wang WC, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest. 2007;117:1391–1398. doi: 10.1172/JCI30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C792–C801. doi: 10.1152/ajpcell.00425.2001. [DOI] [PubMed] [Google Scholar]
- 13.Camoretti-Mercado B, Pauer SH, Yong HM, Smith DC, Deshpande DA, An SS, Liggett SB. Pleiotropic effects of bitter taste receptors on [Ca2+]i mobilization, hyperpolarization, and relaxation of human airway smooth muscle cells. PLoS One. 2015;10:e0131582. doi: 10.1371/journal.pone.0131582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krzywinski M, Altman N, Blainey P. Points of significance: nested designs: for studies with hierarchical noise sources, use a nested analysis of variance approach. Nat Methods. 2014;11:977–978. doi: 10.1038/nmeth.3137. [DOI] [PubMed] [Google Scholar]
- 15.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. Distinct β-arrestin– and G protein–dependent pathways for parathyroid hormone receptor–stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. β-arrestin–dependent, G protein–independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 17.Liggett SB. Bitter taste receptors on airway smooth muscle as targets for novel bronchodilators. Expert Opin Ther Targets. 2013;17:721–731. doi: 10.1517/14728222.2013.782395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liggett SB. Bitter taste receptors in the wrong place: novel airway smooth muscle targets for treating asthma. Trans Am Clin Climatol Assoc. 2014;125:64–74; discussion 74–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Beasley R, Pearce N, Crane J, Burgess C. β-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;104:S18–S30. doi: 10.1016/s0091-6749(99)70270-8. [DOI] [PubMed] [Google Scholar]
- 20.Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting β2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327:1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 21.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 22.Kraan J, Koëter GH, vd Mark TW, Sluiter HJ, de Vries K. Changes in bronchial hyperreactivity induced by 4 weeks of treatment with antiasthmatic drugs in patients with allergic asthma: a comparison between budesonide and terbutaline. J Allergy Clin Immunol. 1985;76:628–636. doi: 10.1016/0091-6749(85)90786-9. [DOI] [PubMed] [Google Scholar]
- 23.Lipworth BJ. Airway subsensitivity with long-acting β2-agonists: is there cause for concern? Drug Saf. 1997;16:295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Salpeter SR, Wall AJ, Buckley NS. Long-acting β-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322–8.e2. doi: 10.1016/j.amjmed.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Sears MR, Taylor DR. The β2-agonist controversy: observations, explanations and relationship to asthma epidemiology. Drug Saf. 1994;11:259–283. doi: 10.2165/00002018-199411040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein–coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA. 2008;105:5230–5235. doi: 10.1073/pnas.0801319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: new targets, and new ways to target the old ones. Pharmacol Ther. 2016;164:82–96. doi: 10.1016/j.pharmthera.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eason MG, Kurose H, Holt BD, Raymond JR, Liggett SB. Simultaneous coupling of α2-adrenergic receptors to two G-proteins with opposing effects: subtype-selective coupling of α2C10, α2C4, and α2C2 adrenergic receptors to Gi and Gs. J Biol Chem. 1992;267:15795–15801. [PubMed] [Google Scholar]
- 29.Eason MG, Liggett SB. Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the α2A-adrenergic receptor: evidence for distinct structural determinants that confer Gs versus Gi coupling. J Biol Chem. 1995;270:24753–24760. doi: 10.1074/jbc.270.42.24753. [DOI] [PubMed] [Google Scholar]
- 30.He W, Danilova V, Zou S, Hellekant G, Max M, Margolskee RF, Damak S. Partial rescue of taste responses of α-gustducin null mice by transgenic expression of α-transducin. Chem Senses. 2002;27:719–727. doi: 10.1093/chemse/27.8.719. [DOI] [PubMed] [Google Scholar]
- 31.Costa-Neto CM, Parreiras-E-Silva LT, Bouvier M. A pluridimensional view of biased agonism. Mol Pharmacol. 2016;90:587–595. doi: 10.1124/mol.116.105940. [DOI] [PubMed] [Google Scholar]
- 32.Boyer JL, Graber SG, Waldo GL, Harden TK, Garrison JC. Selective activation of phospholipase C by recombinant G-protein α- and β γ-subunits. J Biol Chem. 1994;269:2814–2819. [PubMed] [Google Scholar]
- 33.Blank JL, Brattain KA, Exton JH. Activation of cytosolic phosphoinositide phospholipase C by G-protein β γ subunits. J Biol Chem. 1992;267:23069–23075. [PubMed] [Google Scholar]
- 34.Hakonarson H, Herrick DJ, Grunstein MM. Mechanism of impaired β-adrenoceptor responsiveness in atopic sensitized airway smooth muscle. Am J Physiol. 1995;269:L645–L652. doi: 10.1152/ajplung.1995.269.5.L645. [DOI] [PubMed] [Google Scholar]
- 35.Hakonarson H, Herrick DJ, Serrano PG, Grunstein MM. Mechanism of cytokine-induced modulation of β-adrenoceptor responsiveness in airway smooth muscle. J Clin Invest. 1996;97:2593–2600. doi: 10.1172/JCI118708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hakonarson H, Maskeri N, Carter C, Hodinka RL, Campbell D, Grunstein MM. Mechanism of rhinovirus-induced changes in airway smooth muscle responsiveness. J Clin Invest. 1998;102:1732–1741. doi: 10.1172/JCI4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunstein MM, Hakonarson H, Maskeri N, Chuang S. Autocrine cytokine signaling mediates effects of rhinovirus on airway responsiveness. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1146–L1153. doi: 10.1152/ajplung.2000.278.6.L1146. [DOI] [PubMed] [Google Scholar]
- 38.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol. 1999;276:L405–L411. doi: 10.1152/ajplung.1999.276.3.L405. [DOI] [PubMed] [Google Scholar]
- 39.Gasic S, Green A. Gi down-regulation and heterologous desensitization in adipocytes after treatment with the α2-agonist UK 14304. Biochem Pharmacol. 1995;49:785–790. doi: 10.1016/0006-2952(94)00537-v. [DOI] [PubMed] [Google Scholar]
- 40.Jewell-Motz EA, Donnelly ET, Eason MG, Liggett SB. Agonist-mediated downregulation of G α i via the α2-adrenergic receptor is targeted by receptor-Gi interaction and is independent of receptor signaling and regulation. Biochemistry. 1998;37:15720–15725. doi: 10.1021/bi980999r. [DOI] [PubMed] [Google Scholar]
- 41.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 42.Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit α-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23:9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conklin BR, Bourne HR. Structural elements of G α subunits that interact with G β γ, receptors, and effectors. Cell. 1993;73:631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]