Abstract
Background
The role of CD4+CD25highCD127− T-reg cells in solid-organ Transplant (Tx) acceptance has been extensively studied. In previous studies on kidney and liver recipients, peripheral T-reg cell counts were associated to graft survival, while in lung Tx, there is limited evidence for similar findings. This study aims to analyze long term peripheral kinetics of T-reg-cells in a cohort of lung recipients and tests its association to several clinical variables.
Methods
From jan 2009 to dec 2014, 137 lung Tx recipients were submitted to an immunological follow up (median: 105.9 months (6.7–310.5)). Immunological follow up consisted of a complete blood peripheral immuno-phenotype, inclusive of CD4+CD25highCD127− T and FOXP3+ cells. We tested the association between T-reg and relevant variables by linear OR regression models for repeated measures, adjusting for time from Tx. Also, by ordered logistic models for panel data, the association between Chronic Lung Allograft Dysfuncton (CLAD) onset/progression and T-reg counts in the previous 3 months was tested.
Results
Among all variables analyzed at multivariate analysis: Bronchiolitis Obliterans Syndrome (OR −6.51, p < 0.001), Restrictive Allograft Syndrome (OR −5.19, p = 0.04) and Extracorporeal photopheresis (OR −5.65, p < 0.001) were significantly associated to T-reg cell. T-reg cell counts progressively decreased according to the severity of CLAD. Furthermore, patients with higher mean T-reg counts in a trimester had a significantly lower risk (OR 0.97, p = 0.012) of presenting CLAD or progressing in the graft dysfunction in the following trimester.
Conclusions
Our present data confirm animal observations on the possible role of T-reg in the evolution of CLAD.
Keywords: Lung transplantation, Immunology, T-regulatory cell, Long term follow-up
Background
Lung transplant (Tx) is the only therapy for patients with end-stage respiratory failure, but despite the use of potent immunosuppressive protocols, the long-term survival of lung graft is hampered by the occurrence of chronic lung allograft dysfunction that occurs in nearly 50% of patients by the 5th post-Tx year [1]. According to recent classification, there are two major clinical phenotypes of chronic irreversible allograft dysfunction (CLAD): the so called obstructive form: bronchiolitis obliterans syndrome (BOS), (nearly 70% of cases) [2], and the newly described restrictive allograft syndrome (RAS) [3, 4]. CLAD is considered a complex multifactorial process [5], which leads to chronic airway inflammation and tissue injuries and ultimates in a fibro-reparative response. This can involve either the airway lumen in BOS, or the interstitial and airways in RAS [6]. Although recent evidence ascribes an important pathogenic role in CLAD to aspecific inflammatory mechanisms [7–10], the importance of specific immunity can’t be denied. Several previous studies support a crucial role for allo and auto-reactive T cells in BOS pathogenesis [11–14]. T cell response as well as the production of allo or auto-antibodies have been described, and are dependent on the migration of antigen-presenting cells in secondary lymphoid organs and on the direct stimulation of T cells within graft [15, 16]. All of this experimental and clinical evidence strongly points out the difficulties in achieving immunologic tolerance, either central or peripheral, of lung graft [17–19].
Since 1995, when Sakaguchi described a CD4+CD25+T cell subset displaying regulatory properties, several subsets of T-reg cells have been described and classified as natural T-reg, thymus-derived and naturally committed to immunoregulation, and inducible T-reg, generated in the periphery during immune response. So far the best characterised T-reg population is the thymus-derived CD4 subset, constitutively expressing CD25 (at a high rate) and FoxP3, a transcription regulator which controls the maturation and function of T-reg cells. Although a specific and exclusive marker of T-reg activity has not yet been identified, the diminished surface expression of CD127, as well as the expression of CD39 or CD152, are considered characteristic of CD4+CD25+ T-reg cells. [20, 21]These molecules can be expressed, even if transiently, as Foxp3, by other cell types including recently activated effector T cells. However, experimental evidence suggests that most of the Foxp3+ T-reg cells are within the CD4+CD25highCD127− cell population, so that sorting of this subset is actually considered the best method for the isolation and in vitro amplification of these cells [22].
The role of CD4+CD25highCD127− T-reg cells in Tx acceptance has been studied in experimental models and also in clinical settings. Animal studies demonstrated that CD4+CD25+ Foxp3+ T-reg cells are expanded in tolerized animals, that tolerance can be infectiously transferred to naïve animals, and appears to be a specific and localized phenomenon. These data have been partially confirmed in kidney and liver Tx recipients, showing a positive correlation between graft survival and the number of circulating CD4+CD25+ T-reg cells, as well as a correlation between their peripheral fluctuation and the occurrence of acute and chronic rejection [18, 23, 24].
As for lung Tx, evidence is limited and somewhat contradictory [25]. In previous cross- sectional studies, we showed that lung Tx recipients with BOS had significantly lower peripheral CD4+CD25high T-reg cells than clinically stable lung recipients, and demonstrated their functional regulatory profile, in vitro [26, 27]but subsequesnt studies failed to demonstrate a correlation between T-reg cell counts and long term lung Tx outcome [28–30].
To date, however, little is known about the long term evolution of peripheral CD4+CD25highCD127− regulatory T cells in lung Tx. The present study aims at analyzing their long-term kinetic on a cohort of lung recipients and at testing its association with several clinical and pharmacological variables (CLAD, treatment, infections, kidney dysfunction or neoplasia).
Methods
Patients
In our Center, LTx has been performed since 1991. The study was conducted from 1st January 2009 to 31st December 2014. During this period 137 lung recipients entered the immunological follow-up, with a median follow-up of 105.9 months (range 6,7–310,5). All patients were submitted to the assessment of a complete peripheral immune phenotype at least twice a year.
Our immune suppression protocol has undergone some changes over time. No patients underwent induction treatment at time of transplantation. All patients transplanted between 2001 and 2007 were treated with a triple immunosuppressive regimen including cyclosporine, azathioprine, and prednisone, whereas patients transplanted since January 2007 received a modified standard triple regimen, with tacrolimus, mycophenolate mofetil, and prednisone. In case of refractory acute rejection (AR), patients were switched from cyclosporine to tacrolimus and from azathioprine to mycophenolate mofetil. In the presence of documented renal dysfunction, patients were treated with low- dose tacrolimus plus everolimus.
All patients underwent surveillance and on-need bronchoscopies; biopsy-proven episodes of AR following criteria [31] were treated with steroid boluses and, in case of AR recurrence or persistence, with a standard anti-thymoglobulin course and a modulation of the IS regimen. Our surveillance protocol has been reported in previous studies [32]. BOS diagnosis and severity grades has been assessed according to published guidelines [6, 33–35]. The CLAD subtype RAS has been retrospectively re-classified for patients diagnosed before 2013, according to radiological (CT scan showing a pattern of persistent interstitial/upper lobe fibrosis) and functional criteria (persistent decline in forced expiratory volume in 1 s (FEV 1) of >20% compared to the best post Tx value and a decline in total lung capacity of >10% compared to baseline) [33–35]. In case of a BOS 0p or early RAS diagnosis, patients were prescribed a 3-month course of chronic low-dose azithromycin. At the same time, patients underwent gastro-esophageal reflux assessment and maximization of anti-reflux medical treatment. In case of a further decline consistent with a CLAD diagnosis, since 2003, patients are referred to the Apheresis Unit for compassionate ECP treatment [36]. Severity of CLAD has been graduated according to the degree of functional impairment as described for BOS [6, 33–35]. Our cytomegalovirus surveillance protocol has been detailed in previous studies [37].
The ethics review committee of the IRCCS Policlinico San Matteo of Pavia approved the research n° ICS 30.4/RF00.65.
Flow cytometric determination of peripheral lymphocyte subsets
Flow cytometry was performed on a Beckman Coulter Navios using Kaluza software (Beckman Coulter). Briefly, 50 μl of fresh whole blood was incubated with the appropriate amounts of fluorochrome-labeled monoclonal antibodies CD45 APC Alexa Fluor 750 (Allophycocyanin-Alexa Fluor 750; clone J33), CD4 APC (Allophycocyanin; clone 13B8.2), CD69ECD (R Phycoerythrin-Texas Red; clone TP1.55.3), CD25 PE (R Phycoerythrin; clone B1.49.9), CD127 PC5 (R Phycoerythrin-Cyanine 5.1; clone R34.34) for the T-reg and CD45 APC Alexa Fluor 750, CD4 APC, CD3 FITC (Fluorescein isothiocyanate; clone UCHT1), CD8 PE (R Phycoerythrin; clone B9.11), CD56 PC5 (; clone N901), CD16 PC5 (PC5.1; clone 3G8), CD19 PC7 (PC 7; clone J3–119) for the lymphocytic population (Beckman Coulter) at room temperature in the dark for 15 min using appropriate mouse immunoglobulin isotypes as a control. Following incubation, 1 ml erythrocyte lysing solution (VersaLyse, Beckman Coulter) was added to the samples and incubated under the same conditions for 20 min. In some samples, peripheral blood mononuclear cells (PBMC) were stained with CD4 APC, CD25 FITC (clone B1.49.9), and CD127 Alexa Fluor 647 (clone HIL-7R-M21) (BD Pharmingen), fixed and permeabilized, followed by intracellular staining with Foxp3 PE (clone PCH101) or control IgG1 (Human Regulatory T cell Staining kit, eBioscience) for 30 min. Finally, the cells were characterized by flow cytometry analysis.
T-reg cell analysis
Peripheral blood assessment of regulatory T cell subsets has significantly evolved in the last years, even more so during the realization of this study. For this reason, we started the protocol performing the analysis of peripheral CD4+CD25highCD127− cell subset, and later on we also started to quantify FOXP3+ cells.
Therefore, we could determine that peripheral CD4+CD25highCD127− T-reg cell subset included a mean of 93.15% (±4.34) FOXP3+ cells (Fig. 1). We also found a high significant correlation between peripheral CD4+CD25highCD127− T-reg cell counts and FOXP3+ cell counts over the whole cohort. For this reason, and in accordance with published evidence [20] we included CD4+CD25highCD127− cell determinations in the final statistical analysis, expressed this subset as absolute number (n°/μl peripheral blood) and named these cells approvedCD4+CD25highCD127− T-reg cells. Analysis performed with CD4+CD25highCD127− T-reg cells expressed as percentage of the whole CD4 subset, gave analogous results.
Fig. 1.
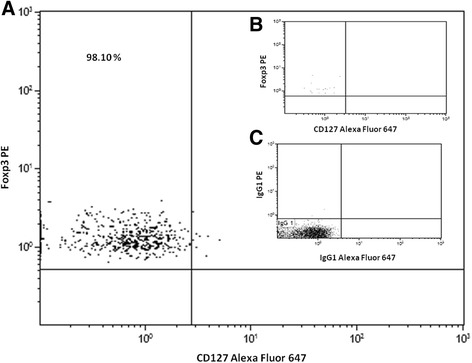
a. Representative dot plot of intracellular staining Foxp3 in CD4 + CD25highCD127 - T-reg cell.b. Representative dot plot of intracellular staining Foxp3 in CD4 + CD25-CD127pos (0%) T-reg cell. c. Representative dot plot of isotope control
Statistical analysis
Descriptive statistics were produced for demographic, clinical and laboratory characteristics of cases. Mean and standard deviation (SD) are shown for normally distributed variables, and median and interquartile range (IQR) for non-normally distributed variables, numbers and percentages for categorical variables.
The association between T-reg and a number of predictors was explored by means of bivariate and multivariate linear mixed models, with patient and time since Tx as random effects and predictors and time (also) as fixed effects. Clinical and Immunological variables that were included in the analysis are shown in Table 1.
Table 1.
demographic and clinical features of the patients enrolled in this study
| N° of patients = 137 | |
|---|---|
| Mean age at Tx (years ± SD) | 46.1 ± 12.86 |
| Sex (M:F) | 91:46 |
| Lenght of post-Tx follow-up (months/range, median) | 105.9 / 6.7–310.5 |
| Tx Indications | |
| Emphysema / Alpha1 antitrypsin Deficiency | 27 |
| Primary graft disfunction | 4 |
| Bronchiectasis / Cystic Fibrosis | 26 |
| Interstitial lung disease | 52 |
| Pulmonary hypertension / Ebstein’s disease/Eisenmenger Syndrome / Mounier-Kuhn Syndrome | 25 |
| Rare pulmonary conditions | 3 |
| Type of Tx | |
| Single lung Tx | 46 |
| Double lung Tx | 83 |
| Heart and Lung Tx | 8 |
| Immunosuppression therapy | |
| Cyclosporine | 23 |
| Tacrolimus | 119 |
| Azathioprine | 15 |
| Mycophenolate mofetil | 88 |
| Rapamycin | 37 |
| Prednisone | 137 |
| Extracorporeal photopheresis (ever) | 39 |
| Azitromycin (ever) | 98 |
| Total of determination = 1943 | |
| Stable | 796 |
| CLAD | 1147 |
| BOS | 838 |
| RAS | 309 |
| BOS 0p | 320 |
| CLAD 1 | 412 |
| CLAD 2 | 169 |
| CLAD 3 | 246 |
CLAD chronic lung allograft dysfunction, BOS bronchiolitis obliterans syndrome, RAS restrictive allograft syndrome. BOS 0p according to published guidelines [6, 30–32] CLAD 1: includes both BOS1 and RAS patients with FEV1 80–65% + FVC < 80%; CLAD 2: includes both BOS2 and RAS patients with FEV1 64–50% + FVC < 80%; CLAD 3: includes both BOS3 and RAS patients with FEV1 < 50% + FVC < 80%
To assess whether CD4+CD25highCD127− T-reg cell count was associated to CLAD occurrence or higher CLAD grade in the subsequent 3 months, we fit ordered logistic models for panel data. When a dependent variable has more than two categories and the values of each category have a meaningful sequential order where a value is indeed ‘higher’ than the previous one, then we can use ordinal logistic regression. In this instance, we are comparing patients in CLAD grade > k versus ≤k. The interpretation is: for a one unit change in the predictor variable (e.g. T-reg cell count in previous trimester), likelihood of having CLAD grade > k instead of ≤k is an OR times higher.
In all cases, tests were two-tailed, and the p-value cut-off for significance was set at 0.05.
Stata computer software version 14.0 (Stata Corporation, 4905 Lakeway Drive, College Station, Texas 77,845, USA) was used for statistical analysis.
Results
Patients
Overall 137 patients were included in this retrospective study and followed-up for a median of 105.9 months (6.7–310.5). Demographics and clinical features of included patients are listed in Table 1, including gender, age at Tx, Tx indication, type of Tx, length of follow-up and type of immunosuppressive drugs used. Some patients (27%) were enrolled in the immunological follow- up at time of Tx, while the others entered the study later in the FU period (median follow-up months at first determination in the latter group: 82,4 months, range 14,0–275,7) this, as stated above, was considered in the statistical analysis.
Being a prospective immunological FU, the overall number of included samples is high: n° 1943 with a median of 14 sample/patient.
Variables associated to T-reg cell counts
Results of bivariate and multivariate analysis are shown in Table 2. As for acute rejection, the limited number of samples obtained during an episode of acute cellular (Agrade ≥2, any B grade) or humoral rejection (globally < than 15) did not allow us to assess any statistical association with peripheral T-reg cell number. All tested immunological variables (CD3+,CD4+, CD8+, CD19+ and CD16 + CD56+ cells) resulted positively correlated associated to T-reg (Table 2) at the initial bivariate analysis. Among clinical variables only the presence of CLAD, treatment with azathioprine and ECP were significantly associated to peripheral CD4+CD25highCD127− T-reg cell counts. Given the lack of association with T-reg cell counts with the majority of tested variables only azathioprine, ECP, and CLAD, as well as BOS or RAS and CLAD severity grades were included in the multivariate model. At multivariate analysis, only the association with azathioprine did not retain its significance (Table 2). Furthermore, when BOS severity grade was considered, a significant progression of CD4+CD25highCD127− T-reg cell decline was observed (Fig. 2). Of note, no difference was detectable between the 2 CLAD phenotypes, BOS and RAS (Fig. 3). The significant negative association of T-reg cell counts with ECP was confounded on the presence and severity of CLAD, and thus was not considered clinically relevant.
Table 2.
bi- and multi-variate linear regression analysis per CD4+CD25highCD127−
| Bivariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | Coef | 95% CI | P-value | Coef | 95% CI | P-value |
| Time | Per each month since Tx | 0.16 | 0.10 to 0.22 | <0.001 | 0.18 | 0.12 to 0.24 | <0.001 |
| CLAD | RAS | −6.60 | −11.594 to-1.603 | 0.01 | −5.19 | −10.2 to −0.16 | 0.04 |
| BOS | −8.14 | −11.807 to −4.473 | <0.001 | −6.51 | −10.26 to −2.77 | 0.001 | |
| BOS grade | 0p | −2.05 | −4.165 to 0.069 | 0.06 | |||
| 1 | −2.53 | −5.122 to 0.068 | 0.06 | ||||
| 2 | −6.49 | −9.724 to −3.261 | <0.001 | ||||
| 3 | −4.90 | −8.731 to −1.066 | 0.01 | ||||
| Lymphocytic population | cd3 | 0.009 | 0.008 to 0.01 | <0.001 | |||
| cd 3 cd4 | 0.021 | 0.019 to 0.023 | <0.001 | ||||
| cd 3 cd8 | 0.01 | 0.008 to 0.011 | <0.001 | ||||
| cd19 | 0.05 | 0.038 to 0.057 | <0.001 | ||||
| cd1 6 cd56 | 0.01 | 0.005 to 0.008 | <0.001 | ||||
| cd4cd25high | 0.60 | 0.585 to 0.62 | <0.001 | ||||
| Immunosuppressive therapy | Cyclosporine | 0.20 | −3.598 to 4.007 | 0.92 | |||
| Tacrolimus | −2.71 | −5.783 to 0.367 | 0.08 | ||||
| Azathioprine | 4.65 | 0.438 to 8.856 | 0.03 | −2.12 | −6.73 to 2.48 | 0.336 | |
| Mycophenolate | 0.04 | −1.735 to 1.823 | 0.96 | ||||
| Rapamycin | −0.75 | −3.202 to 1.703 | 0.55 | ||||
| Prednisone | −6.32 | −15.329 to 2.687 | 0.17 | ||||
| Azithromycin therapy | −0.74 | −2.505 to 1.016 | 0.41 | 0.58 | −1.28 to 2.43 | 0.542 | |
| Extracorporeal photopheresis | −6.03 | −8.259 to −3.804 | <0.001 | −5.65 | −8.05 to −3.25 | <0.001 | |
| Kidney failure | −1.36 | −3.62 to 0.907 | 0.24 |
Bold characters mean statistically significant variable
Linear mixed models were fitted, with patient and time since Tx as random effects, and individual predictors and time as fixed effects
CLAD chronic lung allograft dysfunction, RAS restrictive allograft syndrome, BOS bronchiolitis obliterans syndrome
Fig. 2.
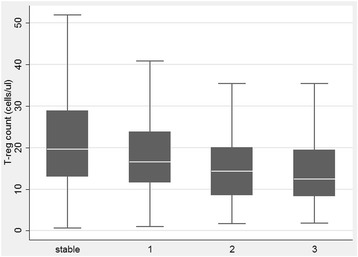
Relation between T-reg cells count and BOS grade (calculated according to BOS severity classification [30]). Figure is purely descriptive. Median, IQR and min/max are depicted. *p < 0.001; §p = 0.01
Fig. 3.
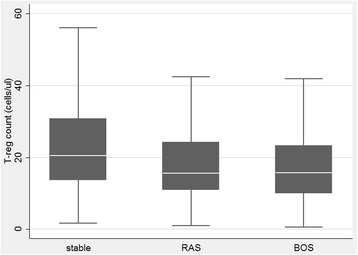
Relation between T-reg cells count and stable, BOS (p < 0.001) or RAS (p = 0.01) patients’ (BOS was diagnosed according to published guidelines [6, 30–32]. RAS has been retrospectively re-classified according to radiological and functional criteria [30–32]).). Figure is purely descriptive. Median, IQR and min/max are depicted
Prediction of CLAD
In patients with higher mean peripheral CD4+CD25highCD127− T-reg counts, the risk of presenting CLAD or progressing in the graft dysfunction in the subsequent trimester was significantly lower (OR 0.97, 95%CI 0.95–0.99, p = 0.012). Variables were included in the model if clinically relevant or statistically significant (at the 0.05 level) at bivariate analysis, without further selection (Table 3).
Table 3.
ordered logistic regression analysis for association between CLAD grade and a number of predictors, including T-reg in the previous trimester
| CLAD grade | OR | P-value | 95% Conf. interval |
|---|---|---|---|
| CD4 + CD25highCD127- | 0.97 | 0.012 | 0.95 to 0.99 |
| Azithromycin therapy | 5.80 | <0.001 | 2.81 to 11.99 |
| Prednisone | 0.22 | <0.001 | 0.13 to 0.39 |
| Trimester | 1.01 | <0.001 | 1.01 to 1.02 |
NB in ordered logistic regression models, OR > 1 implies higher risk of being in a higher category (in this case: CLAD grade, calculated for all CLAD patients according to BOS severity classification [30]) rather than in any of the lower categories; an OR <1, implies a lower risk
Discussion
On the basis of our results we can confirm and extend our previous observation on the role of CD4 + CD25high T-reg cells in lung graft acceptance/rejection [27]. To our knowledge, this is the first longitudinal study reporting the long-term kinetics of peripheral CD4+CD25highCD127− T-reg cells in lung recipients, and the first observation, that variation of peripheral T-reg cell counts can predict CLAD onset/progression.
Although, during the study period, a methodological evolution in the identification of T-reg cells subsets occurred, we clearly demonstrated that CD4+CD25highCD127− subset is significantly enriched with FOXP3+ cells, thus inferring that it is endowed with regulatory functions. Based on these observations, we have referred to these cells as CD4+CD25highCD127− T-reg cells.
Experimental evidence in animals have strongly suggested that lung graft acceptance is associated to an intra-graft [16, 38] and peripheral [39] T-reg cell expansion and that adoptive transfer of “in vitro” generated and expanded T-reg cells attenuates airway obliteration in a rat OB model [40].
Conversely, human studies have provided, to date, sub-optimal evidence on the protective role of T-reg cells towards chronic graft rejection. Most studies, in fact, have explored peripheral CD4 + CD25+ T-reg cell counts early after Tx, suggesting that a low number of these effectors might predict subclinical or clinical acute kidney rejection [41, 42], and can be significantly affected by the type of immunosuppressive regimen [43, 44]. A few studies are available on lung Tx recipients with conflicting results. Neujahr and colleagues found higher BAL CD4 + CD25high cell counts in patients with biopsy-proven AR, but failed to find a correlation with peripheral blood findings [45]. On the contrary, lower CD4 + CD25high T cell counts were detected both at peripheral and BAL level of BOS patients with respect to stable lung recipients [27, 46].Moreover, an increase in BAL CCR7(+) T-reg cell percentage was found to correlate to a reduced risk of BOS by Gregson and colleagues [47]. Krustrup and colleagues, analyzing CD4 + CD25 + FOXP3+ cell at tissue level, found higher frequencies during acute rejection episodes [29], but failed to demonstrate an association between these cells and the risk of CLAD occurrence [30]. We believe that prediction studies on peripheral T-reg in Tx recipients are invalidated by the high fluctuation of blood-T-reg cell in time, possibly in relation to a number of confounding variables including drugs, infectious complications, neoplasia, kidney failure and ECP treatment.
Thanks to the large number of determinations that we included in this long term study, we took into account this variability in the analysis, thus we succeeded in demonstrating that: 1) peripheral counts of CD4+CD25highCD127− T-reg cells significantly decrease in CLAD patients; 2) the degree of their decrease is associated with the severity of BOS and, most noteworthy; 3) CLAD onset or GRADE is significantly associated to mean T-reg cell counts in the previous trimester.
Finally, we also tested the association of CD4+CD25highCD127− T-reg cell counts with a number of clinical variables, including also infections and different immunosuppressive drugs. Interestingly, only azathioprine and ECP were found to be significantly associated to them at the univariate analysis, while, in the multivariate model, only the ECP effect was significant. However, the ECP effect was confounded on CLAD and CLAD severity, and is therefore to be considered irrelevant from the clinical point of view.
Interestingly, unlike previous evidence in literature [26, 48]we could not detect any significant variation of T-reg cell counts with respect to other specific immunosuppressive drugs such as cyclosporine A, tacrolimus or everolimus. The effect of maintenance drugs on T-reg cell counts has been poorly evaluated in humans, the role of CsA and tacrolimus has been studied in mice with controversial results [44] while everolimus has been shown to enhance the number of peripheral T-reg cells in liver recipients following a tacrolimus to everolimus conversion [48]. However, it was recently observed that sirolimus, another mTor inhibitor, did not expand peripheral T-reg cells in a cohort of de novo kidney recipients [49], in analogy to our present observation. Finally, in the present study we could not assess the possible role of lympho-depleting strategies, since induction treatment is not performed at our center.
We must acknowledge a number of limitations. First, although larger than other cohorts, our sample is not sufficiently large to identify small associations, especially in some subgroups. Second, patients started FU at different times from Tx; although this was taken into account in the analysis, we cannot be certain that bias would not impact on results. Third, although data were collected prospectively, this study was designed retrospectively as an exploratory analysis; therefore, patients were sampled at irregularly spaced times, and sometimes upon clinical basis, so there might be unobserved variables that might have affected results. In addition, we performed all the analysis using both the absolute number and the percentage of T-reg in peripheral blood, but, although both had the same trends over time and were significantly correlated with CLAD, we decided to show only the results concerning absolute number analysis because they had the best correlation with the analyzed variables. Finally, a relevant issue, from the biological point of view, is that graft tolerance is a results of a balance between regulatory and effector clones. In this study we couldn’t take into account the weight of allospecific effectors at different time points.
Conclusion
In conclusion, given all the above limitations, we could find an association between T-reg cell counts and CLAD onset/progression, including both BOS and RAS phenotype, thus confirming animal observations on the protective role of T-reg cells with respect to CLAD.
Acknowledgments
- The authors thank Dr. Katherine O’Donohoe for careful revision of the manuscript.
- Gruppo Comunale AIDO “Luigi Stabile” Castegnato for the economical support.
Funding
Not applicable.
Availability of data and materials
The dataset used and analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AR
Acute rejection
- BAL
Bronchoalveolar lavage
- BOS
Bronchiolitis obliterans syndrome
- CLAD
Chronic lung allograft dysfunction
- CsA
Cyclosporine A
- CT
Computed tomography
- ECP
Extracorporeal photopheresis
- FEV1
Forced expiratory volume in 1 second
- FU
Follow-up
- GER
Gastro esophageal reflux
- IS
Immunosuppressant
- LTx
Lung transplant
- RAS
Restrictive allograft syndrome
- T-reg
T regulatory cells
- Tx
Transplant
Authors’ contributions
D.P. participated in the writing of the paper, participated in the performance of the research and in performing clinical follow-up, participated in data analysis. M.M. participated in research design, participated in the writing of the paper, participated in the performance of the research, participated in data analysis, contributed new reagents or analytic tools. S.M. participated in the performance of the research, contributed new reagents or analytic tools. A.B. participated in the performance of the research, contributed new reagents or analytic tools. L.S. participated in the writing of the paper, participated in data analysis. E.C. participated in the performance of the research, contributed new reagents or analytic tools. T.O. participated in the performance of the research and in performing clinical follow-up. G.S. participated in the performance of the research and in performing clinical follow-up. C.T. participated in data analysis. F.A. participated in performing surgical transplant and clinical follow-up. A.M.D’A. chief of surgical unit, participated in performing surgical transplant. F.M. Participated in research design, participated in the writing of the paper, participated in the performance of the research and in performing clinical follow-up, participated in data analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The ethics review committee of the IRCCS Policlinico San Matteo of Pavia approved the research n° ICS 30.4/RF00.65.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Davide Piloni, Phone: +390382501017, Email: davidepiloni@live.it.
Monica Morosini, Email: m.morosini@smatteo.pv.it.
Sara Magni, Email: sara.magni04@gmail.com.
Alice Balderacchi, Email: alice.balderacchi@libero.it.
Luigia Scudeller, Email: l.scudeller@smatteo.pv.it.
Emanuela Cova, Email: e.cova@smatteo.pv.it.
Tiberio Oggionni, Email: t.oggionni@smatteo.pv.it.
Giulia Stella, Email: g.stella@smatteo.pv.it.
Carmine Tinelli, Email: c.tinelli@smatteo.pv.it.
Filippo Antonacci, Email: f.antonacci@smatteo.pv.it.
Andrea Maria D’Armini, Email: am.darmini@smatteo.pv.it.
Federica Meloni, Email: f.meloni@smatteo.pv.it.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Ohmori-Matsuda K, Saito T, et al. Time-dependent changes in the risk of death in pure bronchiolitis obliterans syndrome (BOS) J Heart Lung Transplant. 2013;32(5):484–491. doi: 10.1016/j.healun.2013.01.1054. [DOI] [PubMed] [Google Scholar]
- 3.Sato M, Hwang DM, Waddell TK, Singer LG, Keshavjee S. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2013;32(1):23–30. doi: 10.1016/j.healun.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Ofek E, Sato M, Saito T, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26(3):350–356. doi: 10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 5.Weigt SS, DerHovanessian A, Wallace WD, Lynch JP, 3rd, Belperio JA. Bronchiolitis obliterans syndrome: the Achilles’ heel of lung transplantation. Semin Respir Crit Care Med. 2013;34(3):336–351. doi: 10.1055/s-0033-1348467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verleden SE, Vasilescu DM, McDonough JE, et al. Linking clinical phenotypes of chronic lung allograft dysfunction to changes in lung structure. Eur Respir J. 2015;46(5):1430–9. [DOI] [PubMed]
- 7.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8(9):1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 8.Evers A, Atanasova S, Fuchs-Moll G, et al. Adaptive and innate immune responses in a rat orthotopic lung transplant model of chronic lung allograft dysfunction. Transpl Int. 2015;28(1):95–107. doi: 10.1111/tri.12444. [DOI] [PubMed] [Google Scholar]
- 9.Kreisel D, Goldstein DR. Innate immunity and organ transplantation: focus on lung transplantation. Transpl Int. 2013;26(1):2–10. doi: 10.1111/j.1432-2277.2012.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verleden SE, Ruttens D, Vos R, et al. Differential cytokine, chemokine and growth factor expression in phenotypes of chronic lung allograft dysfunction. Transplantation. 2015;99(1):86–93. doi: 10.1097/TP.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 11.Grossman EJ, Shilling RA. Bronchiolitis obliterans in lung transplantation: the good, the bad, and the future. Transl Res. 2009;153(4):153–165. doi: 10.1016/j.trsl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Snell GI, Westall GP, Paraskeva MA. Immunosuppression and allograft rejection following lung transplantation: evidence to date. Drugs. 2013;73(16):1793–1813. doi: 10.1007/s40265-013-0136-x. [DOI] [PubMed] [Google Scholar]
- 13.Angaswamy N, Tiriveedhi V, Sarma NJ, et al. Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection. Hum Immunol. 2013;74(11):1478–1485. doi: 10.1016/j.humimm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharat A, Mohanakumar T. Autoimmunity and lung transplantation. Front Biosci (Elite Ed) 2012;4:2378–2388. doi: 10.2741/e549. [DOI] [PubMed] [Google Scholar]
- 15.Gracon AS, Wilkes DS. Lung transplantation: chronic allograft dysfunction and establishing immune tolerance. Hum Immunol. 2014;75(8):887–894. doi: 10.1016/j.humimm.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–554. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalev I, Selzner N, Shyu W, Grant D, Levy G. Role of regulatory T cells in the promotion of transplant tolerance. Liver Transpl. 2012;18(7):761–770. doi: 10.1002/lt.23458. [DOI] [PubMed] [Google Scholar]
- 18.Issa F, Robb RJ, Wood KJ. The where and when of T cell regulation in transplantation. Trends Immunol. 2013;34(3):107–113. doi: 10.1016/j.it.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Issa F, Wood KJ. Translating tolerogenic therapies to the clinic - where do we stand? Front Immunol. 2012;3:254. doi: 10.3389/fimmu.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Guo H, Lu L, et al. Sequential monitoring and stability of ex vivo-expanded autologous and nonautologous regulatory T cells following infusion in nonhuman primates. Am J Transplant. 2015;15(5):1253–1266. doi: 10.1111/ajt.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segundo DS, Ruiz JC, Izquierdo M, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82(4):550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 24.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34(2):74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Neujahr DC. Intragraft regulatory T cells and lung transplant outcomes--we are still at square one. Clin Transpl. 2015;29(3):177–178. doi: 10.1111/ctr.12503. [DOI] [PubMed] [Google Scholar]
- 26.Meloni F, Morosini M, Solari N, et al. Peripheral CD4+ CD25+ T-reg cell expansion in lung transplant recipients is not affected by calcineurin inhibitors. Int Immunopharmacol. 2006;6(13–14):2002–2010. doi: 10.1016/j.intimp.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Meloni F, Vitulo P, Bianco AM, Paschetto E. Regulatory CD4+CD25+ T cells in the peripheral blood of lung transplant recipients: correlation with transplant outcome. Transplantation. 2004;77(5):762–766. doi: 10.1097/01.TP.0000116565.86752.6B. [DOI] [PubMed] [Google Scholar]
- 28.Nakagiri T, Warnecke G, Avsar M, et al. Lung function early after lung transplantation is correlated with the frequency of regulatory T cells. Surg Today. 2012;42(3):250–258. doi: 10.1007/s00595-011-0087-3. [DOI] [PubMed] [Google Scholar]
- 29.Krustrup D, Iversen M, Martinussen T, Andersen CB. Time elapsed after transplantation influences the relationship between the number of regulatory T cells in lung allograft biopsies and subsequent acute rejection episodes. Transpl Immunol. 2014;31(1):42–47. doi: 10.1016/j.trim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Krustrup D, Madsen CB, Iversen M, Engelholm L, Ryder LP, Andersen CB. The number of regulatory T cells in transbronchial lung allograft biopsies is related to FoxP3 mRNA levels in bronchoalveolar lavage fluid and to the degree of acute cellular rejection. Transpl Immunol. 2013;29(1–4):71–75. doi: 10.1016/j.trim.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Stewart S, Fishbein MC, Snell GI, et al. Revision of the1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Meloni F, Solari N, Miserere S, et al. Chemokine redundancy in BOS pathogenesis. A possible role also for the CC chemokines: MIP3-beta, MIP3-alpha, MDC and their specific receptors. Transpl Immunol. 2008;18:275–280. doi: 10.1016/j.trim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–444. doi: 10.1164/rccm.200201-003PP. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 35.Verleden GM, Vos R, Vanaudenaerde B, et al. Current views on chronic rejection after lung transplantation. Transpl Int. 2015;28(10):1131–9. [DOI] [PubMed]
- 36.Del Fante C, Scudeller L, Oggionni T, et al. Long-term off-line extracorporeal Photochemotherapy in patients with chronic lung allograft rejection not responsive to conventional treatment: a 10-year single-Centre analysis. Respiration. 2015;90(2):118–128. doi: 10.1159/000431382. [DOI] [PubMed] [Google Scholar]
- 37.Lilleri D, Gerna G, Bruno F, et al. Systemic and local human cytomegalovirus-specific T-cell response in lung transplant recipients. New Microbiol. 2013;36:267–277. [PubMed] [Google Scholar]
- 38.Sommer W, Knöfel AK, Madrahimov N, et al. Allogeneic CD4+CD25high T cells regulate obliterative bronchiolitis of heterotopic bronchus allografts in both porcinized and humanized mouse models. Transplantation. 2015;99(3):482–91. [DOI] [PubMed]
- 39.Shi Q, Niu Y, Cao H, et al. CD28 superagonist antibody treatment attenuated obliterative bronchiolitis in rat allo-orthotopic tracheal transplantation by preferentially expanding Foxp3-expressing regulatory T cells. Transplant Proc. 2012;44(4):1060–1066. doi: 10.1016/j.transproceed.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Shi Q, Cao H, Liu J, et al. CD4+ Foxp3+ regulatory T cells induced by TGF-β, IL-2 and all-trans retinoic acid attenuate obliterative bronchiolitis in rat trachea transplantation. Int Immunopharmacol. 2011;11(11):1887–1894. doi: 10.1016/j.intimp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Oh EJ, Ghee JY, et al. Clinical significance of monitoring circulating CD4+CD25+ regulatory T cells in kidney transplantation during the early posttransplant period. J Korean Med Sci. 2009;24(Suppl):S135–S142. doi: 10.3346/jkms.2009.24.S1.S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giaretta F, Bussolino S, Beltramo S, et al. Different regulatory and cytotoxic CD4+ T lymphocyte profiles in renal transplants with antibody-mediated chronic rejection or long-term good graft function. Transpl Immunol. 2013;28(1):48–56. doi: 10.1016/j.trim.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Krystufkova E, Sekerkova A, Striz I, Brabcova I, Girmanova E, Viklicky O. Regulatory T cells in kidney transplant recipients: the effect of induction immunosuppression therapy. Nephrol Dial Transplant. 2012;27(6):2576–2582. doi: 10.1093/ndt/gfr693. [DOI] [PubMed] [Google Scholar]
- 44.Wang XJ, Leveson-Gower D, Golab K, et al. Influence of pharmacological immunomodulatory agents on CD4(+)CD25(high)FoxP3(+) T regulatory cells in humans. Int Immunopharmacol. 2013;16(3):364–370. doi: 10.1016/j.intimp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Neujahr DC, Cardona AC, Ulukpo O, et al. Dynamics of human regulatory T cells in lung lavages of lung transplant recipients. Transplantation. 2009;88(4):521–527. doi: 10.1097/TP.0b013e3181b0e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhorade SM, Chen H, Molinero L, et al. Decreased percentage of CD4+FoxP3+ cells in bronchoalveolar lavage from lung transplant recipients correlates with development of bronchiolitis obliterans syndrome. Transplantation. 2010;90(5):540–546. doi: 10.1097/TP.0b013e3181e8dabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregson AL, Hoji A, Palchevskiy V, et al. Protection against bronchiolitis obliterans syndrome is associated with allograft CCR7+ CD45RA- T regulatory cells. PLoS One. 2010;5(6):e11354. doi: 10.1371/journal.pone.0011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levitsky J, Mathew JM, Abecassis M, et al. Systemic immunoregulatory and proteogenomic effects of tacrolimus to sirolimus conversion in liver transplant recipients. Hepatology. 2013;57(1):239–248. doi: 10.1002/hep.25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Libetta C, Esposito P, Gregorini M, et al. Sirolimus vs cyclosporine after induction with basiliximab does not promote regulatory T-cell expansion in de novo kidney transplantation: results from a single-center randomized trial. Transpl Immunol. 2015;33(2):117–124. doi: 10.1016/j.trim.2015.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed during the current study available from the corresponding author on reasonable request.


