SUMMARY
The study evaluated the antibacterial and anti-inflammatory efficacy, domiciliary oral hygiene, of a mouthrinse containing Tea Tree Oil (TTO) comparing it with two mouthrinses containing chlorhexidine 0,12% respectively and essential oils, and a placebo.
Materials and methods
A pilot study, randomized 4 × 4, controlled, cross-over, double-blind. 16 subjects with gingivitis (7 males and 9 females) aged 21–37 years, were randomly divided into four groups based on mouthwash that had to be used for domiciliary oral hygiene: mouthwash with essential oils, mouthwash with chlorhexidine 0,12 %, mouthwash containig tea tree oil and mouthwash placebo.
Clinical evaluation was performed by: Full Mouth Plaque Score (FMPS), Full Mouth Bleeding Score (FMBS), Gingival Index (GI), discolorations, language examination and alteration of taste. The data were recorded before and 2 weeks after treatment.
Results
Statistical analysis shows that treatments with tea tree oil, essential oils and chlorhexidine are effective. Comparing treatments should be noted that the tea tree oil gives a greater improvement in the GI and FMBS, while it is the least effective in the control of bacterial plaque.
Conclusion
Although further studies are needed, the anti-inflammatory properties of the mouthwash made from TTO would seem to be a valuable non-toxic adjunct in the management of gingivitis.
Keywords: essential oil, dental plaque, gingivitis
Introduction
Experimental clinical studies in human have shown that, with the removal of bacterial plaque in subjects with gingivitis, you get a complete resolution of inflammation and restitutio ad integrum tissue (1–3).
Furthermore, as an integral part of the supporting therapy after periodontal treatment, the efficient plaque control can stop the progression and recurrence of pathology (4–6).
The rational mechanical removal of the plaque daily appears to be the only practical means for a long-term improvement of oral hygiene, although it may be flanked by a chemical control (5–7).
The effectiveness of chemical agents is extremely variable and their action is mainly preventive rather than therapeutic. Most of them, in fact, inhibit the development of plaque and of gingival inflammation but it is a limited effect, or at least slow. Currently there are several antimicrobial on the market, but the need to identify products without the side effects of synthetic drugs, is directing research towards natural remedies.
Tea tree oil
Tea Tree Oil (TTO) is the essential oil derived from the distillation of the leaves of Melaleuca alternifolia. The tree belongs to the Myrtaceae family and contains about 230 species, almost all native of Australia. The Melaleuca name comes from greek mèlas, black, and leukòs, white, from the contrast between the dark green of leaves and the white bark. Defined by Aborigines as ‘the most versatile healer of nature’, Melaleuca oil is used since ancient times; its traditional use has recently been validated by scientific research. In fact, numerous studies, in medical field, have reported its powerful antimicrobial activity (8). The first studies date back to the late nineteenth century: Belaiche mentioned it in 1895 as a remedy against Candida (8).
Currently, the TTO is among the few essential oils to be contextually embedded in the 7th European Pharmacopoeia and, simultaneously, is included in a monograph by the European Medicine Agency (EMA) that has recognized its traditional use and published its monograph (9) foreseeing the traditional use in products found herbalist’s shop and pharmacies (10). In 2006, some Australian microbiologists took stock of the situation on the properties and uses of oil of Melaleuca alternifolia and have condensed their impressions on the prestigious Clinical Microbiology Reviews (8).
The phyto components characterizing the TTO are monoterpenes and alcohol-monoterpenic. The international standard ISO 4730 sets maximum and/or minimum for 14 oil components (11) (Table 1) and requires two distinctive components: the terpinol-4 and 1,8-cineol (Figures 1, 2). The terpinol must have a minimum content of 30% and a maximum and the cineole a content of 15% in order to obtain a good therapeutic activity and a very low irritative action at the skin level. The composition of TTO can greatly vary during storage, with increased levels of cineol, increase and decrease of terpinol (12). It’s insoluble in water and soluble in two volumes of ethanol 85% at 20° C.
Table 1.
Composition of tea tree oil.
| Component | Composition (%) | |
|---|---|---|
|
| ||
| ISO 4730 rangea | Typical compositionb | |
| Terpinen-4-ol | ≥30c | 40.1 |
| γ-Terpinene | 10–28 | 23.0 |
| α-Terpinene | 5–13 | 10.4 |
| 1,8-Cineole | ≤15d | 5.1 |
| Terpinolene | 1.5–5 | 3.1 |
| ρ-Cymene | 0.5–12 | 2.9 |
| α-Pinene | 1–6 | 2.6 |
| α-Terpineol | 1.5–8 | 2.4 |
| Aromadendrene | Trace–7 | 1.5 |
| δ-Cadinene | Trace–8 | 1.3 |
| Limonene | 0.5–4 | 1.0 |
| Sabinene | Trace–3.5 | 0.2 |
| Globulol | Trace–3 | 0.2 |
| Viridiflorol | Trace–1.5 | 0.1 |
IOS 4730. International Organization for Standardization standard no. 4730 (from reference 89).
From reference 25.
No upper limit is set, although 48% has been proposed.
No lower limit is set.
Figure 1.
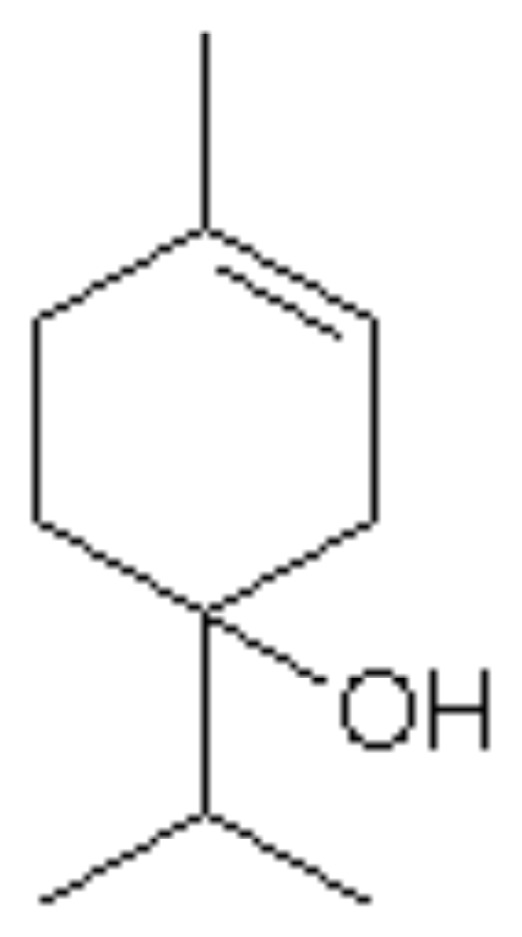
Terpinol-4.
Figure 2.
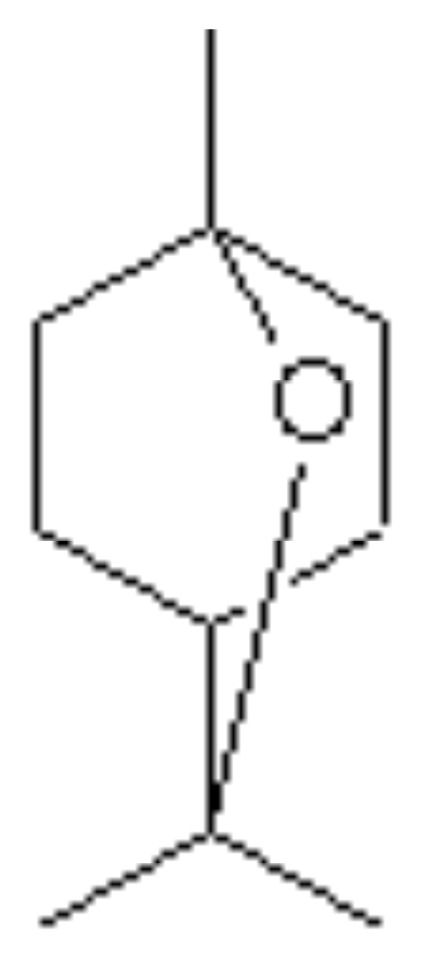
1–8 Cineol.
Biological properties
The TTO has been shown to have a number of therapeutic properties including anti-inflammatory activity (13–15). However, it is best known for its antimicrobial activity against a broad spectrum of microorganisms, including oral ones, for example Staphylococcus aureus and some viruses, including herpes simplex virus and flu viruses.
The TTO also has potent antifungal activity (16) and has also been successfully used as a topical agent for wound infections (17). Of all the properties claimed for the TTO, its antimicrobial activity has received more attention. Penfold published the first results on the antimicrobial activities of the TTO in a series of documents in 1920 and 1930 (18).
The antimicrobial activity of Melaleuca oil was compared with carbolic acid, or phenol, the gold standard of that time with a Rideal-Walker Coefficient test known as (RW), and the result was that the activity of the TTO was 11 times more active (18). Accordingly the TTO was promoted as a therapeutic agent.
In the medical field excellent properties are attributed to the essential oil: anti-inflammatory, antiseptic, immunostimulating and soothing, antibacterial, antifungal, healing, antiviral and fungicidal, apart from a slight numbing action.
In vitro studies have determined that the MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) of TTO range is from 0.003 to 2.0% (v/v). While most of the bacteria are sensitive to the TTO in concentrations of 1.0% or less, MIC above 2% have been reported for organisms such as staphylococci and micrococci diners, Enterococcus faecalis and Pseudomonas aeruginosa (19, 20).
The studies indicate that various oral bacteria are sensitive, suggesting that the TTO and/or components can be used in products for oral hygiene and in maintaining oral hygiene (16). The TTO, the α-terpineol and the terpinene-4-ol prove to have antibacterial activity against the growth of S. aureus and E. coli at a concentration about 0.78%. Terpinene-4-ol seems to have the most potent activity (21).
Studies carried out in the last decade have shown that TTO striking a series of immune responses, both in vitro and in vivo. For example, the soluble components in water of TTO can inhibit the production of lipopolysaccharide induced by inflammatory mediators which Tumor Necrosis Factor (TNF), interleukin-1 (IL-1), IL-10 and prostaglandin E2 (22).
Saxer et al. (23) have shown that the ‘action of the main components of TTO, 1,8-cineol and terpinen-4-ol, as well as lead to a reduction in inflammation of the oral tissues, also means less plaque formation’ as reported by Takarada et al., which demonstrated that TTO is able to inhibit the adhesion of S. mutans and Porphyromonas gingivalis (24).
Therefore, the TTO can have a role in the treatment of gingivitis and oral candidiasis (24–26) and there are also some preliminary evidences to suggest the reduction of levels of different compounds associated with bad breath (27).
Oral toxicity
TTO may be toxic if ingested, as evidenced by studies with animals and human cases of poisoning.
Accidental poisonings after ingestion of an overdose, 10–25 ml, have shown that the Melaleuca alternifolia causes depression of the central nervous system and muscle weakness (28, 29). In all cases, the patients responded to supportive therapy; the symptoms usually resolved within 36 hours, with no apparent sequelae. There are no human deaths due to TTO reported in the literature.
Interaction with other medicinal products and other forms of interaction haven’t been reported. Safety has not been established during pregnancy and lactation. In the absence of sufficient data, the use during pregnancy and lactation is not recommended.
Materials and methods
The aim of this study is to compare the antibacterial and anti-inflammatory efficacy, in domiciliary oral hygiene, of a mouthwash containing Tea Tree Oil (TTO) with two mouthwashes containing respectively chlorhexidine 0,12% and essential oils.
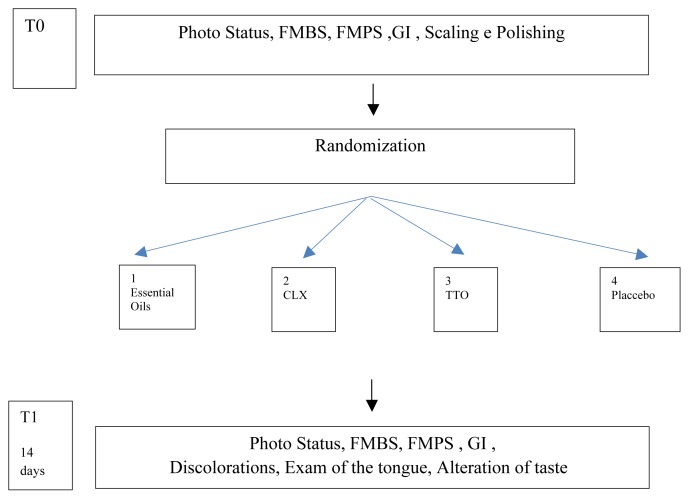
Design of the study (Figure 3)
Figure 3.
Flow Chart of the study.
A pilot study, randomized 4×4, controlled, cross-over, double-blind.
The study has been realized according with the ethical standards established in the Declaration of Helsinki and the informed consent gotten by all the participants before their enlistment to the study.
The allocation concealment has been gotten through seal opaque closed envelopes, open immediately after the treatment of scaling and polishing.
To eliminate the possible subjective error of evaluation, the study has been conducted in double blind.
After the treatment of professional oral hygiene, the patients have been divided in 4 groups in base to the mouthwash that you/they had to use for the domiciliary oral hygiene:
Treatment 1: mouthwash with essential oils, composed by thymol, menthol, eucalyptol, limonene, fluoride of sodium, zinc and xilitolo.
Treatment 2: mouthwash with chlorhexidine 0.12%.
Treatment 3: mouthwash of Tea Tree Oil (TTO) (Tebodont, DrWild and Co AG, Switzerland) (22, 30–32). Solution to 1,5% of tea tree oil.
Treatment 4: mouthwash placebo, prepared diluting in 2 liters of water 100 mls of dye to feed, of red color, coloring containing E122, preservative E211, proofreader of acidity E260.
Selection of subjects
The study included 16 patients, 7 males and 9 females, aged 21–37 years, selected from among people affected by gingivitis related to Operative Unit of Odontostomatology “Fra G.B. Orsenigo Hospital St. Pietro”, Rome, directed by Professor Marco Gargari.
Inclusion criteria
They included patients aged 18–70 years with the presence of:
- At least 20 teeth, to exclusion of 3rd molars
- PSR with values of 1 or 2
- PPD ≤ 3 mm
- Lack of periodontitis and orthodontic appliances
- No known allergy to any of the components of mouthwashes.
Exclusion criteria
They excluded subjects from the study who exhibited the following characteristics:
- Last 6 months < periodontal treatment
- Patients with systemic diseases that may affect the therapy such as: diabetes mellitus, tumors, metabolic bone diseases, disorders that affect the healing, radiations, immunosuppressive diseases and anticoagulant therapies
- Presence of orthodontic appliances
- Patients with periodontal disease PPD > 3 mm
- 6 months taking antibiotics <
- Taking 3 months < anti-inflammatory
- Assumption of cortisone
- Pregnant or taking contraceptive hormones
- Patients with mental or physical limitations that may restrict oral hygiene at home
- The lack of consent to the study.
The smoke was reported, but it was not a criterion for exclusion.
Clinical parameters for effectiveness evaluation:
- Full Mouth Plaque Score (FMPS)
- Full Mouth Bleeding Score (FMBS)
- Gingival Index (GI)
- Discolouring: 4 areas were considered for each tooth, buccal, oral, mesial and distal, the evaluation used a dichotomous method, presence/absence. The average value of tooth discoloration was shown as a percentage.
-
- Exam language: was examined the back of the tongue, using gauze to grasp the tip, giving 4 values:
0 - no visible patina on the dorsum of the tongue
1 - presence of patina on the posterior third of the back of the tongue
2 - presence of patina all over the back of the tongue, which do not cover the mucosal color
3 - presence of patina all over the back of the tongue, thick masking the mucosa of the tongue.
Clinical stage
Each patient was treated by the same operator, who used for every patient the same tools for the duration of all therapy.
Oral hygiene program: the patient has not changed the method of hygiene at home though uncorrect.
Non-surgical treatment: clinicians study participants have been operating independently of one another respecting a chronological sequence.
1st visit: made from 1° Clinical operator.
In first visit is collected on specific card:
- Medical history
- Informed consent
- Photo Status
- Gingival Index (GI)
- Full mouth plaque score (FMPS)
- Full mouth bleeding score (FMBS).
-
Instrumental treatment: carried out by the 2nd Clinical operator
Consisting of supragingival scaling with ultrasounds (EMS, inset A) and Scaler LM 301–302 and 311–312; curette Langer LM 1–2,3–4, 5–6; Polishing with synthetic fiber brush and prophy paste Cleanic of Kerr.
Photo Status, after the session of oral hygiene
Delivery of mouthwash: after treatment of professional hygiene through a sealed envelope, the 2° operator gave the patient the mouthwash that had to use home hygiene
Revaluation to 2 weeks: made from 1° the operator choosing the timing which was dictated by the historical study of Loe of 1965 (2). They were again collected GI values, FMPS, FMBS, and status of photography, evaluated any alteration on hard and soft oral tissues (discolouring, status of lingual papillae, etc.) and to patient requested to evaluate the mouthwash using a special assessment scale (VAS).
Results
The clinical data collected during the first visit (T0) of the subjects of the various groups, are collected in Table 2 below:
Table 2.
Data at T0.
| Patient | GI | FMPS % | FMBS % | |
|---|---|---|---|---|
| Group 1- OILS | 1 | 1.3 | 81 | 33 |
| 2 | 1.4 | 62.5 | 37 | |
| 3 | 1.3 | 88 | 34 | |
| 4 | 1.2 | 69 | 30 | |
|
| ||||
| Group 2- CLX | 1 | 1.3 | 66 | 36 |
| 2 | 1.7 | 85 | 61 | |
| 3 | 1.8 | 80 | 78 | |
| 4 | 1.1 | 63 | 24 | |
|
| ||||
| Group 3- TTO | 1 | 1.8 | 100 | 69 |
| 2 | 1.6 | 73 | 54 | |
| 3 | 2 | 100 | 77 | |
| 4 | 1.7 | 65 | 60 | |
|
| ||||
| Group 4 - Placebo | 1 | 1.6 | 68 | 64 |
| 2 | 1.6 | 55 | 63 | |
| 3 | 1.7 | 88 | 60 | |
| 4 | 1.4 | 82 | 45 | |
All of the patients showed a significant accumulation of plaque, as evidenced by the FMPS, despite they having reported to regularly use the toothbrush, 3 times a day, and dental floss or interdental cleaner, for interproximal hygiene, 1 time per day. FMPS values are considerably higher than the threshold value, 20%, considered compatible with periodontal health.
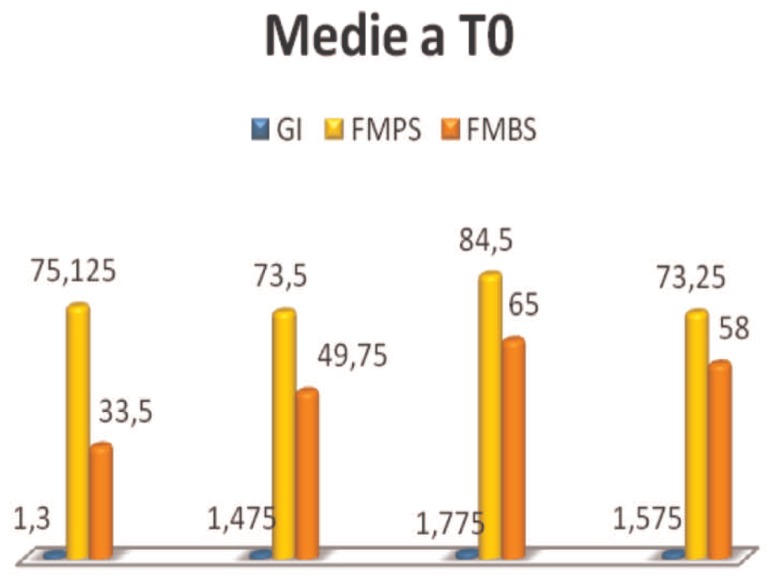
FMBS, index values of active inflammation, are above the threshold, 20% (30). Gingival index, GI, allows to assess the degree of gingival inflammation in relation to morphological parameters of color, shape and texture. The examinees had a clinical picture consistent, values describe in fact in almost all subjects a mild inflammation (Figure 4).
Figure 4.
Average values at T0.
After sitting professional hygiene to patients are asked to keep the same professional hygiene methods to not determine a further variable, integrating them with the use of mouthwash assigned, for 2 weeks. At the end of that period, all patients were reassessed.
The new data collected at T1, have been compared with those at T0 in Table 3.
Table 3.
Data at T0 and T1.
| T0 | T1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Patient | GI | FMPS % | FMBS % | GI | FMPS % | FMBS % | Discolorations % | Tongue | Taste | ||
| Group 1- OILS | 1 | 1.3 | 81 | 33 | 0.7 | 28 | 12 | 7 | 1 | No | |
| 2 | 1.4 | 62.5 | 37 | 1.3 | 49 | 31 | 0.4 | 3 | No | ||
| 3 | 1.3 | 88 | 34 | 1.2 | 14 | 18 | 0.8 | 1 | No | ||
| 4 | 1.2 | 69 | 30 | 1 | 37 | 18 | 0.4 | 2 | No | ||
|
| |||||||||||
| Group 2- CLX | 1 | 1.3 | 66 | 36 | 0.9 | 39 | 14 | 7 | 0 | No | |
| 2 | 1.7 | 85 | 61 | 1.3 | 78 | 32 | 10 | 3 | Yes | ||
| 3 | 1.8 | 80 | 78 | 1.5 | 52 | 56 | 19 | 3 | No | ||
| 4 | 1.1 | 63 | 24 | 0.9 | 5 | 10 | 24 | 3 | Yes | i | |
|
| |||||||||||
| Group 3- TTO | 1 | 1.8 | 100 | 69 | 1.5 | 79 | 48 | 16 | 2 | No | |
| 2 | 1.6 | 73 | 54 | 1.2 | 19 | 19 | 4.5 | 1 | No | ||
| 3 | 2 | 100 | 77 | 1.5 | 72 | 47 | 6 | 2 | No | ||
| 4 | 1.7 | 65 | 60 | 1.3 | 54 | 32 | 3 | 2 | No | ||
|
| |||||||||||
| Group 4-Placebo | 1 | 1.6 | 68 | 64 | 1.5 | 37 | 50 | 0.6 | 2 | No | |
| 2 | 1.6 | 55 | 63 | 1.4 | 43 | 40 | 0 | 3 | No | ||
| 3 | 1.7 | 88 | 60 | 1.5 | 51 | 51 | 17 | 2 | No | ||
| 4 | 1.4 | 82 | 45 | 1.3 | 28 | 30 | 0.4 | 3 | No | ||
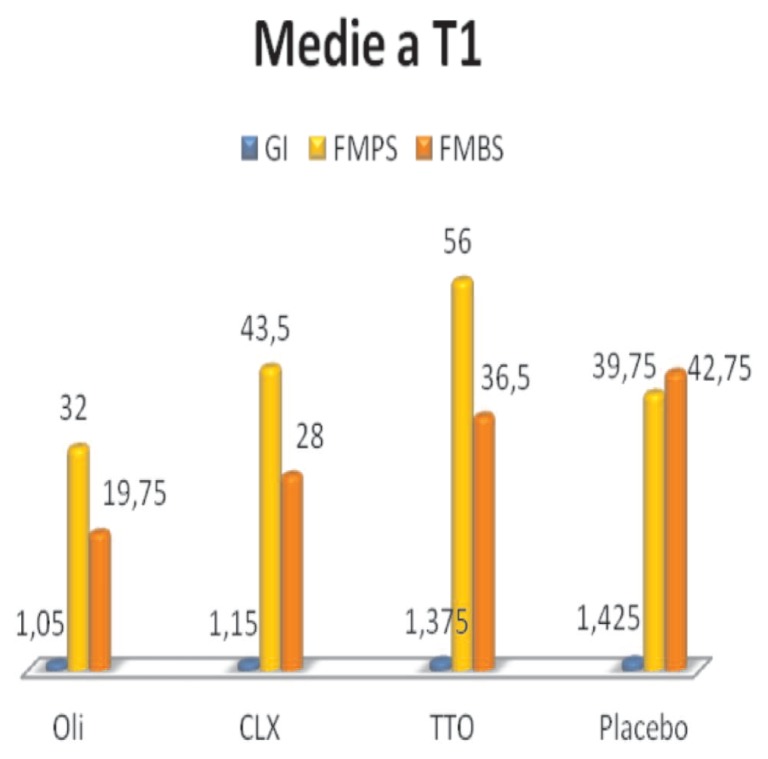
The GI values, FMPS and FMBS are shown in the graph (Figure 5).
Figure 5.
Average values at T1.
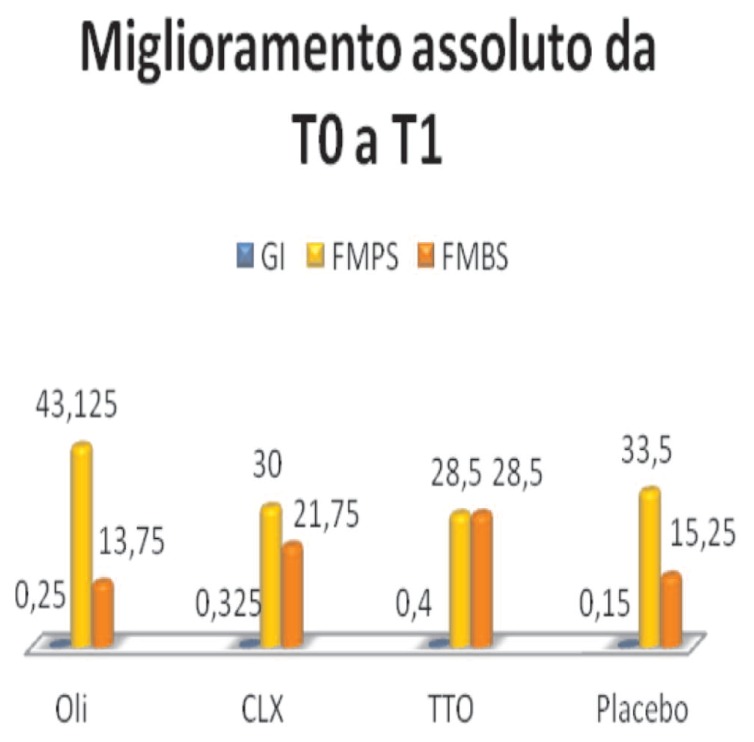
Comparing the data you can see an improvement, in the three indices considered, in all 4 groups (Figure 6).
Figure 6.
Absolute improvement.
In detail:
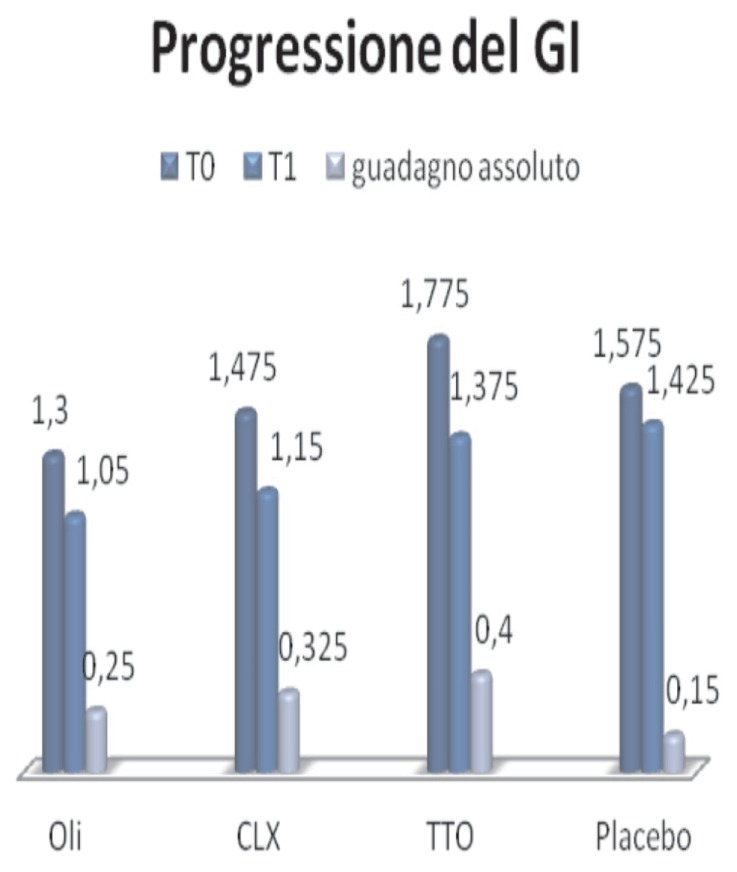
GI (Figure 7):
Figure 7.
Progression of GI.
- Essential oils, the mean changes from 1.3, at T0, at 1.05, T1, with an absolute improvement of 0.25
- Clx, from 1,475, TO, to 1.15 at T1, with an absolute improvement 0325
- TTO, from 1,575, TO, to 1,425 in T1, with an improvement of 0.4
- Placebo, from 1,775, a TO, to 1,375 to T1, with an absolute improvement of 0.15
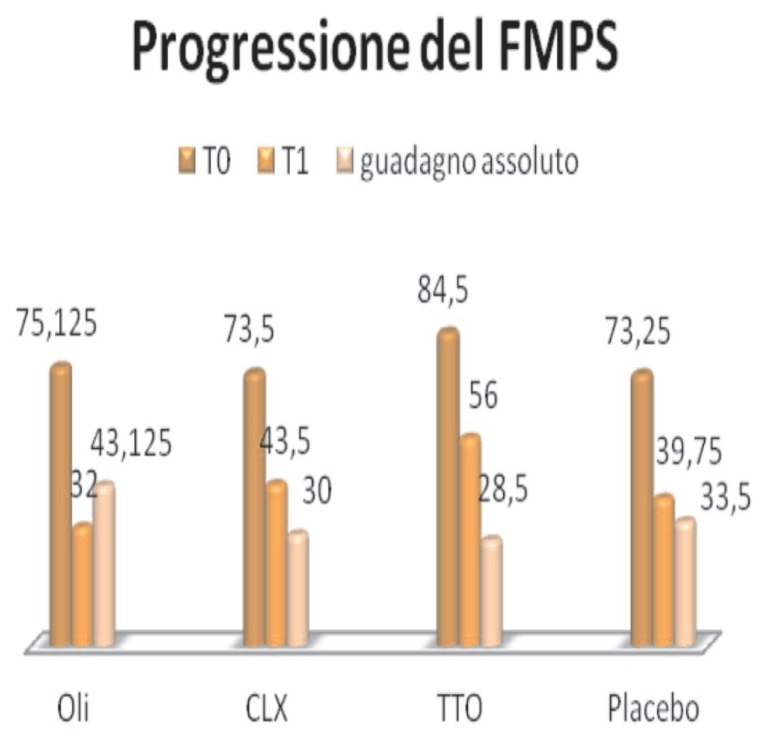
FMPS (Figure 8):
Figure 8.
Progression of FMPS.
- Essential oils, the average percentage value changes from 75.12, T0, T1, 32, with an absolute improvement of 43,125
- Clx, from 73.5, a TO, at 43.5 at T1, with an improvement of 30
- TTO, from 73.25, a TO, to 39.75 to T1, with an absolute improvement of 28.5
- Placebo, from 84.5, a TO, 56 to T1, with an absolute improvement of 33.5
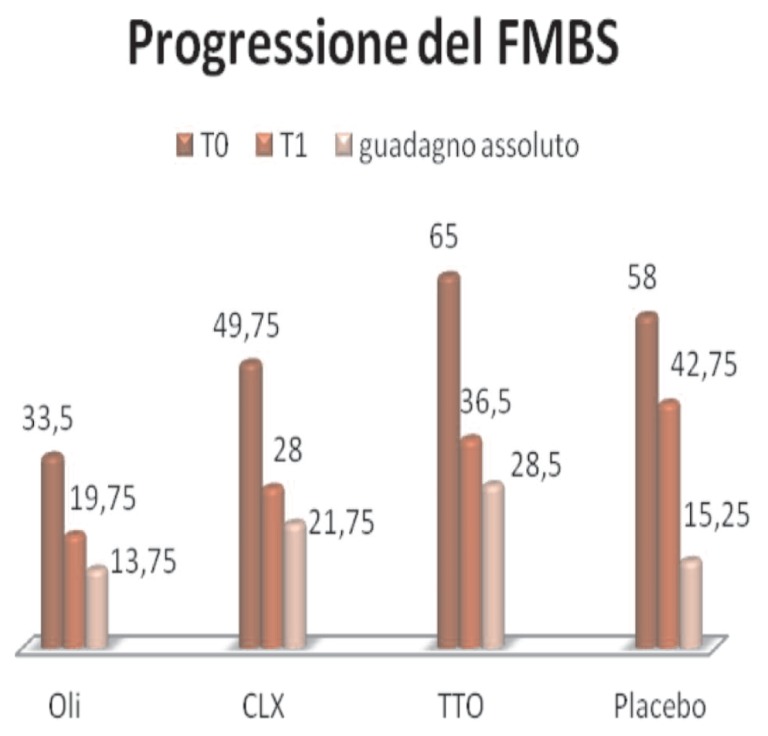
FMBS (Figure 9):
Figure 9.
Progression of FMBS.
- Essential oils, the average percentage value changes from 33.5, T0, T1, 19.75, improving overall 13.75
- Clx, from 49.75, a T0, to 28 at T1, with absolute improvement of 21.75
- TTO, 58, a T0, to 42.75 to T1, with an absolute improvement of 28.5
- Placebo, from 65, T0, to 36.5 in T1, with an absolute improvement of 15.25.
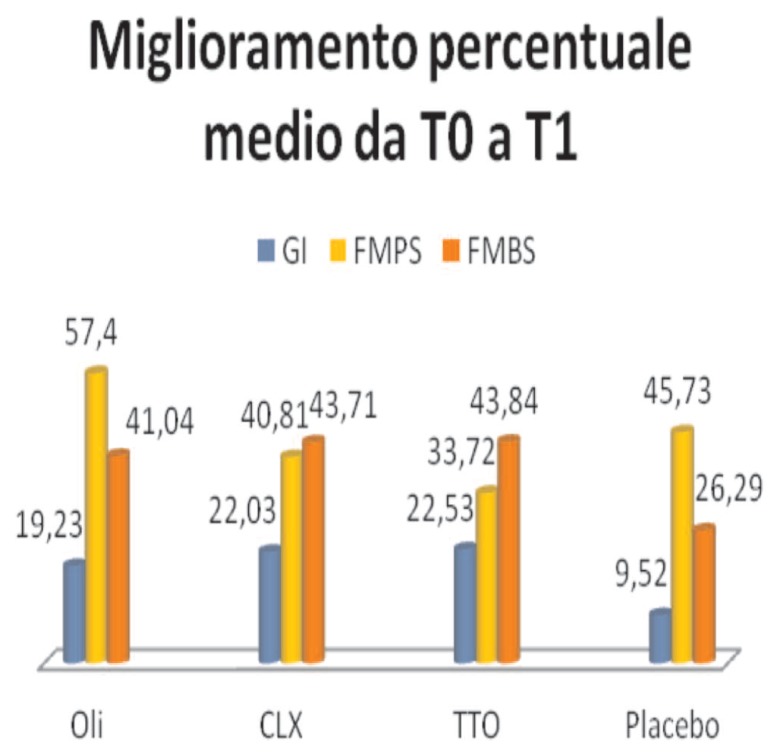
Analyzing the percentage improvement of 4 and mouthwash (Figure 10), it should be noted that the major improvement of the GI occurred in the group of TTO, 22.53, followed by CLX, 22.03, and from essential oils, 19.23; the placebo instead shows lower values. These results remain in the FMBS, with TTO 43.84, CLX 43.71, essential oils 41.04 and placebo 26.29.
Figure 10.
Percentage improvement.
The FMPS instead shows follow a different course: the greater percentage improvement is evident in the Group of essential oils, 57.4, followed by placebo, 45.73, CLX 40.81 and finally TTO with 33.72.
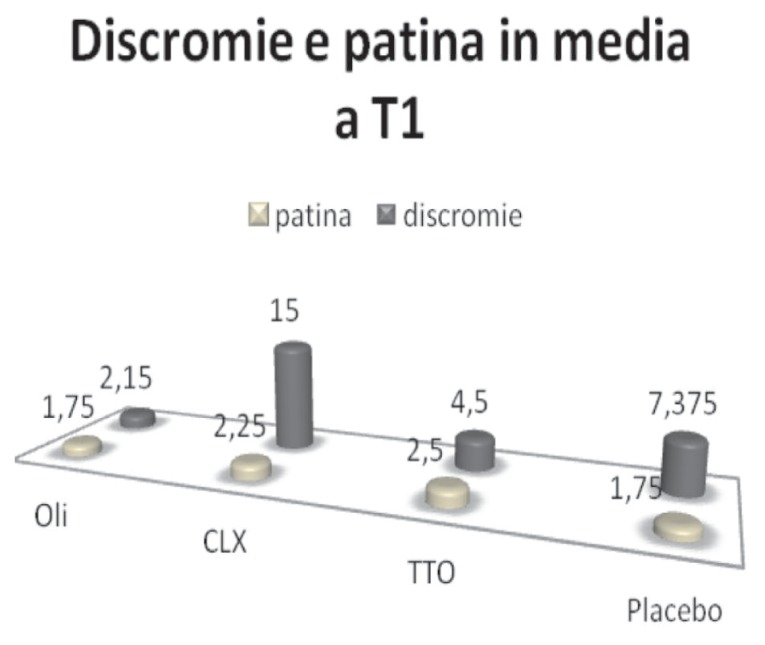
The analysis of the tongue coating and discolourings (Figure 11) shows that, while there is no significant difference in the quality and quantity of coating between the components of the 4 groups, the use of chlorhexidine determines after 15 days, the appearance of discoloration on the tooth surfaces, as well as documented in the literature (Figure 11).
Figure 11.
Discoloration and tongue coating.
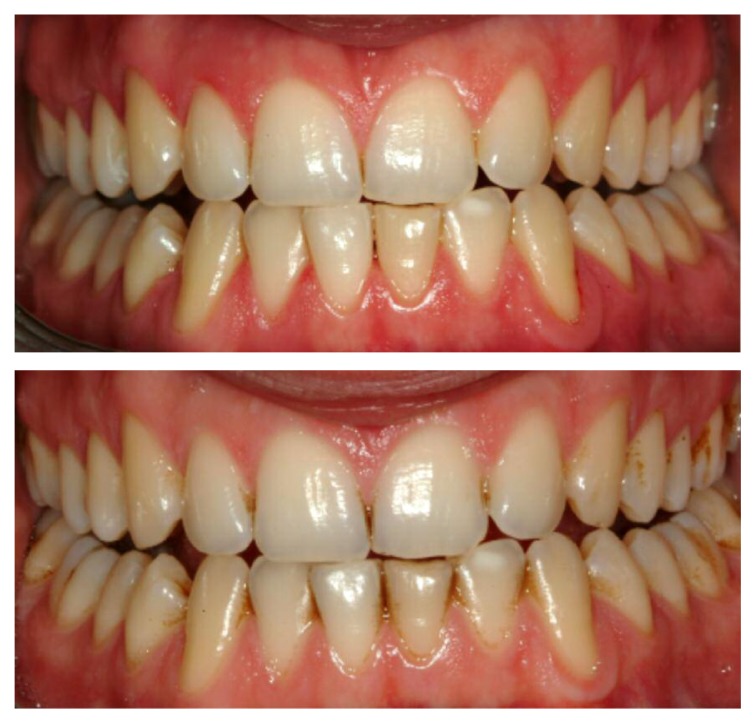
Figures 12 and 13 show images of a patient at T0 and T1, before and after treatment with mouthwash based CLX.
Figure 12–13.
Images of a patient at T0 and T1, before and after treatment with mouthwash based CLX.
Mouthwash based CLX is the only one to determine alteration of taste, in 50% of subjects in the respective group.
Discussion and conclusions
Gingivitis is a very common disease and affects a wide range of population. At the same time, the increasing “social” value of the mouth, has led to a boom of the production of products, synthetic or natural, can facilitate the attainment of the objective.
Such a wide range of products requires that dental workers, dentists and dental hygienists are able to give precise information to patients on the selection and use of chemical principals.
This study aimed to demonstrate the efficacy of a new mouthwash, Tebodont, based on essential oil of Melaleuca alternifolia, in the control of bacterial plaque and inflammatory response, cause and expression of gingivitis.
The tested product formulation, according to the instructions of the manufacturer, would provide an antimicrobial and anti-inflammatory effect.
The data obtained, despite the small amount of sample, suggest a more effectiveness in the treatment of gingivitis. The effectiveness of the mouthwash seems to act not so much through as an antibacterial action, rather in the reduction of the inflammatory response and the bleeding.
The analysis of data on the other hand, reveals extremely discordant values: while GI and FMBS seem to have a homogeneous trend, the FMPS deviates significantly to the point that there seems not to be correlation between cause and effect.
The proposed work is to be considered a pilot study given the small sample size; however, the values, in all their inconsistency, give a glimpse of the therapeutic possibilities of the product tested.
To obtain more statistically significant data it would be important to conduct further studies with a larger sample, and a follow-up of at least six months to determine the full action of Tebodont.
References
- 1.Garmyn P, van Steenberghe D, Quirynen M. Efficacy of plaque control in the maintenance of gingival health: Plaque control in primary and secondary prevention. In: Lang NP, Attstrom R, Loe H, editors. Proceedings of the European workshop on mechanical plaque control. Berlin: Quintessence; 1998. [Google Scholar]
- 2.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. Journal of Periodontology. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal-disease in adults. Journal of Clinical Periodontology. 1978;5:133–151. doi: 10.1111/j.1600-051x.1978.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 4.Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal-disease in adults – results after 6 years. Journal of Clinical Periodontology. 1981a;8:239–248. doi: 10.1111/j.1600-051x.1981.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson P, Lindhe J. Effect of oral hygiene instruction and professional tooth-cleaning on caries and gingivitis in school-children. Community Dentistry and Oral Epidemiology. 1981b;9:251–255. doi: 10.1111/j.1600-0528.1981.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 6.Axelsson P, Lindhe J. The significance of maintenance care in the treatment of periodontal-disease. Journal of Clinical Periodontology. 1981c;8:281–294. doi: 10.1111/j.1600-051x.1981.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 7.Axelsson P, Lindhe J, Nystrom B. On the prevention of caries and periodontal-disease – results of a 15-year longitudinal- study in adults. Journal of Clinical Periodontology. 1991;18:182–189. doi: 10.1111/j.1600-051x.1991.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 8.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006 Jan;19(1):50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Assessment Report on Melaleuca alternifolia (Maiden and Betch) Cheel, M. linariifolia Smith, M. dissitiflora F. Mueller and/or other species of Melaleuca, aetheroleum. London: EMA; 2013. (EMA/HMPC/320930/2012) [Google Scholar]
- 10.Scientific Committee on Consumer Products Opinion on tea tree oil. Brussels: European Commission; 2008. (SCCP/1155/08) [Google Scholar]
- 11.International Organisation for Standardisation. Oil of Melaleuca, terpinen-4-ol type (tea tree oil) International Organisation for Standardisation; Geneva, Switzerland: 2004. ISO 4730:2004. [Google Scholar]
- 12.Brophy JJ, Davies NW, Southwell IA, Stiff IA, Williams LR. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree) J Agric Food Chem. 1989;37:1330–1335. [Google Scholar]
- 13.Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay-Jones JJ. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000 Nov;49(11):619–26. doi: 10.1007/s000110050639. [DOI] [PubMed] [Google Scholar]
- 14.Koh KJ, Pearce AL, Marshman G, Finlay-Jones JJ, Hart PH. Tea tree oil reduces histamine-induced skin inflammation. Br J Dermatol. 2002 Dec;147(6):1212–7. doi: 10.1046/j.1365-2133.2002.05034.x. [DOI] [PubMed] [Google Scholar]
- 15.Pearce AL, Finlay-Jones JJ, Hart PH. Reduction of nickel-induced contact hypersensitivity reactions by topical tea tree oil in humans. Inflamm Res. 2005 Jan;54(1):22–30. doi: 10.1007/s00011-004-1317-6. [DOI] [PubMed] [Google Scholar]
- 16.Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003a;95:853–86010. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 17.Syed TA, Qureshi ZA, Ali SM, Ahmad S, Ahmad SA. Treatment of toenail onychomycosis with 2% bute-nafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Trop Med Int Health. 1999 Apr;4(4):284–7. doi: 10.1046/j.1365-3156.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 18.Penfold AR, Grant R. The germicidal values of some Australian essential oils and their pure constituents, together with those for some essential oil isolates, and synthetics. Part III. J R Soc New South Wales. 1925;59:346–349. [Google Scholar]
- 19.Banes-Marshall L, Cawley P, Phillips CA. In vitro activity of Melaleuca alternifolia (tea tree) oil against bacterial and Candida spp. Isolates from clinical specimens. Br J Biomed Sci. 2001;58:139–145. [PubMed] [Google Scholar]
- 20.Hammer KA, Carson CF, Riley TV. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil) Am J Infect Control. 1996;24:186–189. doi: 10.1016/s0196-6553(96)90011-5. [DOI] [PubMed] [Google Scholar]
- 21.Budzyńska A, Wieckowska-Szakiel M, Sadowska B, Kalemba D, Rozalska B. Antibiofilm activity of selected plant essential oils and their major components. Pol J Microbiol. 2011;60(1):35–41. [PubMed] [Google Scholar]
- 22.Saxer UP, Stauble A, Szabo SH, Menghini G. Effect of mouthwashing with tea tree oil on plaque and inflammation. Schweiz Monatsschr Zahnmed. 2003;113:985–96. [PubMed] [Google Scholar]
- 23.Groppo FC, Ramacciato JC, Simoes RP, Florio FM, Sartoratto A. Antimicrobial activity of garlic, tea tree oil, and chlorhexidine against oral microorganisms. Int Dent J. 2002;52:433–437. doi: 10.1111/j.1875-595x.2002.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 24.Takarada K. The effects of essential oils on periodon-topathic bacteria and oral halitosis. Oral Dis. 2005;11:115. [Google Scholar]
- 25.Arweiler NB, Donos N, Netuschil L, Reich E, Sculean A. Clinical and antibacterial effect of tea tree oil—a pilot study. Clin Oral Investig. 2000;4:70–73. doi: 10.1007/s007840050118. [DOI] [PubMed] [Google Scholar]
- 26.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott C. Tea tree oil poisoning. Med J Aust. 1993;159:830–831. doi: 10.5694/j.1326-5377.1993.tb141370.x. [DOI] [PubMed] [Google Scholar]
- 28.Seawright A. Tea tree oil poisoning. Med J Aust. 1993;159:831. doi: 10.5694/j.1326-5377.1993.tb141370.x. [DOI] [PubMed] [Google Scholar]
- 29.De Groot AC, Weyland JW. Systemic contact dermatitis from tea tree oil. Contact Dermatitis. 1992;27:279–280. doi: 10.1111/j.1600-0536.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- 30.Rylander H, Lindhe J. Cause-related periodontal therapy. In: Lindhe J, Karring T, Lang NP, editors. Clinical Periodontology and Implant Dentistry. Munksgaard; 1997. pp. 438–44. [Google Scholar]
- 31.Shapiro S, Meier A, Guggenheim B. The antimicrobial activity of essential oils and essential oil components towards oral bacteria. Oral Microbiol Immunol. 1994;9:202–208. doi: 10.1111/j.1399-302x.1994.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 32.Kulik E, Lenkeit K, Meyer J. Antimikrobielle Wirkung von Teebaumol (Melaleuca alternifolia) auf orale Mikroorganismen. Schweiz. Monatsschr Zahnmed. Acta Med Dent Helv. 2000;5:125, 11. [PubMed] [Google Scholar]