Abstract
Background
In Japan, nafamostat mesylate (NM) is frequently used as an anticoagulant during continuous renal replacement therapy (CRRT). The dialyzer membrane AN69ST has been reported to adsorb NM and affect the management of anticoagulant therapy. However, the adsorbed amount has not yet been quantitatively assessed. Therefore, in this study, we evaluated the pre- and post-hemofilter prolongation of the activated clotting time (ACT) in patients with AN69ST and PS membranes. We also measured the adsorption of NM in three types of CRRT membranes using an experimental model.
Methods
In a study of patients who underwent CRRT using AN69ST or PS membranes in 2015 at the Advanced Emergency and Critical Care Center, Okayama University Hospital, pre- and post-hemofilter ACT measurements were extracted retrospectively, and the difference was calculated. In addition, AN69ST (sepXiris100), PS (HEMOFEEL SHG-1.0), and PMMA membranes (HEMOFEEL CH-1.0N) were used in an in vitro model of a dialysis circuit, and the concentrations of NM were measured in pre- and post-hemofilter membranes and filtrates.
Results
The ACT difference was significantly lower in the group using AN69ST membranes (p < 0.01). In the in vitro model (n = 4) with adsorption and filtration, the post-hemofilter and filtrate concentrations of NM in AN69ST membranes were significantly lower than those in the PS and PMMA membranes (p < 0.01). The NM adsorption clearance of the AN69ST membrane was significantly higher than that of the PS and PMMA membranes.
Conclusions
The AN69ST membrane had higher NM adsorption than the PS and PMMA membranes. This may have resulted in the lower ACT difference in patients undergoing CRRT using the AN69ST membrane than in patients undergoing CRRT using PS or PMMA membranes.
Keywords: AN69ST, Nafamostat mesylate, Continuous renal replacement therapy, Adsorption, Anticoagulant therapy
Background
Continuous renal replacement therapy (CRRT) has a milder impact on hemodynamics than intermittent hemodialysis therapy and enables strict management of the body fluid balance, acid-base equilibrium, electrolytes, and plasma osmotic pressure. Accordingly, CRRT has been widely used in the treatment of acute renal injury [1]. However, CRRT requires long-term anticoagulation management [2]. In Japan, nafamostat mesylate (NM) is used in most patients receiving CRRT because it does not affect the patient’s coagulability in vivo [3]. NM is a serine protease inhibitor with a molecular weight of 539 Da that exerts non-antithrombin III-mediated anticoagulant effects by strongly inhibiting activated coagulation factors, such as factor IIa (thrombin), factor Xa, and factor XIIa. In addition, NM also has an inhibitory effect on platelet coagulability. In the blood, NM is rapidly degraded by carboxylesterase; as a result, its active half-life in the blood (β-phase) is 23.1 min. NM can also be eliminated through dialysis. Therefore, NM exerts anticoagulant effects only in extracorporeal circulation circuits, whereas in vivo, NM is quickly inactivated, enabling safe management of coagulation [4].
The AN69ST membrane is currently attracting attention in the field of intensive care because of its ability to adsorb cytokines, making it potentially beneficial in the treatment of patients with septic shock [5, 6]. The AN69ST membrane was developed based on the AN69 membrane, which was released in the market in France in 1969. Because the AN69 membrane has a strong negative charge, it poses problems such as induction of bradykinin production [7] and adsorption of pharmacological agents such as NM [8]. Therefore, with the AN69ST membrane, surface treatment has been performed to neutralize the negative charge at the surface of the membrane. Additionally, reduction of bradykinin production has been achieved by reducing the zeta potential at the point of contact between the blood and the surface of the membrane to a level that is lower than that found in the AN69 membrane [9]. However, few studies have examined the adsorption of drugs by the AN69ST membrane, and no previous studies have quantitatively assessed the amount of adsorption.
Therefore, in this study, the pre- and post-hemofilter difference in the activated clotting time (ACT) in patients using the AN69ST membrane was calculated, and the values were compared with those in patients who used PS membranes. In addition, the amounts of NM adsorbed on AN69ST, PS, and PMMA membranes were quantitatively measured and compared in an in vitro model, and the capacity of the AN69ST membrane to adsorb NM was evaluated.
Methods
Measurement of the ACT and calculation of its difference
This study was performed in patients who were hospitalized at the Advanced Emergency and Critical Care Center, Okayama University Hospital in 2015, and who were receiving CRRT with AN69ST or PS membranes. In addition, we extracted data for patients whose respective pre- and post-hemofilter ACT was measured simultaneously after initiation of CRRT, and we calculated the ACT difference (ACT difference [s] = ACT post-hemofilter − ACT pre-hemofilter). For the measurement of the ACT, we used a Hemochron Response device (Heiwa Bussan, Co. Ltd., Tokyo, Japan) and Hemochron ACT tubes (Celite ACT; FTCA510; Heiwa Bussan, Co. Ltd.; Fig. 1). This study was approved by the Ethics Committee of Okayama University Hospital (approval no. Ken1610-510).
Fig. 1.
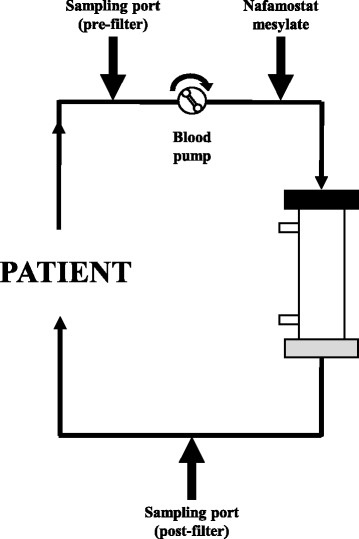
Activating clotting time (ACT) sampling sites
In vitro model of a dialysis circuit and measurement of NM levels
The following hemofilters were used (n = 4 each): AN69ST membrane (sepXiris100; Baxter Co. Ltd., Tokyo, Japan), PS membrane (Hemofeel SHG1.0; Toray Medical Co., Ltd., Tokyo, Japan), and PMMA membrane (Hemofeel CH1.0N; Toray Medical Co., Ltd.; Table 1). The device used in this study was a JUN-505 (Junken Medical, Co., Ltd., Tokyo, Japan). Only normal saline solution was used as the filling solution to eliminate the influence of other substances. In the clinical setting, heparin coating was performed by adding heparin sodium to the filling solution; however, because the presence or absence of heparin has been reported to not result in any differences in the amount of adsorption of high-mobility group box-1 [10], heparin priming was not performed in our study.
Table 1.
Characteristics of the three hemofilters
| Dialysis membrane | Material | Structure | Surface area (m2) | Inner diameter (μm) | Wall thickness (μm) | Priming volume (mL) |
|---|---|---|---|---|---|---|
| sepXiris100 | Polyacrylonitrile (surface treated) | Symmetric | 1.0 | 240 | 50 | 69 |
| Hemofeel SHG-1.0 | Polysulfone | Asymmetric | 1.0 | 200 | 40 | 67 |
| Hemofeel CH-1.0N | Polymethylmethacrylate | Symmetric | 1.0 | 200 | 30 | 58 |
To calculate the NM clearance, pre- and post-hemofilter samples and filtrates were collected under the following conditions: blood pump, 80 mL/min; filtrate pump, 1000 mL/h; NM, 100 mg/h (Fig. 2). Although pre-hemofilter samples were collected from the site before administering NM in the clinical setting to observe excess or shortage of anticoagulant, we collected pre-hemofilter samples from the site after administering NM in an in vitro setting to quantify NM adsorption by each membrane. To normalize the sample concentrations, the timing of blood sampling was aligned with that of the time elapsed since the activation of the pump. The timing for sampling was considered as the time when the NM had fully reached the sampling port, which was determined based on measurement of the priming volume of the blood circuit.
Fig. 2.
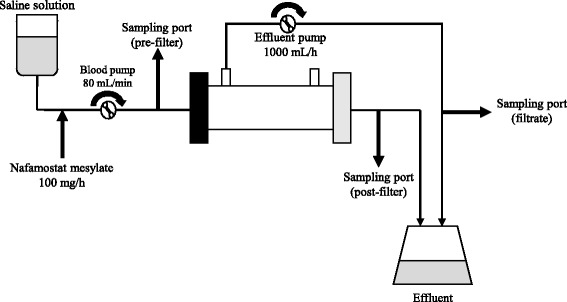
Diagram of in vitro experimental setup (single pass). The heights of all sites of measurement of the inlet pressure, filtration pressure, and return pressure were aligned with the heights of the sites of connection between the dialyzer and the patient. Adjustments were made to maintain a fixed/constant pressure inside the circuit. During sampling, caution was taken not to apply negative pressure. Extracorporeal ultrafiltration method (ECUM) model
The NM used in this study was nafamostat mesylate (MEEK; Meiji Seika Pharma Co. Ltd, Tokyo, Japan), and the normal saline solution used in this study was Terumo (Terumo Corp., Tokyo, Japan). Nafamostat mesylate (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) was used as the standard substance, and ethyl p-hydroxybenzoate (Wako Pure Chemical Industries, Ltd) was used as the internal standard substance.
NM was quantified by high-performance liquid chromatography (HPLC). Measurement devices from Shimadzu Emit Co., Ltd. were used (pump: LC-20AT; ultraviolet/visible detector: SPD-20A; column oven: CTO-20AC; degasser: DGU-20A5R). The HPLC conditions were as follows: flow rate, 1.0 mL/min; wavelength, 260 nm; injection volume, 100 μL; C18-Supersphar column (4 μm, 125 mm × 4 mm); and column temperature, 40 °C. The mobile phase consisted of 0.1 M acetic acid (sodium 1-heptanesulfonate 6.07 g/L):acetonitrile (70:30). An isocratic analysis was performed.
Various equations were used for calculation of NM clearance (Table 2). In addition, for the AN69ST, PS, and PMMA membranes, the sieving coefficient (SC) was 1.0.
Table 2.
Clearance formulas
| Solution clearance (mL/min) | CLs = (CBi − CBo)/CBi × (QB − QF) + QF |
| Filtrate clearance (mL/min) | CLf = CF/CBi × QF |
| Adsorption clearance (mL/min) | CLad = CLs − CLf |
| Sieving coefficient | SC = 2CF/(CBi + CBo) |
C Bi inlet (pre-hemofilter) solute concentration, C Bo outlet (post-hemofilter) solute concentration, CF filtrate solute concentration, QB blood flow, QF filtration flow
Statistical analysis
All data are expressed as the mean ± standard deviation (SD) and were compared using chi-squared tests, Mann-Whitney U tests, or one-way analysis of variance followed by Tukey’s post-test where appropriate. p < 0.05 was considered significant. All statistical calculations were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
Results
The difference in ACT was significantly lower with the AN69ST membrane
There were 122 and 37 ACT measurements in 19 AN69ST-treated patients and 11 PS-treated patients, respectively. Table 3 shows the characteristics of the patients participating in this study. The proportion of sepsis patients was significantly higher in the AN69ST group. Figure 3a shows the pre- and post-filter average ACT in each membrane. Compared with the PS group, the AN69ST group had higher and lower pre- and post-filter ACT values, respectively (p < 0.01). Figure 3b shows the pre- and post-filter ACT difference in the AN69ST and PS membrane groups. The ACT difference was significantly lower in the AN69ST membrane group (p < 0.01). The amounts of NM used with the AN69ST and PS membranes were 16.8 ± 7.1 and 17.7 ± 8.4 mg/h, respectively, and this difference was not significant.
Table 3.
Patient summary
| Dialysis membrane | AN69ST | PS | P value |
|---|---|---|---|
| Age (years) | 69.8 ± 11.4 | 67.4 ± 19.6 | N.S. |
| Sex (male:female) | 13:6 | 6:5 | N.S. |
| Body weight | 58.2 ± 14.6 | 62.3 ± 16.9 | N.S. |
| SOFA | 11.1 ± 4.0 | 9.5 ± 4.4 | N.S. |
| APACHE II | 27.6 ± 10.0 | 26.9 ± 10.4 | N.S. |
| Mortality | 6/19 (32%) | 3/11 (27%) | N.S. |
| Sepsis | 13/19 (68%) | 3/11 (27%) | 0.03 |
| QB (mL/min) | 95.9 ± 8.9 | 95.1 ± 15.4 | N.S. |
| QD (mL/h) | 934.8 ± 329.6 | 1422.4 ± 602.5 | <0.01 |
| QS (mL/h) | 517.7 ± 152.5 | 602.6 ± 312.8 | N.S. |
| QF (mL/h) | 1471.7 ± 414.7 | 1852.4 ± 662.2 | <0.01 |
Data are the mean and standard deviation or number. For intergroup testing, the Mann-Whitney U test or the χ 2 test was performed at suitable locations. AN69ST polyacrylonitrile (surface treated), PS polysulfone, N.S. not significant, SOFA Sequential Organ Failure Assessment, APACHE II Acute Physiology and Chronic Health Evaluation II score, QB blood pump flow rate, QD dialysate pump flow rate, QF filtration pump flow rate, QS substitution fluid pump flow rate
Fig. 3.
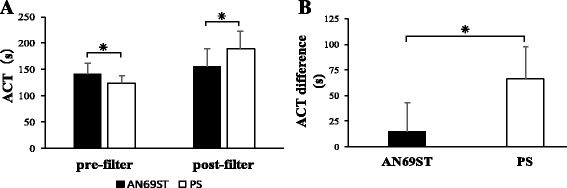
Comparison of ACT difference. a Pre- and post-filter ACT for the AN69ST (n = 122) and PS (n = 37) hemofilters. b Pre- and post-hemofilter ACT difference. Data represent the mean (SD) of four independent experiments. Asterisk indicates p < 0.01 by Mann-Whitney U test
NM adsorption was significantly higher with the AN69ST membrane
Figure 4 shows the NM concentrations in pre- and post-hemofilter samples and in filtrate samples when both the blood pump and filtrate pump were activated. The concentrations of NM in the filtrate and post-hemofilter samples were significantly lower in the circuit using the AN69ST membrane than in circuits using the two other membranes (p < 0.05; Fig. 4). Finally, we calculated the clearance of NM with each type of hemofilter (Fig. 5). The highest clearance of NM was found when the AN69ST membrane was used (p < 0.01); in comparison with the findings obtained with the other membranes, the adsorption clearance (CLad) accounted for a significantly larger proportion, whereas the filtration clearance (CLf) did not contribute substantially (p < 0.01). Comparable CLf values were found with the PS and PMMA membranes, whereas the CLad tended to be higher with the PMMA membrane.
Fig. 4.
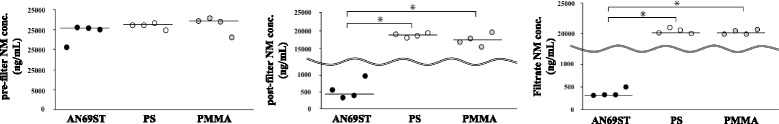
Results of the in vitro assay (single pass). AN69ST polyacrylonitrile (surface treated), PS polysulfone, PMMA polymethylmethacrylate, conc. concentration. Study of the adsorbed/filtered amounts of NM (ECUM). Data represent the mean (SD) of four independent experiments. Asterisk indicates Tukey’s post-tests that were used to determine the significant differences between groups
Fig. 5.
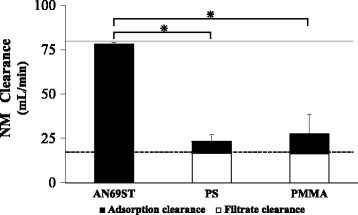
Nafamostat mesylate (NM) clearance. Data represent the mean (SD of total clearance) of four independent experiments. Asterisk indicates Tukey’s post-tests that were used to determine differences between the groups. The theoretical maximum value of the filtration clearance is shown as a dotted line, and the theoretical maximum value of the adsorption clearance is shown as a gray line. AN69ST polyacrylonitrile (surface treated), PS polysulfone, PMMA polymethylmethacrylate
Discussion
AN69 membranes are copolymers of acrylonitrile and sodium methallyl sulfonate and are characterized by their strong negative charge. As a result, positively charged cytokines and NM are adsorbed into the hemofilter through ion binding. For generation of AN69ST membranes, AN69 membranes were subjected to surface treatment with biocompatible polyethyleneimine, resulting in a weaker negative charge [11]. In the clinical setting, heparin priming is generally performed, where the membrane surface is coated with heparin [12]; thus, the negative charge is attenuated by addition of positive charges to the membrane. However, because some negative charges still remain, NM is adsorbed.
The basic principles of substance removal by hemofilters involve the following three elements: dialysis (diffusion), filtration (convection), and adsorption. Given that there is no NM adsorption, diffusion would be the main removal mechanism because NM is small with a molecular weight of 539 Da. Diffusion is correlated with the ratio between the blood pump flow rate (QB) and dialysate pump flow rate (QD). In this study, however, we found a significantly smaller ACT difference between pre- and post-AN69ST hemofilter application, despite the significantly lower QD, indicating low diffusion capability. This finding suggested that NM was removed by adsorption to the AN69ST membrane.
The substance removal capability of each hemofilter can be calculated using the clearance equation. In the model that we used in our experiment, the dialysate was not used to eliminate the effects of solutes other than NM. Therefore, the elimination of NM was examined as a phenomenon that was due to filtration and adsorption. CLad is defined by the structure and charge of the membrane; the interactions among the charge, half-life, and size of the target substance; and the blood pump flow rate QB. Accordingly, the value of QB is considered to be the maximum value for CLad [13]. Our experiment was performed with a QB of 80 mL/min; therefore, the maximum value of CLad was 80 mL/min. In our experimental results, the CLad of the AN69ST membrane reached a level equivalent to the theoretical maximum value, showing the strength of the membrane’s adsorptive power with regard to NM. CLf represents the product of the filtration pump flow rate (QF) and the sieving coefficient (SC), and the SC is the percentage of solutes that pass through the pores of the hemofilter as a result of the filtration of substances. The three types of hemofilters that we used in our study had a maximal SC value of nearly 1.0 for NM (539.58 Da); therefore, CLf = QF × 1.0, and theoretically, the maximum value of CLf was equal to the QF value [14, 15]. The experiments conducted in our study were performed with a QF of 1000 mL/h, i.e., 17 mL/min. The CLf of the AN69ST membrane was extremely low because most of the NM was lost through adsorption (CLf = 0.3 mL/min). Additionally, for both the PS and PMMA membranes, the CLf reached the theoretical maximum value, showing that these membranes had high filtration capacity in the filtration of NM. The PMMA membrane had a neutral to weakly negative charge and had the ability to adsorb substances in the pores inside the membrane. As a result, the CLad of the PMMA membrane tended to be higher than that of the PS membrane.
There are some limitations in this study. First, the patient sample size was small. The proportions of men differed greatly; however, there were no significant differences between groups, possibly because of the small sample size. Second, the proportion of patients with sepsis was significantly higher in the AN69ST group. Sepsis-associated factors may have affected the NM adsorption ability of the AN69ST filter. Third, further studies are necessary to determine how the adsorption capacity of the circuit model used in our study may change over time. Additionally, only NM was measured in this study. Accordingly, measurements were performed using normal saline solution as the filling solution. In the clinical setting, various substances and drugs in the blood are also believed to be adsorbed. Therefore, NM concentrations may need to be measured from samples collected from dialysis circuits attached to actual patients.
Conclusions
In summary, a comparison of pre- and post-hemofilter findings revealed that in dialysis patients, the ACT difference was significantly lower with the AN69ST membrane than with the PS membrane. Moreover, our study showed that this was due to the high NM adsorption capacity of the AN69ST membrane. We suggest that in dialysis circuits using AN69ST membranes, administration of additional post-hemofilter doses of NM may be useful for the management of anticoagulant therapy.
Acknowledgements
We would like to thank Mr. Satoru Ezumi at the Department of Pharmacy, Okayama University Hospital; Dr. Tetsuya Yumoto at the Department of Emergency and Critical Care Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences; and Mr. Jun-ichi Ono at the Kawasaki University of Medical Welfare for substantial contributions to data management.
Funding
This study was supported by the Department of Traumatology and Emergency Intensive Care Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and the Department of Pharmacy, Okayama University Hospital.
Availability of data and material
The datasets supporting the conclusion of this article are included within the article.
Abbreviations
- ACT
Activated clotting time
- AN69
Polyacrylonitrile
- AN69ST
Polyacrylonitrile (surface treated)
- CLad
Adsorption clearance
- CLf
Filtration clearance
- CRRT
Continuous renal replacement therapy
- ECUM
Extracorporeal ultrafiltration method
- NM
Nafamostat mesylate
- PMMA
Polymethylmethacrylate
- PS
Polysulfone
- QB
Blood pump flow rate
- QD
Dialysate pump flow rate
- QF
Filtration pump flow rate
- QS
Substitution fluid pump flow rate
- SC
Sieving coefficient
- SDs
Standard deviations
Authors’ contributions
TH designed the study protocols, acquired the data, and performed the statistical analysis. NN helped with the statistical analysis and completed the manuscript for publication. YO and SU helped with acquiring data and advised the study methodology. YK, TS, TU, and AN supervised the interpretation of the results and writing of the reports. All authors have read and approved the final version of the manuscript.
Ethical approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki because our research included a study that used human data. Our study was approved by the Ethics Committee of Okayama University Hospital (approval no. Ken1610-510). The study includes retrospective data with the highest privacy policy standards; therefore, the requirement of informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takahiro Hirayama, Phone: 81-86-235-7427, Email: ce-hirayama@cc.okayama-u.ac.jp.
Nobuyuki Nosaka, Email: nosaka@okayama-u.ac.jp.
Yasumasa Okawa, Email: pfwy9q3b@okayama-u.ac.jp.
Soichiro Ushio, Email: s-ushio@okayama-u.ac.jp.
Yoshihisa Kitamura, Email: kitamura-y@cc.okayama-u.ac.jp.
Toshiaki Sendo, Email: sendou@md.okayama-u.ac.jp.
Toyomu Ugawa, Email: ugawat@gmail.com.
Atsunori Nakao, Email: qq-nakao@okayama-u.ac.jp.
References
- 1.Forni LG, Hilton PJ. Continuous hemofiltration in the treatment of acute renal failure. N Engl J Med. 1997;336:1303–9. doi: 10.1056/NEJM199705013361807. [DOI] [PubMed] [Google Scholar]
- 2.Morabito S, Guzzo I, Solazzo A, Muzi L, Luciani R, Pierucci A. Continuous renal replacement therapies: anticoagulation in the critically ill at high risk of bleeding. J Nephrol. 2003;16:566–71. [PubMed] [Google Scholar]
- 3.Ohtake Y, Hirasawa H, Sugai T, Oda S, Shiga H, Matsuda K, et al. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol. 1991;93:215–7. doi: 10.1159/000420222. [DOI] [PubMed] [Google Scholar]
- 4.Akizawa T, Koshikawa S, Ota K, Kazama M, Mimura N, Hirasawa Y. Nafamostat mesilate: a regional anticoagulant for hemodialysis in patients at high risk for bleeding. Nephron. 1993;64:376–81. doi: 10.1159/000187357. [DOI] [PubMed] [Google Scholar]
- 5.Shiga H, Hirasawa H. Continuous hemodiafiltration with a cytokine-adsorbing hemofilter in patients with septic shock: a preliminary report. Blood Purif. 2014;38:211–8. doi: 10.1159/000369377. [DOI] [PubMed] [Google Scholar]
- 6.Hattori N, Oda S. Cytokine-adsorbing hemofilter: old but new modality for septic acute kidney injury. Renal Replace Ther. 2016;41:2. [Google Scholar]
- 7.Renaux J, Thomas M, Crost T, Loughraieb N, Vantard G. Activation of the kallikrein-kinin system in hemodialysis: role of membrane electronegativity, blood dilution, and pH. Kidney Int. 1999;55:1097–103. doi: 10.1046/j.1523-1755.1999.0550031097.x. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki O, Nishian Y, Iwaki R, Nakagawa K, Takamitsu Y, Fujita Y. Adsorption of nafamostat mesilate by hemodialysis membranes. Artif Organs. 1992;16:553–8. doi: 10.1111/j.1525-1594.1992.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 9.Désormeaux A, Moreau ME, Lepage Y, Chanard J, Adam A. The effect of electronegativity and angiotensin-converting enzyme inhibition on the kinin-forming capacity of polyacrylonitrile dialysis membranes. Biomaterials. 2008;29:1139–46. doi: 10.1016/j.biomaterials.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishida O, Yumoto M, Moriyama K, Shimomura Y, Miyasho T, Yamada S. Possible adsorption mechanism of high mobility group box 1 protein on polyacrylonitrile (AN69ST) membrane filter. Crit Care. 2012;16:135–6. doi: 10.1186/cc11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacques C, Sylvie L, Randoux C, Rieu P. New insights in dialysis membrane biocompatibility: relevance of adsorption properties and heparin binding. Nephrol Dial Transplant. 2003;18:252–7. doi: 10.1093/ndt/18.2.252. [DOI] [PubMed] [Google Scholar]
- 12.Chanard J, Lavaud S, Paris B, Toure F, Rieu P, Renaux JL, et al. Assessment of heparin binding to the AN69 ST hemodialysis membrane: I. Preclinical studies. ASAIO J. 2005;51:342–7. doi: 10.1097/01.mat.0000169119.06419.ed. [DOI] [PubMed] [Google Scholar]
- 13.Nishida O. A reconsideration of effective mediator removal based on hemofiltration principles—taking the HMGB1, notable alarmin, removal by hemofiltration for instance. J Jpn Soc Blood Purif Crit Care. 2011;2:52–60. [Google Scholar]
- 14.Moriyama K, Soejima Y. Continuous hemodiafiltration using PMMA membrane: clinical efficacy and its mechanisms. Contrib Nephrol. 1999;125:222–32. doi: 10.1159/000059941. [DOI] [PubMed] [Google Scholar]
- 15.Yumoto M, Nishida O. In vitro evaluation of high mobility group box 1 protein removal with various membranes for continuous hemofiltration. Ther Apher Dial. 2011;15:385–93. doi: 10.1111/j.1744-9987.2011.00971.x. [DOI] [PubMed] [Google Scholar]


