Dysfunction of the microvasculature is a cardinal feature of sepsis pathophysiology, leading to abnormal perfusion and tissue edema that can progress to significant injury in multiple organs. Within the lung, sepsis frequently causes inflammation-induced disruption of the pulmonary vasculature, alveolar edema, refractory hypoxemia, and ultimately respiratory failure, a devastating process defined as acute respiratory distress syndrome (ARDS) (1). Recent work has highlighted a critical role of the glycocalyx/endothelial surface layer (ESL), a previously underappreciated proteoglycan-rich layer that lines the vascular lumen, in sepsis-induced organ dysfunction, including ARDS (2, 3). The ESL consists of proteoglycans, hydrated glycosaminoglycan polymers, and other structural components that form a protective lining along the interior of blood vessels. It includes polymerized heparin sulfate (HS), which undergoes extensive processing, including epimerization and sulfation, which provides the negative charge that facilitates interactions with positively charged proteins that are necessary for its critical signaling functions (4). Accumulating evidence indicates that injury to the endothelial glycocalyx is an early step in sepsis and other inflammatory conditions. These observations suggest biomarkers produced by ESL disruption may be useful diagnostic tools. For example, plasma levels of syndecan-1, a heparan sulfate proteoglycan component of the ESL, were significantly elevated in a cohort of patients with sepsis who died compared with survivors, as well as in those who developed acute kidney injury (5). Moreover, levels of glycosaminoglycan fragmentation in urine correlated with acute kidney injury and mortality in patients with septic shock or ARDS (6). In addition, the observation that sepsis-induced ESL injury occurs early in the disease process suggests interventions that inhibit or reverse this event may have powerful therapeutic effect. Outcomes are improved in preclinical mouse models of sepsis by inhibition of heparinase, which degrades the glycocalyx, or administration of heparin sulfate-like compounds that resist degradation by heparinase (7, 8). At this time, there is no definitive evidence that targeting the ESL will be beneficial in humans afflicted with sepsis. Interventions such as corticosteroids, activated protein C, and N-acetylcysteine all have been reported to exert positive effects on glycocalyx function, but lack proven benefit in patients with sepsis (9). Nevertheless, the hypothesis that agents that promote ESL protection and reconstitution may have clinical use in sepsis remains a plausible concept worthy of further exploration.
To that end, in this issue of the Journal, Yang and colleagues (pp. 727–737) build on their previous work in this area to characterize mechanisms responsible for restoration of the pulmonary ESL after injury (10). Using sophisticated intravital lung microscopy techniques, they observe that the rate of recovery of the ESL is dependent on the type of injurious stimulus, as repair is delayed in a mouse model of sepsis-induced lung injury compared with sterile lung injury caused by heparinase III infusion. This delayed recovery was attributed to impaired reconstitution of the ESL, rather than continued degradation, because inhibition of heparinase, which is implicated in cecal ligation and puncture-mediated ESL destruction (8), did not accelerate recovery. They have identified that ESL repair requires fibroblast growth factor (FGF) signaling, which is suppressed during sepsis (10). Heparin sulfate within the ESL has well-described interactions with FGF ligands and receptors, and FGF signaling has important roles in the response to vascular damage (11, 12). Using both genetically engineered endothelial specific FGFR1/2 knockout mice and pharmacologic inhibition of FGFR1, the authors convincingly demonstrate that FGF signaling mediates ESL reconstitution. To determine whether HS fragments could activate FGF signaling, the authors conducted mass spectroscopy experiments to characterize the HS fragments that resulted from heparinase III degradation. These fragments were able to promote downstream Erk phosphorylation and induce expression of the HS polymerizing enzyme, exostosin-1. Moreover, exostosin-1 was induced by heparinase III infusion, but suppressed after CLP. Taken together, these data demonstrate that the delay in ESL recovery after sepsis occurs as a result of suppression of FGFR1 expression and the subsequent downstream exostosin-1 expression (Figure 1).
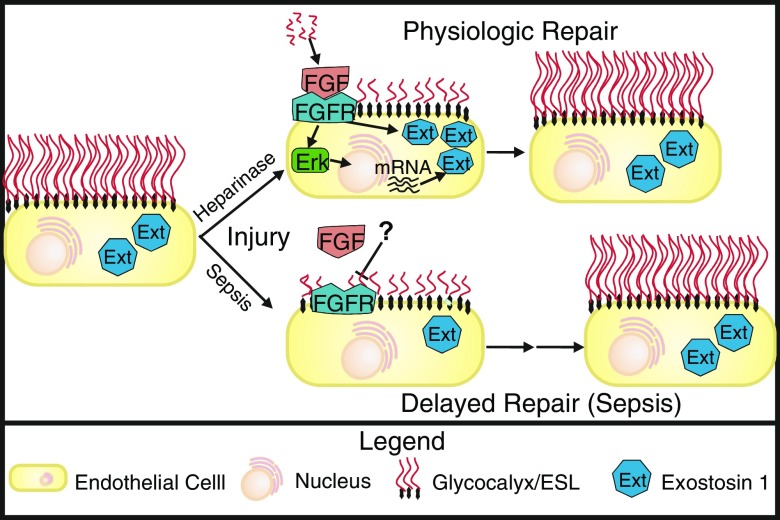
Figure 1.
Sepsis inhibits fibroblast growth factor (FGF)-mediated repair of the pulmonary endothelial glycocalyx. At baseline, pulmonary endothelial cells project a long glycocalyx/endothelial surface layer (ESL) into the vessel lumen that is maintained in part via exostosin-1 (Ext) expression (left). Injury by either infusion of heparinase or sepsis causes disruption of the pulmonary endothelial ESL and release of heparin sulfate (HS) fragments. Physiologic repair of the ESL after heparinase injury occurs rapidly because HS fragments activate FGF signaling, which leads both to activation of existing Ext as well as Erk-mediated up-regulation of additional Ext, resulting in rapid reconstitution of the ESL. In contrast, repair after sepsis-induced injury is significantly delayed because sepsis leads to inhibition of fibroblast growth factor receptor 1 (FGFR) signaling and subsequent suppression of the FGF-mediated pathway that is necessary for Ext activation and up-regulation.
This interesting work highlights the importance of the ESL in protecting the lung from edema formation and elucidates key signaling mechanisms that may facilitate the development of pharmacologic agents to promote ESL repair. Achieving this goal is likely to require further identification of the mechanisms responsible for suppression of FGF signaling, as well as a more thorough understanding of the complex regulation of exostosin-1 expression, which remain unclear and are a limitation of the current study. Also, the observation that inhibition of FGFR1 signaling did not completely suppress ESL recovery suggests the presence of other reparative pathways that merit further investigation. These key details, in addition to an understanding of the mechanisms underlying the large differences in the reported rates of ESL reconstitution in different vascular beds (8, 13), are not characterized in the current work. Support for the clinical relevance of this proposed mechanism also will require verification of decreased FGFR1 expression in patients with sepsis, as the authors’ data indicate that HS fragments pooled from the plasma of humans with sepsis are capable of significantly augmenting FGFR1 signaling above that of samples collected from control patients. Thus, the delay in ESL repair during sepsis is more likely a result of decreased FGFR1 expression than a deficiency in FGF receptor ligands.
In summary, Yang and colleagues have substantially advanced our understanding of the mechanisms of ESL reconstitution and have identified a key difference in the pathophysiology of sepsis-induced lung injury compared with sterile injury, suggesting a potential role for FGF-promoting agents to accelerate ESL recovery in patients with sepsis (10). This work adds to our growing appreciation for the importance of heterogeneity in patients categorized by our current definitions as having sepsis or ARDS (14). This heterogeneity has been a major impediment to past efforts to develop broadly effective therapeutic interventions for these syndromes, but hopefully the current work represents another step toward the goal of enhancing the precision of our approaches to sepsis and ARDS. On the basis of this work, future investigations in this area are warranted to elucidate additional mechanistic details that will help develop pharmacologic interventions to enhance FGF signaling and ESL repair, with the goal of building a protective glycocalyx wall within the pulmonary vasculature in patients with sepsis.
Footnotes
Support provided by National Institutes of Health Grants F30 HL121982 (A.N.R.) and P01 HL126609 and P01 HL098050 (S.M.D.).
Author contributions: A.N.R. and S.M.D. drafted and edited the manuscript for important content.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin L, Koczera P, Zechendorf E, Schuerholz T. The endothelial glycocalyx: new diagnostic and therapeutic approaches in sepsis. Biomed Res Int. 2016;2016:3758278. doi: 10.1155/2016/3758278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013;1:23494. doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haeger SM, Yang Y, Schmidt EP. Heparan sulfate in the developing, healthy, and injured lung. Am J Respir Cell Mol Biol. 2016;55:5–11. doi: 10.1165/rcmb.2016-0043TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puskarich MA, Cornelius DC, Tharp J, Nandi U, Jones AE. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J Crit Care. 2016;36:125–129. doi: 10.1016/j.jcrc.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, Ammons LA, Hiller TD, Suflita MA, Yu Y, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JW, Zullo JA, Liveris D, Dragovich M, Zhang XF, Goligorsky MS. Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Exp Ther. 2017;361:115–121. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19:26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Haeger SM, Suflita MA, Zhang F, Dailey KL, Colbert JF, Ford JA, Picon MA, Stearman RS, Lin L, et al. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol. 2017;56:727–737. doi: 10.1165/rcmb.2016-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Liaw L, Prudovsky I, Brooks PC, Vary C, Oxburgh L, Friesel R. Fibroblast growth factor signaling in the vasculature. Curr Atheroscler Rep. 2015;17:509. doi: 10.1007/s11883-015-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104:1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaver CM, Bastarache JA. Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clin Chest Med. 2014;35:639–653. doi: 10.1016/j.ccm.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]