Abstract
Background
Many very preterm (i.e., <32 weeks of gestation) newborns fail to mount an adequate adrenocortical response to stress or illness, termed relative adrenal insufficiency. Conversely, later in life these infants show features of increased glucocorticoid bioactivity, such as abdominal adiposity, insulin resistance, raised blood pressure, shorter stature and internalizing problem behavior.
Summary
Studies suggested that very preterm newborns have impairments along multiple levels of the hypothalamus-pituitary-adrenal (HPA) axis. Among the impairment were defects in: (1) the pituitary responsiveness to exogenous corticotropin-releasing hormone, (2) 11β-hydroxylase activity, and (3) the interconversion between cortisol and inert cortisone. There is some evidence suggesting that later in life these infants have an increased basal secretion rate of cortisol and adrenal hyperandrogenism. However, the response to acute (psychosocial) stress was blunted rather than enhanced in them. The mechanisms explaining this switch in HPA axis activity are complex and not yet fully understood.
Key Messages
Very preterm newborns have several impairments along the HPA axis that could impede an adequate adrenocortical response to stress or illness. Later in life, these infants are predisposed to increased HPA axis activity, which could partially explain their phenotype.
Keywords: Cortisol, Adrenal insufficiency, Preterm birth, Hypercortisolism
Introduction
There is emerging evidence linking preterm birth to cardiometabolic risk factors such as abdominal adiposity, insulin resistance and raised blood pressure [1, 2, 3, 4], as well as to shorter stature [5] and internalizing problem behavior [6]. The clustering of these features resembles effects of increased glucocorticoid bioactivity, such as in Cushing's syndrome.
A lower activity of the placental barrier enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) type 2 – induced by maternal stress, disease or licorice consumption – allows a larger proportion of maternal cortisol to reach the fetus, which has been associated with intrauterine growth restriction (IUGR) and shorter gestation [7]. In addition, there is some evidence suggesting that resetting of the hypothalamus-pituitary-adrenal (HPA) axis by a greater transfer of cortisol through the placenta could result in long-lasting consequences for the offspring, such as neurodevelopmental problems and cardiometabolic diseases [7].
For several reasons, these mechanisms cannot wholly explain the triad of preterm birth, permanent alteration in HPA axis activity, and adverse outcomes. First, several studies in children and adults born very preterm (i.e., <32 weeks of gestation) or with very-low-birth-weight (VLBW; i.e., <1,500 g) showed that insulin sensitivity was equally reduced in IUGR and non-IUGR subjects [2, 3]. Second, in the early-postnatal phase a considerable proportion of very preterm infants manifested clinical signs of adrenocortical insufficiency [8]. Such observations pose the question as to whether the HPA axis in preterms could be programmed postnatally by adversities related to preterm birth.
Relative Adrenal Insufficiency
Very preterm newborns encounter serious problems, including hypotension and hypoglycemia. Among the causes of hypotension are abnormalities in the regulation of the vascular tone, left-to-right shunting through a persistent ductus arteriosus, volume depletion and myocardial dysfunction [9]. Hypoglycemia is often attributed to a combination of factors, including small energy depots, poor availability of gluconeogenic substrates and impairments in activities of glucose-6-phosphatase (which is the final step of both glycogenolysis and gluconeogenesis), lipolysis and ketogenesis [10]. Hypotension and hypoglycemia typically emerge during acute illness in the first weeks of life, and relative adrenal insufficiency (RAI) has been postulated to play a role [11]. Cortisol raises the blood pressure, mainly by its permissive effect on the vascular tone [9]. It increases the serum glucose level by its (direct and indirect) effect on energy depots, hepatic glucose production and peripheral insulin resistance.
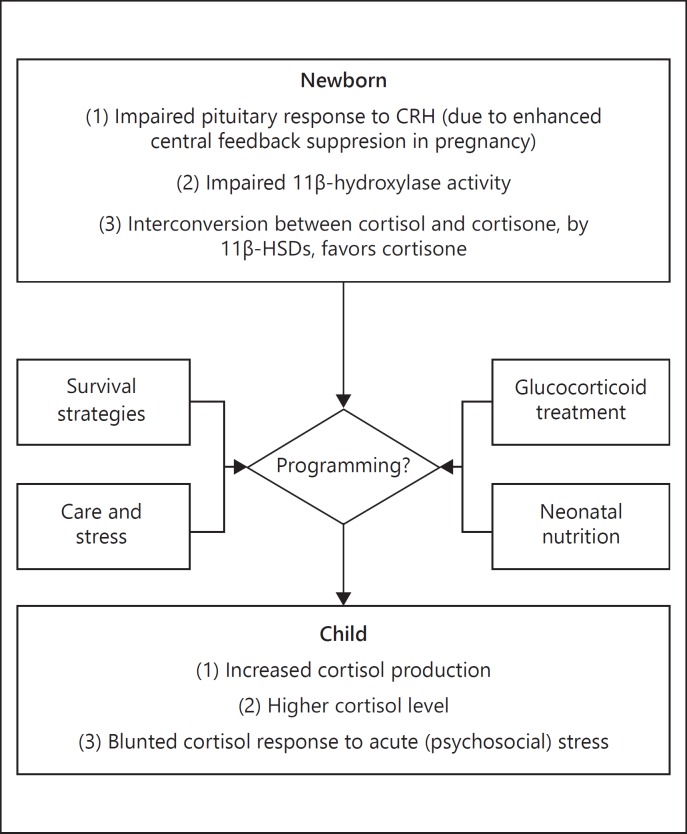
RAI is the term given to inadequate production of glucocorticoids for the degree of stress or illness, and its causes are multifactorial (Fig. 1). During pregnancy, the placental barrier enzyme 11β-HSD type 2 protects the fetus from overexposure to maternal cortisol [7]. Decreased activity of placental 11β-HSD type 2 has been implicated to play a role in preterm delivery [7]. Furthermore, late in gestation the placenta secretes increasing amounts of corticotropin-releasing hormone (CRH), which stimulates the fetal adrenal to secrete cortisol in a self-perpetuating positive feedback loop [11]. Both these mechanisms can lead to a suppression of fetal hypothalamic CRH that may continue after birth. Indeed, studies showed that the pituitary response to exogenous CRH was impaired in preterm newborns [12], suggestive of enhanced central feedback suppression by glucocorticoids. Another mechanism contributing to RAI is the immaturity of adrenal cortex enzymes, notably decreased 11β-hydroxylase activity, which is necessary for the conversion of 11-deoxycortisol to cortisol [13, 14]. Furthermore, there is some evidence suggesting that the interconversion between cortisol and cortisone, by 11β-HSD isozymes in peripheral tissues, favors cortisone with decreasing gestational age [15].
Fig. 1.
The HPA axis in very preterm newborns and how it develops with age.
Synthetic glucocorticoids have proven to be effective for the prevention and treatment of hypotension after preterm birth [16, 17, 18]. Furthermore, antenatal glucocorticoid treatment for fetal lung maturation has been associated with a lower risk of hypotension in the first days after very preterm birth [19], and with markers of insulin resistance in cord blood [20]. The latter might protect the brain against glucose deprivation.
Adrenocortical Function in Childhood and Its Clinical Correlates
The onset of puberty was probably age-appropriate in cross-sectional studies of 11- to 15-year-old boys and girls with VLBW [25, 26]. There is some evidence suggesting that puberty progresses more rapidly in them, with data linking VLBW to a younger age at take-off of pubertal height velocity [26] and a more advanced bone age at adolescence [25, 26]. However, girls with VLBW were no different from girls born at term in age at menarche [25, 26, 27], which argues against a major contribution of the hypothalamus-pituitary-gonadal axis to these associations. Two studies showed that adrenal androgens were higher in young adults born preterm [28, 29].
Several studies have addressed the long-term impact of prematurity on HPA axis activity [12]. Evidence encompassed a higher corticosteroid production rate [30], and increases in the serum or salivary cortisol concentration during early morning, at nadir, or throughout the day in subjects born preterm were observed (Fig. 1) [28, 31, 32]. However, 2 out of 3 studies that performed social stress tests found blunted rather than enhanced cortisol responses at lower gestational ages (Fig. 1) [33, 34, 35]. These observations suggest that HPA axis activity in subjects with a history of preterm birth is characterized by an increased basal secretion rate and a poorer response to acute (psychosocial) stress.
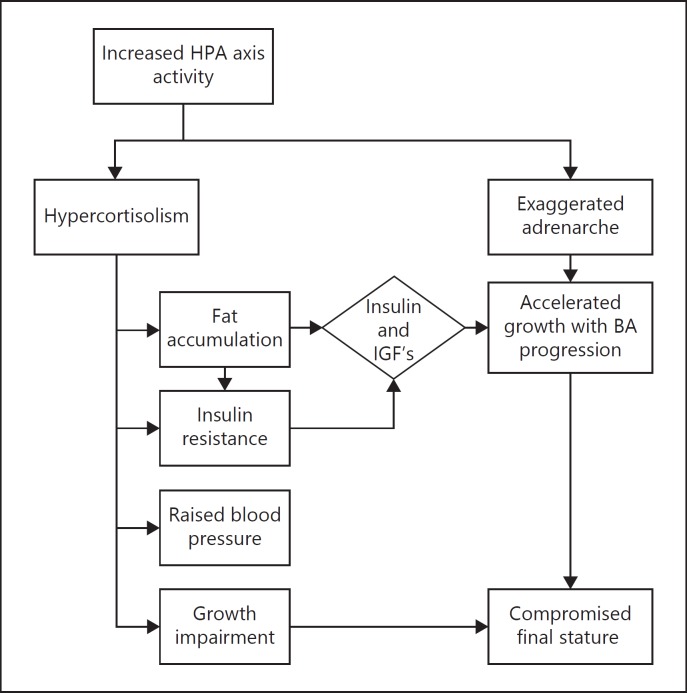
In summary, increased HPA axis activity might explain, at least in part, the clustering of cardiometabolic risk factors in preterm survivors (Fig. 2). Furthermore, after an age-appropriate adrenarche, the production of adrenal androgens is suggested to increase rapidly, which may contribute to an earlier pubertal growth spurt and advancement of bone maturation at adolescence, resulting in premature epiphyseal closure and shorter adult stature (Fig. 2).
Fig. 2.
Schematic representation of putative mechanisms of the associations between very preterm birth, child HPA axis activity and long-term cardiometabolic outcomes. IGFs, insulin-like growth factors.
Exploring Associations between Prematurity and Long-Term Outcomes with Focus on HPA Axis Activity
Exogenous Glucocorticoids
Antenatal glucocorticoids have proven to be effective in the prevention of respiratory distress syndrome and associated complications [36], but animal studies have raised concerns about their long-term cardiometabolic and neurodevelopmental effects [37]. However, findings from randomized trials and observational studies that had followed subjects exposed to a single course of antenatal glucocorticoids until adolescence or young adulthood were inconsistent, especially for cardiometabolic outcomes [38, 39, 40, 41, 42]. In the project on preterm and small-for-gestational-age cohort, we found that carriers of a glucocorticoid receptor (GR) polymorphism, which has been associated with an increased sensitivity to glucocorticoids, had a greater waist circumference and poorer cognitive test scores at age 19 years after exposure to synthetic glucocorticoids antenatally [43, 44]. Thus, whether a single course of antenatal glucocorticoids could produce long-lasting deleterious effects might be dependent on the sensitivity of tissues to these hormones. There is a need for long-term follow-up data of children born to mothers who remained at risk of preterm delivery and were, therefore, treated with additional courses of glucocorticoids [45].
Parental Care and Stress
Pups exposed to maternal separation, or non-handling, were found to have increased HPA axis activity and anxiety-like behavior as adults [46]. Other animal experiments showed that poor maternal care had similar effects on offspring through persistent alterations in the epigenome at the level of the hippocampal GR promotor [47]. In humans, poor quality of parental care, such as neglect or emotional or physical maltreatment, early in childhood was associated with greater HPA axis activity, mental illnesses and cardiometabolic disease [48]. In an autopsy study, childhood abuse was associated with increased DNA methylation at the GR promotor and decreased expression of GR mRNA in the hippocampus [49]. Whether such findings could be extrapolated to very preterm babies, who receive less parental care and are exposed to stressors like invasive procedures, pain, interruption of sleep states and noise during their admission to the neonatal intensive care unit, remains to be explored.
Nutrition
In rodents, perinatal undernutrition has been associated with increased HPA axis activity later in life [50]. Whether this also applies to very preterm babies, who accumulate a significant nutrient deficit in the first postnatal weeks [21], is unknown.
We have recently shown that cortisol and cortisone are secreted into human milk in concentrations comparable to those in saliva [51]. We have also demonstrated that their concentrations in milk are lower after preterm delivery, and follow the diurnal rhythm of maternal HPA axis activity [51]. These findings could have important implications for donor milk banks, which often provide preterm infants with milk from mothers who delivered at term and do not take the time of collection into account. Although human milk is considered superior for infant outcomes, it is unknown whether it also protects against RAI. Moreover, to our knowledge there are no studies that have investigated the intestinal absorption of glucocorticoids in preterm infants.
Conclusions
Early in life, the HPA axis of very preterm infants is characterized by an inability to secrete sufficient glucocorticoids for the degree of stress or illness. Emerging evidence suggests that the axis becomes overactive with age, which might offer an explanation for the clustering of features such as increased abdominal fat contents, insulin resistance, raised blood pressure, shorter adult stature and internalizing problem behavior. Research into the mechanisms that may drive this switch in HPA axis activity is ongoing.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, Wit JM, Dekker FW. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr. 2005;81:480–487. doi: 10.1093/ajcn.81.2.480. [DOI] [PubMed] [Google Scholar]
- 2.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–2186. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 3.Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang-Karlsson S, Makitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–2063. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- 4.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Euser AM, de Wit CC, Finken MJ, Rijken M, Wit JM. Growth of preterm born children. Horm Res. 2008;70:319–328. doi: 10.1159/000161862. [DOI] [PubMed] [Google Scholar]
- 6.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 7.Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98:106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- 8.Helbock HJ, Insoft RM, Conte FA. Glucocorticoid-responsive hypotension in extremely low birth weight newborns. Pediatrics. 1993;92:715–717. [PubMed] [Google Scholar]
- 9.Subhedar NV. Treatment of hypotension in newborns. Semin Neonatol. 2003;8:413–423. doi: 10.1016/S1084-2756(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 10.Mitanchez D. Glucose regulation in preterm newborn infants. Horm Res. 2007;68:265–271. doi: 10.1159/000104174. [DOI] [PubMed] [Google Scholar]
- 11.Ng PC. Adrenocortical insufficiency and refractory hypotension in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2016 doi: 10.1136/archdischild-2016-311289. pii:fetalneonatal-2016-311289. [DOI] [PubMed] [Google Scholar]
- 12.Finken MJ, van der Voorn B, Heijboer AC, de Waard M, van Goudoever JB, Rotteveel J. Glucocorticoid programming in very preterm birth. Horm Res Paediatr. 2016;85:221–231. doi: 10.1159/000443734. [DOI] [PubMed] [Google Scholar]
- 13.Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab. 1994;78:266–270. doi: 10.1210/jcem.78.2.8106610. [DOI] [PubMed] [Google Scholar]
- 14.Korte C, Styne D, Merritt TA, Mayes D, Wertz A, Helbock HJ. Adrenocortical function in the very low birth weight infant: Improved testing sensitivity and association with neonatal outcome. J Pediatr. 1996;128:257–263. doi: 10.1016/s0022-3476(96)70404-3. [DOI] [PubMed] [Google Scholar]
- 15.Bolt RJ, van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA. Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res. 2002;52:405–410. doi: 10.1203/00006450-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Gaissmaier RE, Pohlandt F. Single-dose dexamethasone treatment of hypotension in preterm infants. J Pediatr. 1999;134:701–705. doi: 10.1016/s0022-3476(99)70284-2. [DOI] [PubMed] [Google Scholar]
- 17.Ng PC, Lee CH, Bnur FL, Chan IH, Lee AW, Wong E, Chan HB, Lam CW, Lee BS, Fok TF. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics. 2006;117:367–375. doi: 10.1542/peds.2005-0869. [DOI] [PubMed] [Google Scholar]
- 18.Kopelman AE, Moise AA, Holbert D, Hegemier SE. A single very early dexamethasone dose improves respiratory and cardiovascular adaptation in preterm infants. J Pediatr. 1999;135:345–350. doi: 10.1016/s0022-3476(99)70132-0. [DOI] [PubMed] [Google Scholar]
- 19.Moise AA, Wearden ME, Kozinetz CA, Gest AL, Welty SE, Hansen TN. Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics. 1995;95:845–850. [PubMed] [Google Scholar]
- 20.Verhaeghe J, van Bree R, van Herck E, Coopmans W. Exogenous corticosteroids and in utero oxygenation modulate indices of fetal insulin secretion. J Clin Endocrinol Metab. 2005;90:3449–3453. doi: 10.1210/jc.2004-2512. [DOI] [PubMed] [Google Scholar]
- 21.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107:270–273. doi: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- 22.Roggero P, Gianni ML, Amato O, Orsi A, Piemontese P, Morlacchi L, Mosca F. Is term newborn body composition being achieved postnatally in preterm infants? Early Hum Dev. 2009;85:349–352. doi: 10.1016/j.earlhumdev.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Sipola-Leppanen M, Vaarasmaki M, Tikanmaki M, Matinolli HM, Miettola S, Hovi P, Wehkalampi K, Ruokonen A, Sundvall J, Pouta A, Eriksson JG, Jarvelin MR, Kajantie E. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol. 2015;181:861–873. doi: 10.1093/aje/kwu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas EL, Parkinson JR, Hyde MJ, Yap IK, Holmes E, Dore CJ, Bell JD, Modi N. Aberrant adiposity and ectopic lipid deposition characterize the adult phenotype of the preterm infant. Pediatr Res. 2011;70:507–512. doi: 10.1203/PDR.0b013e31822d7860. [DOI] [PubMed] [Google Scholar]
- 25.Peralta-Carcelen M, Jackson DS, Goran MI, Royal SA, Mayo MS, Nelson KG. Growth of adolescents who were born at extremely low birth weight without major disability. J Pediatr. 2000;136:633–640. doi: 10.1067/mpd.2000.104291. [DOI] [PubMed] [Google Scholar]
- 26.Powls A, Botting N, Cooke RW, Pilling D, Marlow N. Growth impairment in very low birthweight children at 12 years: correlation with perinatal and outcome variables. Arch Dis Child Fetal Neonatal Ed. 1996;75:F152–F157. doi: 10.1136/fn.75.3.f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehkalampi K, Hovi P, Dunkel L, Strang-Karlsson S, Jarvenpaa AL, Eriksson JG, Andersson S, Kajantie E. Advanced pubertal growth spurt in subjects born preterm: the Helsinki study of very low birth weight adults. J Clin Endocrinol Metab. 2011;96:525–533. doi: 10.1210/jc.2010-1523. [DOI] [PubMed] [Google Scholar]
- 28.Szathmari M, Vasarhelyi B, Tulassay T. Effect of low birth weight on adrenal steroids and carbohydrate metabolism in early adulthood. Horm Res. 2001;55:172–178. doi: 10.1159/000049991. [DOI] [PubMed] [Google Scholar]
- 29.Meuwese CL, Euser AM, Ballieux BE, van Vliet HA, Finken MJ, Walther FJ, Dekker FW, Wit JM. Growth-restricted preterm newborns are predisposed to functional adrenal hyperandrogenism in adult life. Eur J Endocrinol. 2010;163:681–689. doi: 10.1530/EJE-10-0471. [DOI] [PubMed] [Google Scholar]
- 30.Gohlke B, Wudy SA, Stutte S, Bartmann P, Hartmann MF, Woelfle J. Increased steroid excretion in children with extremely low birth weight at a median age of 9.8 years. Horm Res Paediatr. 2015;84:331–337. doi: 10.1159/000441031. [DOI] [PubMed] [Google Scholar]
- 31.Brummelte S, Chau CM, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, Grunau RE. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology. 2015;51:151–163. doi: 10.1016/j.psyneuen.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Graaf J, van den Akker EL, van Lingen RA, Groot Jebbink LJ, de Jong FH, Grunau RE, van Dijk M, Tibboel D. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. J Pediatr. 2014;165:459–463. doi: 10.1016/j.jpeds.2014.05.047. e2. [DOI] [PubMed] [Google Scholar]
- 33.Buske-Kirschbaum A, Krieger S, Wilkes C, Rauh W, Weiss S, Hellhammer DH. Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J Clin Endocrinol Metab. 2007;92:3429–3435. doi: 10.1210/jc.2006-2223. [DOI] [PubMed] [Google Scholar]
- 34.Kaseva N, Wehkalampi K, Pyhala R, Moltchanova E, Feldt K, Pesonen AK, Heinonen K, Hovi P, Jarvenpaa AL, Andersson S, Eriksson JG, Raikkonen K, Kajantie E. Blunted hypothalamic-pituitary-adrenal axis and insulin response to psychosocial stress in young adults born preterm at very low birth weight. Clin Endocrinol (Oxf) 2014;80:101–106. doi: 10.1111/cen.12251. [DOI] [PubMed] [Google Scholar]
- 35.Wust S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30:591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Raikkonen K, Seckl JR, Pesonen AK, Simons A, Van den Bergh BR. Stress, glucocorticoids and liquorice in human pregnancy: programmers of the offspring brain. Stress. 2011;14:590–603. doi: 10.3109/10253890.2011.602147. [DOI] [PubMed] [Google Scholar]
- 38.Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, Harding JE. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331:665. doi: 10.1136/bmj.38576.494363.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 40.Dessens AB, Haas HS, Koppe JG. Twenty-year follow-up of antenatal corticosteroid treatment. Pediatrics. 2000;105:E77. doi: 10.1542/peds.105.6.e77. [DOI] [PubMed] [Google Scholar]
- 41.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–142. [PubMed] [Google Scholar]
- 42.Finken MJ, Keijzer-Veen MG, Dekker FW, Frolich M, Walther FJ, Romijn JA, van der Heijden BJ, Wit JM. Antenatal glucocorticoid treatment is not associated with long-term metabolic risks in individuals born before 32 weeks of gestation. Arch Dis Child Fetal Neonatal Ed. 2008;93:F442–F447. doi: 10.1136/adc.2007.128470. [DOI] [PubMed] [Google Scholar]
- 43.Finken MJ, Meulenbelt I, Dekker FW, Frolich M, Walther FJ, Romijn JA, Slagboom PE, Wit JM. Abdominal fat accumulation in adults born preterm exposed antenatally to maternal glucocorticoid treatment is dependent on glucocorticoid receptor gene variation. J Clin Endocrinol Metab. 2011;96:E1650–E1655. doi: 10.1210/jc.2011-0288. [DOI] [PubMed] [Google Scholar]
- 44.van der Voorn B, Wit JM, van der Pal SM, Rotteveel J, Finken MJ. Antenatal glucocorticoid treatment and polymorphisms of the glucocorticoid and mineralocorticoid receptors are associated with IQ and behavior in young adults born very preterm. J Clin Endocrinol Metab. 2015;100:500–507. doi: 10.1210/jc.2014-2843. [DOI] [PubMed] [Google Scholar]
- 45.Crowther CA, McKinlay CJ, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2015;7:CD003935. doi: 10.1002/14651858.CD003935.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 48.Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- 49.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–631. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 51.van der Voorn B, de Waard M, van Goudoever JB, Rotteveel J, Heijboer AC, Finken MJ. Breast-milk cortisol and cortisone concentrations follow the diurnal rhythm of maternal hypothalamus-pituitary-adrenal axis activity. J Nutr. 2016;146:2174–2179. doi: 10.3945/jn.116.236349. [DOI] [PubMed] [Google Scholar]