Abstract
Background/Aims
Sensitive skin (SS), a frequently reported condition in the Western world, has been suggested to be underlined by an impaired skin barrier. The aim of this study was to investigate the skin barrier molecular composition in SS subjects using confocal Raman microspectroscopy (CRS), and to compare it with that of non-SS (NSS) individuals as well as atopic dermatitis (AD) and allergic rhinoconjunctivitis (AR) subjects, who frequently report SS.
Methods
Subjects with SS (n = 29), NSS (n = 30), AD (n = 11), and AR (n = 27) were included. Stratum corneum (SC) thickness, water, ceramides/fatty acids, and natural moisturizing factor (NMF) were measured by CRS along with transepidermal water loss and capacitance on the ventral forearm, thenar, and cheek. Sebum levels were additionally measured on the forearm and cheek.
Results
No differences between SS and NSS subjects were found regarding SC thickness, water, and NMF content, yet a trend towards lower ceramides/fatty acids was observed in the cheek. Compared to AD subjects, the SS group showed higher ceramides/fatty acid content in the forearm, whereas no differences emerged with AR. The correlation of macroscopic biophysical techniques and CRS was weak, yet CRS confirmed the well-known lower content of NMF and water, and thinner SC in subjects with filaggrin mutations.
Conclusion
The skin barrier in SS is not impaired in terms of SC thickness, water, NMF, and ceramides/fatty acid content. The failure of biophysical techniques to follow alterations in the molecular composition of the skin barrier revealed by CRS emphasizes a strong need in sensitive and specific tools for in vivo skin barrier analysis.
Keywords: Sensitive skin, Atopic dermatitis, Stratum corneum, Confocal Raman microspectroscopy, Transepidermal water loss, Capacitance, Skin barrier function, Skin barrier molecular composition
Introduction
The skin protects our internal milieu from the external environment due to its low permeability, which is mainly established by specific properties of the stratum corneum (SC) [1]. The cells of the SC are the result of epidermal differentiation, a gradual maturation process of the basal keratinocytes ascending to the cornified layer of the squamous epithelium, functioning as corneocytes [1,2,3,4]. Next to a tough and resilient organization of the flattened corneocytes, the envelope of proteins and lipids surrounding each corneocyte also plays a crucial role in preventing the penetration and diffusion of foreign substances through the skin and loss of internal water. The currently accepted model of the SC, although subject to change [5,6,7], also contains the intercellular lipid lamellae, a product originating from the lamellar bodies of the stratum granulosum, and consisting of ceramides, fatty acids, and cholesterol. Located between the corneocytes, it contributes to the overall skin barrier function [3,8,9,10,11,12]. Furthermore, water-soluble intracorneocyte substances, collectively known as natural moisturizing factor (NMF), contribute to the overall skin barrier function by binding water and limiting water loss from the skin [1]. Impairment of the skin barrier may result in the penetration of allergens and pathogens that contribute to skin diseases, such as atopic dermatitis (AD), and allergic and irritant contact dermatitis.
Sensitive skin (SS) is a condition characterized by the perception of skin discomfort following mild stimuli, frequently without objective signs of skin irritation [13,14,15,16,17,18]. This skin condition has been shown to be highly prevalent in the Western World but, despite extensive research in the past years, no consensus on its definition and pathomechanisms has been reached [19]. Impaired skin barrier function has been suggested to underlie SS [13,20,21], leading to proposals of an association of SS with atopic conditions [22,23,24]. The assessment of the skin barrier is traditionally performed by means of transepidermal water loss (TEWL), which involves indirect macroscopic biophysical techniques. The hydration of the SC, indirectly measured based on capacitance, has also been proposed to be lower in the facial areas of SS subjects [25] and of subjects perceiving stinging responses following application of lactic acid on the nasolabial folds [20]. However, other studies, also using macroscopic biophysical techniques, did not find such differences between the SS and a non-SS (NSS) group [14,18,20,26,27]. This inconsistency between outcomes could on one hand be due to the fact that subjects were included in tests using different criteria, since a definition of SS is still lacking. It might also be possible that indirect assessments of barrier function and hydration by means of TEWL and capacitance lacks sensitivity and specificity if barrier function impairment in SS is only subtle.
Over recent years, confocal Raman microspectroscopy (CRS) has emerged as a powerful tool for the direct and non-invasive assessment of the molecular components of the skin, also at a high spatial and temporal resolution. CRS is an optical technique based on the principle of inelastic (Raman) scattering: when a monochromatic laser beam is focused in the skin, incident photons interact with the vibrational levels of the molecules, transferring to them a part of their energy. The exact amount of energy required to excite a vibrational mode is dependent on the masses of the atoms involved in the vibration and on the chemical bonds between them. The resulting Raman spectra are thus molecule-specific and contain detailed information on their type and amounts [28]. In addition, by using the principle of confocal detection, only the light scattered from the laser focus is detected, providing spatially resolved information, which approaches a subcellular resolution [29]. Raman spectroscopy has already established a strong position in non-invasive skin analysis for many applications, ranging from the detection of non-melanoma skin cancer [30] to evaluation of skin barrier composition in AD [31,32]. As CRS is able to detect differences at the molecular level and at a high spatial resolution, it could provide a breakthrough in the evaluation of barrier function involvement in SS, which so far is not possible with macroscopic biophysical techniques [33].
The primary objective of this study was to evaluate the SC molecular composition using CRS in subjects with SS and to compare the results with those measured on people with NSS, AD, and allergic rhinoconjunctivitis (AR). The underlying hypothesis was that the mechanism of SS could be based on aberrant properties of the SC and could share interface with atopic conditions. The second objective was to perform a comparative study of the penetration kinetics of a solution of glycerol (1%) in water in SS and NSS subjects in order to establish whether a faster penetration of topicals due to an impaired skin barrier might occur in SS. Glycerol is a widely used humectant in dermatologic and cosmetic formulations [34]. In this study, it was chosen as a model for substance penetration for its low toxicity, its known diffusivity in the SC allowing measurement with CRS, its hydrophilic nature, and its undisruptive effect on the intercellular lipid lamellae [34]. The penetration of a lipophilic substance might be heavily influenced by its affinity with the lipid compartment of the skin barrier, which is possibly different between SS and NSS. Meanwhile, the lack of a disruptive effect on the intercellular lipid lamellae implies that differing penetration kinetics of glycerol could be attributed to the properties of the skin barrier rather than on an active effect of glycerol on the barrier. The knowledge gained should be of high value for clinicians and the cosmetic and pharmaceutical industry involved in developing solutions for individuals with SS.
Methods
Subject Selection
The inclusion criteria for this study were an age between 18 and 65 years and skin type II or III (Fitzpatrick scale). Subjects could not use immunosuppressive drugs during the study period. Starting from 2 days before the test, subjects were advised not to use toiletries (e.g. personal care and cosmetic products) in the areas to be investigated and not to excessively expose these areas to sunlight or use artificial tanning methods. Four groups of subjects were included. Subjects with SS and subjects with NSS were selected based on a perception-based questionnaire, as previously described in detail [21,35,36]. Briefly, potential participants filled in questions on self-assessed skin sensitivity and on skin perceptions (i.e. discomfort, stinging, redness, dryness) following exogenous and endogenous triggers (i.e. toiletries, shaving, heat, cold, clothes, emotions). The severity of reactions to each trigger and the corresponding duration were scored on a visual analogue scale and on an ordinal scale (no reaction, seconds, minutes, less than 1 h, hours, days), respectively. The scores of each trigger were summed, and the final score was compared to an upper and lower quartile determined in advance by distributing the questionnaire in a large study cohort [37]. If the final score was above the upper quartile and the subject reported SS, he/she was included in the group with SS. If the final score was below the lower quartile and the subject reported NSS, he/she was included in the group with NSS. In addition, subjects in both the SS and NSS groups did not have a specific history of skin diseases, AD, or other concomitant diseases, including asthma and AR in particular. The other 2 groups included in the study were subjects with AR using oral histamine antagonists when experiencing symptoms and not having a history of asthma, urticaria, AD or other (skin) diseases, and subjects with moderate to severe AD (3-item severity score, TIS ≥3) without a history of asthma, urticaria, AR or other (skin) diseases, currently using no therapy or using solely topical corticosteroids. Subjects with AR and AD did not fill in the full-length questionnaire, but were asked if they perceived their skin as sensitive or non-sensitive. The study was conducted in accordance with the Declaration of Helsinki principles and was approved by the local ethics committee. Experiments were performed at the department of Dermatology of the Radboud University Medical Center in Nijmegen, The Netherlands, between January and March 2015.
Instrumentation
Three body sites were measured: (i) the mid-ventral forearm, (ii) the lateral side of the thenar eminence of the arm, and (iii) the malar eminence of the right cheek on the 90-degree angle crosspoint of the ala of the nose and the lateral canthus of the eye. Forearm and thenar measurements were performed in the non-dominant arm (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000452152). AD subjects did not have active lesions on these body sites. Subjects were acclimatized for 20 min in a temperature- and relative humidity-controlled room (temperature 21 ± 1°C, relative humidity 50 ± 10%).
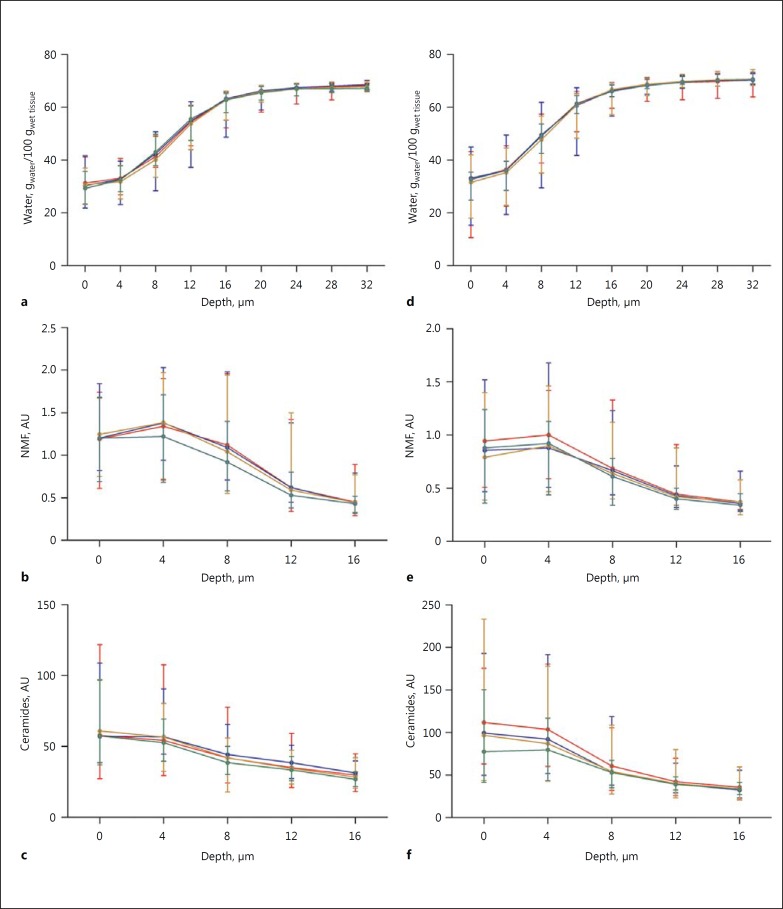
Fig. 1.
Average concentration profiles (median and range) of water (a, d), NMF (b, e), and ceramides/fatty acids (c, f) at different depths (µm) measured on the volar forearm (a-c) and cheek (d-f). Red, NSS; blue, SS; brown, AR; green, AD (colours refer to the online version only).
In vivo CRS measurements were performed using the gen2-SCA performance model of RiverD International B.V. (Rotterdam, The Netherlands). The device has an axial resolution of 5 μm and is equipped with a 785-nm laser source for measurements in the 400-1,800 cm−1 spectral region (NMF, ceramides and glycerol) and a 671-nm laser source for measurements in the 2,400-4,000 cm−1 spectral region (water). The laser power complies with the maximum permissible levels for skin as defined by the international laser safety standard (IEC 60285-1:2007; <30 mW for 785 nm, and <20 mW for 671 nm). In the 400-1,800 cm−1 region, spectra of the cheek and forearm were acquired at 4-μm increments in the axial direction up to a depth of 28 μm using a 5-s acquisition time per point, whereas the thenar spectra were acquired at 15-μm steps up to a depth of 180 μm using a 5-s acquisition time until 100 μm and a 7-s acquisition time until 180 μm. In the 2,400-4,000 cm−1 region, spectra of the cheek and forearm were acquired at 4-μm increments up to a depth of 40 μm using a 2-s acquisition time per point, whereas spectra of the thenar were acquired at 15-μm steps up to a depth of 180 μm for 2 s until 100 μm and for 5 s until 180 μm. Ten measurements per body site were performed in each spectral region.
The macroscopic biophysical techniques employed included: (i) capacitance for the indirect measurement of SC hydration (Corneometer CM825; Courage+Khazaka); (ii) TEWL for the indirect assessment of the skin barrier (Aquaflux AF200; Biox), and (iii) a sebumeter for the indirect measurement of the sebum level at the skin surface (Sebumeter SM815, Courage+Khazaka). Three measurements per body site were performed, except for the SC hydration of the cheek, which was measured 5 times. The sebum measurements were performed on the side contralateral to that used during CRS and were not performed on the thenar.
Penetration Kinetics of Glycerol
A total of 200 µl of a 1% solution of glycerol in demineralized water was applied to the non-dominant volar forearm during 1 min, with a 13-mm-diameter plastic ring used to prevent the spreading of the substance over the skin surface. The solution was wiped off using a tissue afterwards. Measurements in the 400-1,800 cm−1 spectral region were performed every 60 s during the subsequent 15 min using axial steps of 4 μm, up to a depth of 28 μm.
Calculation of Parameters from CRS Measurements
Concentration profiles of SC molecular components relative to keratin were obtained using SkinTools 2.0 software (RiverD International B.V.) using a previously reported fitting algorithm [28]. Briefly, the algorithm consists of a least square fitting of the Raman spectra obtained in vivo to a library of Raman spectra of SC molecular components obtained in vitro, resulting in a set of fit coefficients for the SC constituent spectra. The fit coefficients are subsequently normalized to the fit coefficient for the keratin spectrum in order to compensate for the loss of signal intensity for increasing skin depths [28]. For the measurement of concentration profiles of glycerol, the Raman spectrum of the 1% glycerol solution in water was obtained in vitro and subsequently added to the library of Raman spectra. Obvious outliers in the concentration profiles (due for example to presence of cosmic rays or high background fluorescence in the corresponding Raman spectra) were removed.
The average thickness of the SC was calculated from the water concentration profiles using an implementation in Matlab (The Mathworks, Natick, MA, USA) of the method of Bielfeldt et al. [38], in which the SC thickness was defined as the intercept of 2 straight lines delineating the boundary between the SC and the epidermis. The average water content in the SC was expressed as the area under the curve (AUC) of the water profiles from a 4-μm depth to the SC thickness for the cheek and forearm, and from a 10-µm depth to the SC thickness in the thenar. The average levels of ceramides/fatty acids (treated in SkinTools as a single variable, “ceramides”) and of NMF between a depth of 4 and 12 μm for the forearm, 4 and 8 μm for the cheek, and 10 and 50 µm for the thenar were calculated from the corresponding average concentration profiles. These intervals were chosen in order to avoid influences from skin surface contamination (e.g. sebum) and from washout/desquamation effects at the skin surface, as well as to avoid Raman signals from the viable epidermis [39]. The presence of filaggrin (FLG) mutations was established according to the method of O'Regan et al. [32], in which the cut-off point of 1.07 AU used for FLG mutations (either FLG−/− or FLG+/−) versus no FLG mutation (FLG+/+) was derived from the mean NMF level between 30 and 50 µm measured on the thenar. The presence of exogenous glycerol after topical application was verified by calculating the difference spectrum, obtained by subtraction of the average Raman spectrum where the glycerol signal was not detectable from the average Raman spectrum where glycerol was detected using SkinTools 2.0 software. If the peaks of the Raman spectrum of glycerol were clearly visible in the difference spectrum, the presence of exogenous glycerol was confirmed. If some peaks were missing or were not convincingly above the noise level, the presence of exogenous glycerol was not confirmed.
Statistical Analysis
The results are presented as number (percentage) or as median (minimum-maximum). Differences between the groups (SS, NSS, AR, and AD) were analyzed using the Mann-Whitney exact test for non-parametric independent values. Correlations were investigated using the Spearman ρ. Statistical analyses were performed with SPSS Statistics (v.20, IBM SPSS Inc., Chicago, IL, USA). A p value ≤0.05 was considered statistically significant. Missing values were excluded from the analyses. No corrections for multiple comparisons were applied.
Results
Group Characteristics
In total, 29 subjects were included in the SS group, 30 in the NSS group, 27 in the AR group, and 11 in the AD group. The group characteristics are presented in Table 1. There were no significant differences with respect to age, gender, Fitzpatrick skin type and presence of FLG mutations between the groups. Self-assessed facial and body skin differed between the SS and NSS subjects, with the former reporting more frequently dry or combined (concomitant presence of dry and oily parts) skin, and the latter more frequently reporting normal skin (p = 0.007 and p = 0.000, respectively). Self-assessed SS was reported by two-thirds of AR subjects and by all AD subjects. The measurements of 4 subjects in the volar forearm, of 1 subject in the cheek, and of 2 subjects in the thenar were excluded because of artefacts found in the Raman spectra most probably caused by exogenous agents on the skin.
Table 1.
Population characteristics
| NSS | SS | AR | AD | |
|---|---|---|---|---|
| Self-assessed sensitive skin | 30 (100.0) | 29 (100.0) | 27 (100.0) | 11 (100.0) |
| SS | 0 (0.0) | 29 (100.0) | 18 (66.7) | 11 (100.0) |
| NSS | 30 (100.0) | 0 (0.0) | 9 (33.3) | 0 (0.0) |
| Gender | ||||
| Male | 14 (46.7) | 11 (37.9) | 11 (40.7) | 5 (45.5) |
| Female | 16 (53.3) | 18 (62.1) | 16 (59.3) | 6 (54.5) |
| Age, years | 21.5 (18 – 28) | 21.0 (18 – 32) | 23.0 (19 – 29) | 23.0 (20 – 27) |
| Skin type (Fitzpatrick) | ||||
| Type II | 22 (73.3) | 21 (72.4) | 19 (70.4) | 8 (72.7) |
| Type III | 8 (26.7) | 8 (27.6) | 8 (29.6) | 3 (27.3) |
| FLG mutations | ||||
| FLG–/– or FLG+/– | 3 (10.0) | 3 (10.3) | 1 (3.7) | 3 (27.3) |
| FLG+/+ | 26 (86.7) | 25 (86.2) | 26 (96.3) | 8 (72.7) |
| Missing | 1 (3.3) | 1 (3.4) | 0 (0.0) | 0 (0.0) |
| Facial skin dryness | ||||
| Normal | 17 (56.7) | 6 (20.7) | na | na |
| Dry | 4 (13.3) | 9 (31.0) | ||
| Oily | 1 (3.3) | 2 (6.9) | ||
| Combined (dry and oily) | 8 (26.7) | 12 (41.4) | ||
| Body skin dryness | ||||
| Normal | 25 (83.3) | 9 (31.0) | na | na |
| Dry | 4 (13.3) | 15 (51.7) | ||
| Oily | 0 (0.0) | 0 (0.0) | ||
| Combined (dry and oily) | 1 (3.3) | 5 (17.2) | ||
| Questionnaire score | 29.5 (0.0 – 62.0) | 157.8 (70.3 – 287.0) | na | na |
Values are n (%) or median (range). SS, sensitive skin; NSS, non-sensitive skin; AR, allergic rhinoconjunctivitis; AD, atopic dermatitis; FLG–/–, homozygous filaggrin mutation; FLG+/–, heterozygous filaggrin mutation; FLG+/+, no filaggrin mutation; na, questions about facial and body skin dryness and the questionnaire to determine skin sensitivity were not asked/distributed in the AR and AD groups.
Macroscopic Biophysical Measurements
Macroscopic biophysical measurements of the forearm, cheek, and thenar are reported in Tables 2, 3, 4. In the forearm, SS and AR subjects had lower TEWL compared to NSS and AD subjects (SS vs. NSS: p = 0.076, SS vs. AD: p = 0.082, AR vs. NSS: p = 0.038, AR vs. AD: p = 0.044). Similar findings were found in the thenar (SS vs. NSS: p = 0.016, SS vs. AD: p = 0.058, AR vs. NSS: p = 0.050, AR vs. AD: p = 0.071). No further difference between the groups was found with respect to TEWL measured on the cheek and with respect to SC hydration (based on the capacitance measurement) and sebum level measured on either body site.
Table 2.
Forearm measurements
| NSS |
SS |
AR |
AD |
SS vs. NSS | SS vs. AD | SS vs. AR | NSS vs. AD | NSS vs. AR | AD vs. AR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | median (range) | n | median (range) | n | median (range) | n | median (range) | |||||||
| Biophysical measurements | ||||||||||||||
| TEWL, g/m2/h | 29 | 13.2 (7.1 – 21.9) | 27 | 11.4 (5.8 – 17.7) | 26 | 11.3 (7.0 – 16.2) | 11 | 13.8 (8.9 – 28.4) | 0.076 | 0.082 | ns | ns | 0.038 | 0.044 |
| SC hydration, AU | 29 | 31.7 (19.4 – 55.6) | 27 | 33.9 (16.7 – 57.7) | 26 | 31.7 (19.4 – 53.4) | 11 | 31.3 (18.1 – 39.0) | ns | ns | ns | ns | ns | ns |
| Sebum, AU | 29 | 1.0 (0.0 – 152.0) | 27 | 0.3 (0.0 – 142.3) | 26 | 1.5 (0.0 – 138.7) | 11 | 1.0 (0.0 – 92.3) | ns | ns | ns | ns | ns | ns |
| CRS parameters | ||||||||||||||
| SC thickness, µm | 29 | 16.6 (13.5 – 24.2) | 27 | 16.4 (13.7 – 23.5) | 26 | 16.9 (14.2 – 20.3) | 11 | 16.5 (13.6 – 19.3) | ns | ns | ns | ns | ns | ns |
| AUC Water 4-SC, AU | 29 | 621.2 (478.6 – 1,010.0) | 27 | 600.5 (503.9 – 852.3) | 26 | 619.7 (518.2 – 735.1) | 11 | 582.4 (491.8 – 719.1) | ns | ns | ns | ns | ns | ns |
| Average NMF 4 – 12 µm, AU | 29 | 1.1 (0.5 – 1.8) | 27 | 1.0 (0.7 – 1.8) | 26 | 1.0 (0.6 – 1.7) | 11 | 0.9 (0.6 – 1.3) | ns | ns | ns | ns | ns | ns |
| Average Cer 4 – 12 µm, AU | 29 | 43.8 (27.0 – 81.5) | 27 | 45.1 (38.8 – 68.9) | 26 | 44.4 (24.6 – 58.5) | 11 | 40.9 (36.2 – 54.1) | ns | 0.007 | ns | ns | ns | ns |
AD, atopic dermatitis; AR, allergic rhinoconjunctivitis; AU, arbitrary units; AUC, area under the curve; Cer, ceramides/fatty acids; CRS, confocal Raman microspectroscopy; NMF, natural moisturizing factor; ns, no statistically significant difference; NSS, non-sensitive skin; SC, stratum corneum; SS, sensitive skin; TEWL, transepidermal water loss.
Table 3.
Cheek measurements
| NSS |
SS |
AR |
AD |
SS vs. NSS | SS vs. AD | SS vs. AR | NSS vs. AD | NSS vs. AR | AD vs. AR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | median (range) | n | median (range) | n | median (range) | n | median (range) | |||||||
| Biophysical measurements | ||||||||||||||
| TEWL, g/m2/h | 30 | 23.8 (12.8 – 40.8) | 28 | 22.7 (10.2 – 37.5) | 27 | 23.0 (13.1 – 40.9) | 11 | 23.2 (15.3 – 42.7) | ns | ns | ns | ns | ns | ns |
| SC hydration, AU | 30 | 37.9 (9.0 – 62.3) | 28 | 30.7 (7.3 – 54.4) | 27 | 29.8 (16.2 – 59.0) | 11 | 36.7 (18.5 – 88.2) | ns | ns | ns | ns | ns | ns |
| Sebum, AU | 30 | 48.3 (0.3 – 290.0) | 28 | 50.8 (0.0 – 209.3) | 27 | 46.7 (0.0 – 373.0) | 11 | 31.0 (4.3 – 215.7) | ns | ns | ns | ns | ns | ns |
| CRS parameters | ||||||||||||||
| SC thickness, µm | 30 | 14.1 (11.6 – 17.3) | 28 | 14.0 (10.8 – 21.5) | 27 | 14.3 (12.0 – 19.1) | 11 | 13.7 (12.6 – 15.9) | ns | ns | ns | ns | ns | ns |
| AUC water 4-SC, AU | 30 | 516.9 (417.7 – 639.1) | 28 | 507.5 (393.4 – 780.4) | 27 | 512.1 (425.5 – 777.3) | 11 | 482.9 (433.9 – 623.5) | ns | ns | ns | ns | ns | ns |
| Average NMF 4 – 8 µm, AU | 30 | 0.9 (0.5 – 1.3) | 28 | 0.8 (0.5 – 1.5) | 27 | 0.8 (0.4 – 1.3) | 11 | 0.8 (0.4 – 1.0) | ns | ns | ns | 0.091 | ns | ns |
| Average Cer 4 – 8 µm, AU | 30 | 82.2 (47.5 – 142.9) | 28 | 72.1 (45.1 – 155.1) | 27 | 72.0 (35.4 – 143.2) | 11 | 66.3 (39.2 – 92.0) | 0.077 | ns | ns | 0.097 | ns | ns |
AD, atopic dermatitis; AR, allergic rhinoconjunctivitis; AU, arbitrary units; AUC, area under the curve; Cer, ceramides/fatty acids; CRS, confocal Raman microspectroscopy; NMF, natural moisturizing factor; ns, no statistically significant difference; NSS, non-sensitive skin; SC, stratum corneum; SS, sensitive skin; TEWL, transepidermal water loss.
Table 4.
Thenar measurements
| NSS |
SS |
AR |
AD |
SS vs. NSS | SS vs. AD | SS vs. AR | NSS vs. AD | NSS vs. AR | AD vs. AR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | median (range) | n | median (range) | n | median (range) | n | median (range) | |||||||
| Biophysical measurements | ||||||||||||||
| TEWL, g/m2/h | 29 | 39.6 (21.8 – 80.6) | 28 | 29.9 (19.4 – 67.8) | 27 | 32.6 (16.5 – 58.6) | 11 | 39.9 (21.1 – 60.8) | 0.016 | 0.058 | ns | ns | 0.050 | 0.071 |
| SC hydration, AU | 29 | 32.8 (15.2 – 82.2) | 28 | 27.0 (9.1 – 42.5) | 27 | 33.0 (15.3 – 47.9) | 11 | 29.9 (13.9 – 56.3) | ns | ns | ns | ns | ns | ns |
| CRS parameters SC thickness, µm | 29 | 97.6 (57.1 – 159.1) | 28 | 97.4 (40.1 – 159.6) | 27 | 111.7 (43.9 – 149.7) | 11 | 96.7 (41.4 – 144.2) | ns | ns | ns | ns | ns | ns |
| AUC water 10-SC, AU | 29 | 3,702.2 (2,176.8 – 6,659.5) | 28 | 3,741.9 (1,401.8 – 6,642.8) | 27 | 4,140.7 (1,629.3 – 5,609.8) | 11 | 3,840.5 (1,447.8 – 5,263.0) | ns | ns | ns | ns | ns | ns |
| Average NMF 10 – 50 µm, AU | 29 | 1.7 (1.1 – 2.2) | 28 | 1.8 (1.1 – 2.6) | 27 | 1.9 (1.1 – 2.5) | 11 | 1.9 (0.9 – 2.0) | ns | ns | ns | ns | 0.076 | ns |
AD, atopic dermatitis; AR, allergic rhinoconjunctivitis; AU, arbitrary units; AUC, area under the curve; Cer, ceramides/fatty acids; CRS, confocal Raman microspectroscopy; NMF, natural moisturizing factor; ns, no statistically significant difference; NSS, non-sensitive skin; SC, stratum corneum; SS, sensitive skin; TEWL, transepidermal water loss.
Molecular Composition Measured by CRS
The average concentration profiles of water, NMF, and ceramides in the forearm and cheek for all groups are shown in Figure 1. The SC thickness, SC water content, and average NMF and ceramides levels in the forearm, cheek, and thenar are reported in Tables 2, 3, 4. The numerical values of water, NMF, and ceramides at each depth and body site can be found in the online supplementary Tables S1-S3. No significant differences in SC thickness and SC water content were found between the groups at either body site.
With respect to the average NMF at 4–12 µm measured on the forearm, no significant differences emerged between the groups, yet it is possible to observe a trend towards lower levels in the AD subjects compared to the other subjects (Fig. 1). Similarly, in the cheek, AD subjects showed a trend towards a lower average NMF at 4–8 µm compared to NSS subjects (p = 0.091). AR subjects tended to have higher average NMF in the thenar at 10–50 µm compared to NSS subjects (p = 0.076).
With respect to the average ceramides/fatty acids at 4–12 µm measured on the forearm, SS subjects showed higher levels compared to AD subjects (p = 0.007), whereas no differences emerged between the other groups. In the cheek SS subjects tended to have lower average ceramides at 4–8 µm compared to NSS subjects, yet these values were again higher than in subjects with AD (p = 0.097). This trend was more pronounced at the skin surface (Fig. 1). Ceramides were not detectable in the thenar.
Presence of FLG Mutations
In total, 10 subjects were found to be carriers of FLG mutations (either FLG−/− or FLG+/−; Table 1). Differences between subjects with FLG−/− or FLG+/− and subjects with FLG+/+ were present in the forearm, with the former having lower NMF levels (p = 0.017) and a trend towards lower water content and thinner SC (p = 0.071 and p = 0.058, respectively). Similarly, in the thenar, subjects with FLG−/− or FLG+/− had lower NMF levels, lower water content, and thinner SC (p = 0.000) compared to subjects with FLG+/+. The results found in the cheek are consistent with these findings, albeit no significant differences were present. The numerical results can be found in online supplementary Table S4.
Correlations between Macroscopic Biophysical Measurements and CRS
A moderate to weak negative correlation was found between TEWL and NMF levels in the forearm (ρ = −0.220, p = 0.034, n = 93), in the cheek (ρ = −0.408, p = 0.000, n = 96), and in the thenar (ρ = −0.208, p = 0.044, n = 95). In the thenar, TEWL was also weakly correlated with SC water content (ρ = 0.207, p = 0.044, n = 95) and with SC thickness (ρ = 0.202, p = 0.050, n = 95). Of note, the weak correlation with SC water content remained when controlling for the corresponding SC thickness. No further correlations were found between TEWL and SC water content, SC thickness or ceramides levels measured on the forearm and cheek.
Skin hydration measured with capacitance was not found to correlate with SC water content in either the forearm, cheek or thenar. On the other hand, in the forearm capacitance correlated weakly with water content in the middle part of the SC (4-12 µm) and with the water content up to the upper epidermis (4-32 µm; ρ = 0.222, p = 0.032, and ρ = 0.207, p = 0.046, n = 93, respectively). In the cheek, capacitance weakly correlated only with the NMF levels (ρ = 0.223, p = 0.029, n = 96).
Penetration Kinetics of Glycerol
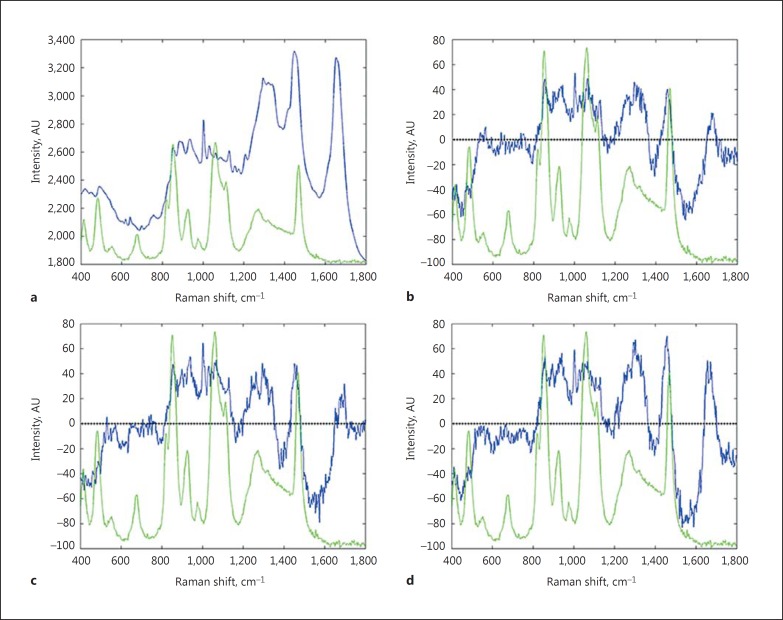
Evidence for the presence of exogenous glycerol was visible only at the skin surface (0-4 µm) and in the first 3 min following application, as verified by the spectra differences shown in Figure 2. Measured levels of glycerol on subjects with NSS and SS are reported in Table 5. NSS subjects showed consistently higher values of glycerol than subjects with SS, reaching statistical significance at 1 min after application (p = 0.030). In both groups, glycerol levels declined slightly from the first to the third minute after application. Baseline levels of endogenous glycerol measured at the skin surface (0-4 µm) were not different between SS and NSS subjects and, in both groups, were significantly lower than the levels measured in the first 3 min after topical glycerol application (p < 0.008).
Fig. 2.
The Raman spectrum of normal skin in the fingerprint region (400-1,800 cm−1; a). The difference of the Raman spectrum obtained by subtraction of the Raman spectra where glycerol was not detectable from the Raman spectra, and where glycerol was detectable at 1 (b), 2 (c), and 3 min (d) after topical glycerol application. The Raman spectrum of glycerol (light green; colours refer to the online version only) is superimposed for clarity.
Table 5.
Levels of glycerol at a depth of 0 – 4 µm at baseline and after application of a solution of 1% glycerol in demineralized water on the volar forearm for 1 min
| NSS |
SS |
||||
|---|---|---|---|---|---|
| n | median (range) | n | median (range) | ||
| Glycerol content, AU | |||||
| Baseline | 20 | 783 (643 – 1,280) | 20 | 816 (656 – 1,008) | ns |
| 1 min | 22 | 1,274 (835 – 2,205) | 20 | 1,039 (783 – 1,545) | 0.030 |
| 2 min | 21 | 1,243 (808 – 2,259) | 20 | 1,038 (646 – 1,962) | ns |
| 3 min | 20 | 1,208 (785 – 2,016) | 20 | 994 (672 – 1,925) | ns |
AU, arbitrary units; ns, no statistically significant difference; NSS, non-sensitive skin; SS, sensitive skin.
Discussion
The main objective of this study was to investigate whether the barrier function is aberrant in SS by directly measuring the molecular composition of the SC by means of CRS. A comparison with the molecular composition of the SC of subjects with NSS, AD, and AR was made, together with the indirect assessment of the skin barrier by means of TEWL and capacitance.
In line with the literature, SS subjects in this study also more frequently reported dry facial and body skin compared to those with NSS. However, we did not find strong evidence to support a hypothesis of impaired skin barrier in SS in terms of SC water and NMF levels in either the forearm, cheek or thenar. SC thickness was also not different between the groups, which is in line with our previous findings [21].
It is interesting, however, that ceramides/fatty acids showed a trend towards lower levels in the cheek of SS subjects compared to the NSS group, whereas non-significant higher values were found in the forearm. Several studies that have suggested the role of an impaired skin barrier function in SS [13,20,21] have in particular demonstrated lower ceramides in the facial skin of subjects with SS [27]. This hypothesis concerning a weaker SC barrier is also supported by previous clinical studies performed by our group, in which the same perception-based questionnaire was used to select volunteers with SS and NSS [21,36]. In those clinical studies, the SC of subjects with SS was shown to be more vulnerable to chemical and mechanical stimuli than the SC of subjects with NSS, suggesting an impaired barrier.
Additional mechanisms to those investigated in this study might thus play a major role in the barrier function impairment in SS, including reduced intercorneocyte adhesion, different organization of lipids in the intercellular lamellae, and increased inflammatory reactions and an altered number of mast cells in response to a range of stimuli, as previously reported by our group [21,34,35]. In addition to barrier function impairment via alterations in the molecular balance of NMF, ceramides, and water, an involvement of the family of transient receptor potential (TRP) channels has been hypothesized to play a role in SS [40]. TRP channels are sensory receptors activated by a variety of external stimuli and known to mediate a range of skin sensations, including pain, itch, and burning feeling. Increased levels or an upregulated sensitivity of these receptors might account for the previously reported increased perception of SS to a variety of stimuli [21,35,36]. Thus, one could also speculate that the more frequent self-reported perception of skin dryness in SS subjects might be primarily mediated by these receptors, because of the lack of significant differences in SC water content and NMF levels in SS compared to NSS subjects demonstrated here.
The possible association of SS with atopic conditions, as proposed previously [22,23,24], remains less clear. We did detect lower NMF and water levels and thinner SC in subjects with FLG mutations, which is well documented in the literature [31,32], albeit indirectly using CRS and in a small populations. However, despite frequently reporting SS, in line with previous findings [37], no differences emerged between the AR and NSS and SS subjects, at least with respect to the inspected parameters. This could be partially due to the overlap in the characteristics of subjects in the groups with respect to skin sensitivity and symptoms, making it difficult to obtain distinct groups since AD and AR subjects frequently report SS [37]. Despite this difficulty, differentiating between subjects with atopy and subjects without atopy is important, since SS cannot solely be explained by having this condition [19]. In fact, higher ceramides/fatty acid levels found in the forearm of SS subjects compared to AD, together with the observation that FLG mutations do not seem to be reported more frequently in the SS compared to the NSS group, do not support the hypothesis of SS being a subclinical form of AD [40]. This is also supported by the lack of association between SS and the dysbiosis of the cutaneous microbiota found in a previous study, in contrast with AD, known to be characterized by an overabundance of Staphylococcus aureus [40,41]. Yet, should the TRP receptor family be involved in underlying SS sensations, this could suggest at least 1 common interface with AD where these receptors were shown to mediate sensory discomfort and inflammation, at least in a murine model [40,42,43,44].
In our previous clinical studies we showed a reduced number of mast cells and a reduced number of tapes required to strip off the total SC in the lower back of SS compared to NSS subjects [21,35,36]. It could be tempting to speculate that the stronger expression of ceramides in the forearm of SS subjects might be due to a compensating mechanism of the primary defect in activation of the innate immune system or impaired intercorneocyte adhesion [21,35,36,45]. This hypothesis might not be valid for the cheek, which is characterized by a higher number of mast cells compared to other body sites, and thus does not need a compensatory mechanism [46].
Despite the different results on ceramides levels between the forearm and cheek, we are of the opinion that the response of SS is rather universal. Farage [47] evaluated answers to determine whether people claiming SS in general also claimed to have SS at specific body sites. Most responses of descriptions of facial and body skin were consistent with the perception of SS in general (60.7 and 68.4% of responders, respectively) or varied by 1 degree out of 4 with respect to severity (36.7 and 31.3% of responders, respectively). Our previous findings also support the hypothesis of a generalized SS based on possible altered immune responses in SS [21].
The second objective of this study was to investigate whether topicals penetrate faster through the skin of subjects with SS compared to subjects with NSS as a result of possible barrier function impairment. We found consistently higher levels of glycerol in the superficial layers of the SC of NSS subjects compared to SS subjects in the first 3 min after application. This might imply a faster spreading of glycerol on the superficial skin of SS subjects because of barrier function impairment. Our results clearly demonstrate that CRS has the potential to detect differences in such assessments provided that a sufficiently longer application time is chosen for the exogenous substance to be applied [48].
As a final remark, we confirm the difficulty of finding correspondences between the measurements performed with the macroscopic biophysical methods, TEWL, and capacitance, and the molecular composition of the SC measured with CRS, as demonstrated by the lack of correlations thereof found in this study. In previous studies, the skin barrier function of SS subjects was predominantly analyzed at baseline or after stimulation using these macroscopic biophysical methods and significant differences between SS and NSS subjects could rarely be detected [13,14,16,18,20,26,49,50]. A range of limitations of these easy-to-use, rapid measurements are known [33], and our study demonstrates that more sensitive and specific tools for the in vivo analysis of the skin barrier in general and in SS in particular are needed.
In conclusion, we propose that SS is not a subclinical form of AD and that the skin barrier is not impaired in terms of SC thickness and in terms of water, NMF, and ceramides content. Treatments of SS solely based on hydration and ceramides supplementation seem not to correspond to the identified SC properties, although benefits have been reported. More research efforts should be directed at unravelling the role of the cutaneous nervous system, in particular the involvement of TRP channels, on the onset of subjective perceptions of sensitive and dry skin. Among other mechanisms to be investigated in SS, we would include the role of an altered immune response and intercorneocyte adhesion.
Statement of Ethics
This study was approved by the local ethics commitee (METC, region Arnhem-Nijmegen) and was conducted conforming to the Declaration of Helsinki principles.
Disclosure Statement
The costs of conducting this study were paid by Philips Electronics Nederland B.V. Natallia Uzunbajakava is an employee of Philips Electronics Nederland B.V. and received a salary for this study.
Acknowledgement
We would like to thank Gabriela de Aquino Santos for assistance during the performance of the study.
References
- 1.Madison KC. Barrier function of the skin: “la raison d'être” of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 2.Wickett RR, Visscher MO. Structure and function of the epidermal barrier. Am J Infect Control. 2006;34:S98–S110. [Google Scholar]
- 3.Marks R, Barton SP. The significance of the size and shape of corneocytes. In: Marks R, Plewig G, editors. Stratum Corneum. Berlin: Springer; 1983. pp. 161–170. [Google Scholar]
- 4.Ya-Xian Z, Suetake T, Tagami H. Number of cell layers of the stratum corneum in normal skin – relationship to the anatomical location on the body, age, sex and physical parameters. Arch Dermatol Res. 1999;291:555–559. doi: 10.1007/s004030050453. [DOI] [PubMed] [Google Scholar]
- 5.den Hollander L, Han H, de Winter M, Svensson L, Masich S, Daneholt B, et al. Skin lamellar bodies are not discrete vesicles but part of a tubuloreticular network. Acta Derm Venereol. 2016;96:303–308. doi: 10.2340/00015555-2249. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg M. A New perspective on the formation of stratum corneum intercellular space. Acta Derm Venereol. 2016;96:291. doi: 10.2340/00015555-2281. [DOI] [PubMed] [Google Scholar]
- 7.Iwai I, Han H, den Hollander L, Svensson S, Ofverstedt LG, Anwar J, et al. The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. J Invest Dermatol. 2012;132:2215–2225. doi: 10.1038/jid.2012.43. [DOI] [PubMed] [Google Scholar]
- 8.Bouwstra JA, Gooris GS, Dubbelaar FE, Weerheim AM, Ijzerman AP, Ponec M. Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipid Res. 1998;39:186–196. [PubMed] [Google Scholar]
- 9.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80((suppl)):44s–49s. [PubMed] [Google Scholar]
- 11.Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17((suppl 1)):6–15. doi: 10.1111/j.1396-0296.2004.04s1001.x. [DOI] [PubMed] [Google Scholar]
- 12.Wertz PW, van den Bergh B. The physical, chemical and functional properties of lipids in the skin and other biological barriers. Chem Phys Lipids. 1998;91:85–96. doi: 10.1016/s0009-3084(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 13.Distante F, Rigano L, D'Agostino R, Bonfigli A, Berardesca E. Intra- and inter-individual differences in sensitive skin. Cosmet Toiletries. 2002;117:39–46. [Google Scholar]
- 14.Kim SJ, Lim SU, Won YH, An SS, Lee EY, Moon SJ, et al. The perception threshold measurement can be a useful tool for evaluation of sensitive skin. Int J Cosmet Sci. 2008;30:333–337. doi: 10.1111/j.1468-2494.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 15.Marriott M, Whittle E, Basketter DA. Facial variations in sensory responses. Contact Dermatitis. 2003;49:227–231. doi: 10.1111/j.0105-1873.2003.0232.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinto P, Rosado C, Parreirao C, Rodrigues LM. Is there any barrier impairment in sensitive skin? A quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Res Technol. 2011;17:181–185. doi: 10.1111/j.1600-0846.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 17.Robinson MK, Perkins MA. Evaluation of a quantitative clinical method for assessment of sensory skin irritation. Contact Dermatitis. 2001;45:205–213. doi: 10.1034/j.1600-0536.2001.450403.x. [DOI] [PubMed] [Google Scholar]
- 18.Schliemann S, Antonov D, Manegold N, Elsner P. Sensory irritation caused by two organic solvents – short-time single application and repeated occlusive test in stingers and non-stingers. Contact Dermatitis. 2011;65:107–114. doi: 10.1111/j.1600-0536.2011.01938.x. [DOI] [PubMed] [Google Scholar]
- 19.Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Physiol. 2015;28:75–83. doi: 10.1159/000363149. [DOI] [PubMed] [Google Scholar]
- 20.An S, Lee E, Kim S, Nam G, Lee H, Moon S, et al. Comparison and correlation between stinging responses to lactic acid and bioengineering parameters. Contact Dermatitis. 2007;57:158–162. doi: 10.1111/j.1600-0536.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- 21.Richters RJ, Uzunbajakava NE, Falcone D, Hendriks JC, Jaspers EJ, van de Kerkhof PC, van Erp PE. Clinical, biophysical and immunohistochemical analysis of skin reactions to acute skin barrier disruption: a comparative trial between subjects with sensitive skin and subjects with non-sensitive skin. Br J Dermatol. 2016;174:1126–1133. doi: 10.1111/bjd.14307. [DOI] [PubMed] [Google Scholar]
- 22.De Jongh CM, Verberk MM, Withagen CE, Jacobs JJ, Rustemeyer T, Kezic S. Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Dermatitis. 2006;54:325–333. doi: 10.1111/j.0105-1873.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 23.Farage MA, Katsarou A, Maibach HI. Sensory, clinical and physiological factors in sensitive skin: a review. Contact Dermatitis. 2006;55:1–14. doi: 10.1111/j.0105-1873.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 24.Willis CM, Shaw S, De Lacharriere O, Baverel M, Reiche L, Jourdain R, et al. Sensitive skin: an epidemiological study. Br J Dermatol. 2001;145:258–263. doi: 10.1046/j.1365-2133.2001.04343.x. [DOI] [PubMed] [Google Scholar]
- 25.Roussaki-Schulze AV, Zafiriou E, Nikoulis D, Klimi E, Rallis E, Zintzaras E. Objective biophysical findings in patients with sensitive skin. Drugs Exp Clin Res. 2005;31((suppl)):17–24. [PubMed] [Google Scholar]
- 26.Diogo L, Papoila AL. Is it possible to characterize objectively sensitive skin? Skin Res Technol. 2010;16:30–37. doi: 10.1111/j.1600-0846.2009.00404.x. [DOI] [PubMed] [Google Scholar]
- 27.Cho HJ, Chung BY, Lee HB, Kim HO, Park CW, Lee CH. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J Dermatol. 2012;39:295–300. doi: 10.1111/j.1346-8138.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 28.Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ. In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001;116:434–442. doi: 10.1046/j.1523-1747.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 29.Uzunbajakava N, Lenferink A, Kraan Y, Volokhina E, Vrensen G, Greve J, et al. Nonresonant confocal Raman imaging of DNA and protein distribution in apoptotic cells. Biophys J. 2003;84:3968–3981. doi: 10.1016/S0006-3495(03)75124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijssen A, Maquelin K, Santos LF, Caspers PJ, Bakker Schut TC, den Hollander JC, et al. Discriminating basal cell carcinoma from perilesional skin using high wave-number Raman spectroscopy. J Biomed Opt. 2007;12:034004. doi: 10.1117/1.2750287. [DOI] [PubMed] [Google Scholar]
- 31.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136–2138. doi: 10.1038/jid.2011.175. [DOI] [PubMed] [Google Scholar]
- 32.O'Regan GM, Kemperman PM, Sandilands A, Chen H, Campbell LE, Kroboth K, et al. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol. 2010;126:574–580. doi: 10.1016/j.jaci.2010.04.038. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falcone D, Uzunbajakava NE, Varghese B, de Aquino Santos GR, Richters RJ, van de Kerkhof PC, et al. Microspectroscopic Confocal Raman and macroscopic biophysical measurements in the in vivo assessment of the skin barrier: perspective for dermatology and cosmetic sciences. Skin Pharmacol Physiol. 2015;28:307–317. doi: 10.1159/000439031. [DOI] [PubMed] [Google Scholar]
- 34.Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159:23–34. doi: 10.1111/j.1365-2133.2008.08643.x. [DOI] [PubMed] [Google Scholar]
- 35.Richters RJ, Hoogedoorn L, Uzunbajakava NE, Janssen LD, Nuijs TAM, van Erp PE, et al. Clinical, biophysical, immunohistochemical, and in vivo reflectance confocal microscopy evaluation of the response of subjects with sensitive skin to home-use fractional non-ablative photothermolysis device. Lasers Surg Med. 2016;48:474–482. doi: 10.1002/lsm.22486. [DOI] [PubMed] [Google Scholar]
- 36.Richters RJ, Hendriks JC, Uzunbajakava NE, Janssen LD, Falcone D, van Erp PE, et al. Responses to sodium dodecyl sulphate as an in vivo human model to study the pathomechanisms underlying sensitive skin. Exp Dermatol. 2016;25:407–409. doi: 10.1111/exd.12973. [DOI] [PubMed] [Google Scholar]
- 37.Richters RJ, Uzunbajakava NE, Hendriks J, Bikker J.-W., van Erp PE, van der Kerkhof. A model for perception-based identification of sensitive skin. J Eur Acad Dermatol Venereol. 2016 doi: 10.1111/jdv.13829. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Bohling A, Bielfeldt S, Himmelmann A, Keskin M, Wilhelm KP. Comparison of the stratum corneum thickness measured in vivo with confocal Raman spectroscopy and confocal reflectance microscopy. Skin Res Technol. 2014;20:50–57. doi: 10.1111/srt.12082. [DOI] [PubMed] [Google Scholar]
- 39.Janssens M, van Smeden J, Puppels GJ, Lavrijsen AP, Caspers PJ, Bouwstra JA. Lipid to protein ratio plays an important role in the skin barrier function in patients with atopic eczema. Br J Dermatol. 2014;170:1248–1255. doi: 10.1111/bjd.12908. [DOI] [PubMed] [Google Scholar]
- 40.Misery L, Loser K, Stander S. Sensitive skin. J Eur Acad Dermatol Venereol. 2016;30((suppl 1)):2–8. doi: 10.1111/jdv.13532. [DOI] [PubMed] [Google Scholar]
- 41.Hillion M, Mijouin L, Jaouen T, Barreau M, Meunier P, Lefeuvre L, et al. Comparative study of normal and sensitive skin aerobic bacterial populations. Microbiol Open. 2013;2:953–961. doi: 10.1002/mbo3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imura K, Yoshioka T, Hirasawa T, Sakata T. Role of TRPV3 in immune response to development of dermatitis. J Inflamm. 2009;6:17. doi: 10.1186/1476-9255-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191:5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller M, Essin K, Hill K, Beschmann H, Rubant S, Schempp CM, et al. Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J Biol Chem. 2008;283:33942–33954. doi: 10.1074/jbc.M801844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raj N, Voegeli R, Rawlings AV, Doppler S, Imfeld D, Munday MR, et al. A fundamental investigation into aspects of the physiology and biochemistry of the stratum corneum in subjects with sensitive skin. Int J Cosmet Sci. 2016 doi: 10.1111/ics.12334. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol. 2003;148:224–228. doi: 10.1046/j.1365-2133.2003.05090.x. [DOI] [PubMed] [Google Scholar]
- 47.Farage MA. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin Exp Dermatol. 2009;34:e521–e530. doi: 10.1111/j.1365-2230.2009.03487.x. [DOI] [PubMed] [Google Scholar]
- 48.Egawa M, Kajikawa T. Changes in the depth profile of water in the stratum corneum treated with water. Skin Res Technol. 2009;15:242–249. doi: 10.1111/j.1600-0846.2009.00362.x. [DOI] [PubMed] [Google Scholar]
- 49.Loffler H, Dickel H, Kuss O, Diepgen TL, Effendy I. Characteristics of self-estimated enhanced skin susceptibility. Acta Derm Venereol. 2001;81:343–346. doi: 10.1080/000155501317140052. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Wang X, Zhou Y, Tan Y, Chen D, Chen Y, et al. Correlation between stinging, TEWL and capacitance. Skin Res Technol. 2003;9:90–93. doi: 10.1034/j.1600-0846.2003.00026.x. [DOI] [PubMed] [Google Scholar]