Abstract
Background:
There is an ongoing debate regarding possible cost and benefits, but also harm of universal screening for the emerging sexually transmitted pathogen Mycoplasma genitalium.
Methods:
From the initial pool of 8665 samples that were tested, a subset of Chlamydia trachomatis-positive and randomly selected C. trachomatis-negative cervical swabs were further interrogated for M. genitalium by real-time polymerase chain reaction, using a 224 bp long fragment of the glyceraldehyde-3-phosphate dehydrogenase gene.
Results:
M. genitalium was detected in 4.8% of C. trachomatis-positive samples and none of C. trachomatis-negative samples. Accordingly, a significant association was shown between M. genitalium and C. trachomatis (P < 0.01), but also between M. genitalium and Mycoplasma hominis infection (P < 0.01).
Conclusions:
Based on the results, routine screening is recommended only for women with one or more identified risk factors. Moreover, younger age does not represent an appropriate inclusion/exclusion criterion for M. genitalium testing in the low-risk female population.
Keywords: Cervical swabs, Chlamydia trachomatis, Mycoplasma genitalium, Mycoplasma hominis, screening, sexually transmitted infections
Introduction
Mycoplasma genitalium represents an emerging cause of sexually transmitted infections (STIs).[1] It is considered as an independent risk factor for cervicitis in women, but its role in pelvic inflammatory disease, spontaneous abortion and infertility has not been ascertained until recently.[2,3] The prevalence of M. genitalium in women ranges from <1% to 42%,[4,5] depending on whether we consider low-risk population (i.e., attendees of general practitioners and public health services) or high-risk population (i.e., sexually transmitted disease clinics attendees or those with specific symptoms).
There is an ongoing debate regarding possible cost and benefits, but also harm of universal screening for M. genitalium among low-risk individuals. Similar to many other countries, M. genitalium infection is not routinely screened for in Croatia and the data of its prevalence in the country are scarce – especially for the female population. Only one study regarding M. genitalium prevalence in Croatia was published thus far, conducted in men attending fertility clinic.[5]
The aim of our study was therefore to determine the prevalence of M. genitalium in cervical swabs admitted to the public health laboratory, as well as to detect co-infection patterns of M. genitalium with Chlamydia trachomatis and other STIs in order to assess the necessity of implementing M. genitalium screening in the low-risk female population.
Methods
Swabs were taken from women with a low-risk for STIs, i.e., asymptomatic attendees of primary care gynecologist searching for screening and prenatal care. An unlinked anonymized method to test routinely collected and stored cervical swabs was used. The samples were collected at primary care and private gynecology offices in the Zagreb region, Croatia, and referred to the public health laboratory for routine C. trachomatis and genital Mycoplasma testing. Uniformity of samples collection was maintained by following the standard protocol and using collection kit comprised of dacron swab and MicroTest™ M4RT® transport medium (Remel Inc., Lenexa, USA). From the total pool of 8,665 samples collected from March 2014 to February 2015, 146 C. trachomatis positive and 168 randomly selected C. trachomatis negative samples were used in the study. Results for routinely tested genital Mycoplasma (Mycoplasma hominis and Ureaplasma spp.) were recorded prior to M. genitalium testing. The study was approved by the Ethics Committee of the institute where the research took place.
For C. trachomatis detection real-time polymerase chain reaction (PCR) was performed using Cobas® Taqman® CT v2.0 test on Cobas® Taqman® Analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Identification of M. hominis and Ureaplasma spp. was performed by Mycoplasma IST 2 kit (bioMérieux SA, Lyon, France). M. genitalium was detected by real-time PCR LightMix® kit Mycoplasma genitalium test (TIB MOLBIOL, GmbH, Berlin, Germany) on LightCycler 480 II Instrument (Roche Diagnostics GmbH, Mannheim, Germany), using a 224 bp long fragment of the glyceraldehyde-3-phosphate dehydrogenase gene. Difference between groups was assessed using Fisher's exact test and strength of association using univariate logistic regression when appropriate. Statistical analysis was performed using STATA/SE ver 11.2 (StataCorp LP, TX, USA).
Results
During 1 year period, in women who were using services of the public health laboratory, C. trachomatis prevalence of 1.9% (165/8665), M. hominis prevalence of 3.2% (277/8665), and Ureaplasma spp. prevalence of 33.3% (2885/8665) were observed. A total of 314 cervical swabs were further selected according to the C. trachomatis status (146 C. trachomatis positive and 168 C. trachomatis negative) and interrogated for M. genitalium. The additional analysis of selected samples on routinely tested genital mycoplasmas (M. hominis and U. urealyticum) revealed that 54 of 314 samples were M. hominis positive, and 181 of 314 were Ureaplasma spp. positive [Table 1]. C. trachomatis positive samples more commonly harbored routinely tested genital Mycoplasma in comparison to C. trachomatis negative samples (101/146; 69.2% vs. 72/168; 42.9% Fisher's exact P < 0.001).
Table 1.
Univariate associations between Mycoplasma genitalium infection and other bacterial sexually transmitted infections
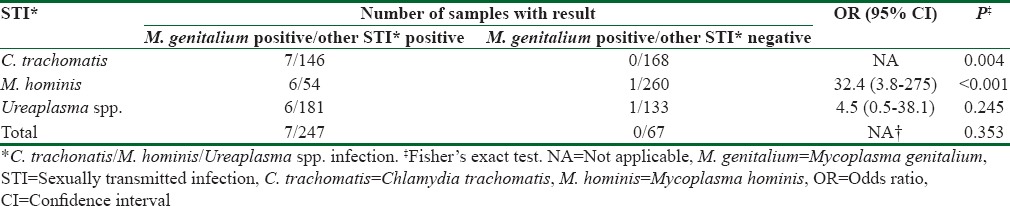
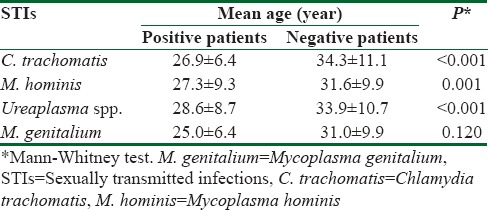
M. genitalium was detected in seven of 314 cervical swabs tested (2.2%, 95% confidence interval = 0.9%–4.5%). However, all positive samples for M. genitalium were also positive for C. trachomatis [Table 1]. Furthermore, five M. genitalium positive samples were detected in cervical swabs with initially proved triple infection (C. trachomatis, M. hominis and Ureaplasma spp.) and two M. genitalium positive specimens were detected in cervical swabs with initially proved dual infection. Eleven percent of those infected with M. hominis, 4.8% of those infected with C. trachomatis and 3.3% of those infected with Ureaplasma spp. were co-infected with M. genitalium. All selected samples negative to other sexually transmitted bacteria were also negative to the M. genitalium. Significant association was shown between M. genitalium and two bacterial STIs: C. trachomatis and M. hominis infection. There was no association between detection of M. genitalium and Ureaplasma spp. [Table 1]. The mean age of investigated women was 30.9 ± 9.9 years (age range: 1–69); women infected with tested bacteria (with the exception of those infected with M. genitalium) were significantly younger when compared to the uninfected women [Table 2].
Table 2.
Mean age of patients with and without detected sexually transmitted infections
Discussion
Such low prevalence of M. genitalium on selected sample of low-risk female population tested for C. trachomatis is comparable to other published studies also conducted on cervical swabs tested for C. trachomatis.[6] However, it seems that the true prevalence of M. genitalium in Croatian low-risk female population is substantially lower, since our study has found M. genitalium only in C. trachomatis positive samples. This presumptive, very low prevalence of M. genitalium infection in low-risk population of women in Croatia is concordant with reported prevalence of 0.8% in French women attending routine screening,[7] but also with previous report of unusually low prevalence of M. genitalium detected in Croatian infertile men and their asymptomatic controls (1.4% and 0%, respectively).[5] Present study also revealed that 4.8% of women with C. trachomatis and 11% of women with M. hominis also had M. genitalium infection, which is lower when compared to 9% of C. trachomatis-M. genitalium co-infected women that underwent population-based screening in London[8] and 11% in a screening study conducted in Norway.[9]
The recommended treatment of uncomplicated C. trachomatis and M. genitalium infection is the same, but unlike C. trachomatis that does not show any homotypic resistance,[10] M. genitalium has a high potential for developing resistance.[1] This is the reason why the treatment of cervicitis or nongonoccocal urethritis should be based upon specific diagnostic testing, and a control PCR should be pursued 4–5 weeks after treatment.[11] Moreover, since the therapy for C. trachomatis may not be effective for M. genitalium, the latter pathogen may represent a “Trojan horse” and hamper successful treatment, which is why it is significant to screen for co-infection.
At the moment, the decision to screen or not to screen is usually based on the discussion between health providers and patients (taking into account personal risk factors), especially aiming to test symptomatic women if molecular methods are available.[1] Although recent meta-analysis has shown that testing high-risk symptomatic women on M. genitalium is warranted,[3] our study suggest that in low-risk population it would be reasonable to implement M. genitalium screening only for those with proven risk factor such as C. trachomatis and/or M. hominis infection. Of course, acquiring precise insights into local M. genitalium epidemiology and tracking antimicrobial resistance development may represent a rationale to undertake screening endeavors regardless of the low prevalence of infection.
Conclusions
Other risk factors (i.e., multiple sexual partners, bacterial vaginosis, being symptomatic, smoking, prior miscarriage, black ethnicity, social class, marital status) including younger age are also associated with M. genitalium infection in the literature.[12] Still, although this study demonstrated that women infected with M. genitalium were younger than woman without the infection, this was not statistically significant– hence younger age would not be an appropriate inclusion/exclusion criterion for M. genitalium screening in the low-risk population. In any case, further studies are needed to confirm or reject results of our investigation, especially those trying to elucidate the relationship between younger age and M. genitalium infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016;30:1650–6. doi: 10.1111/jdv.13849. [DOI] [PubMed] [Google Scholar]
- 2.Ljubin-Sternak S, Meštrovic T. Chlamydia trachomatis and genital mycoplasmas: Pathogens with an impact on human reproductive health. J Pathog. 2014;2014:183167. doi: 10.1155/2014/183167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418–26. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues MM, Fernandes PÁ, Haddad JP, Paiva MC, Souza Mdo C, Andrade TC, et al. Frequency of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma species in cervical samples. J Obstet Gynaecol. 2011;31:237–41. doi: 10.3109/01443615.2010.548880. [DOI] [PubMed] [Google Scholar]
- 5.Plecko V, Zele-Starcevic L, Tripkovic V, Skerlev M, Ljubojevic S, Plesko S, et al. Unusually low prevalence of Mycoplasma genitalium in urine samples from infertile men and healthy controls: A prevalence study. BMJ Open. 2014;4:e005372. doi: 10.1136/bmjopen-2014-005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsen E, Vik E, Røed MA. Low prevalence of Mycoplasma genitalium in patients examined for Chlamydia trachomatis. Tidsskr Nor Laegeforen. 2011;131:2232–4. doi: 10.4045/tidsskr.11.0165. [DOI] [PubMed] [Google Scholar]
- 7.Peuchant O, Le Roy C, Desveaux C, Paris A, Asselineau J, Maldonado C, et al. Screening for Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium should it be integrated into routine pregnancy care in French young pregnant women? Diagn Microbiol Infect Dis. 2015;82:14–9. doi: 10.1016/j.diagmicrobio.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Svenstrup HF, Dave SS, Carder C, Grant P, Morris-Jones S, Kidd M, et al. A cross-sectional study of Mycoplasma genitalium infection and correlates in women undergoing population-based screening or clinic-based testing for Chlamydia infection in London. BMJ Open. 2014;4:e003947. doi: 10.1136/bmjopen-2013-003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulsen LK, Dahl ML, Skaare D, Grude N. Prevalence of M. genitalium and U. urealyticum in urine tested for C. trachomatis. Tidsskr Nor Laegeforen. 2016;136:121–5. doi: 10.4045/tidsskr.14.1574. [DOI] [PubMed] [Google Scholar]
- 10.Ljubin-Sternak S, Mestrovic T, Vilibic-Cavlek T, Mlinaric-Galinovic G, Sviben M, Markotic A, et al. In vitro susceptibility of urogenital Chlamydia trachomatis strains in a country with high azithromycin consumption rate. Folia Microbiol (Praha) 2013;58:361–5. doi: 10.1007/s12223-012-0218-2. [DOI] [PubMed] [Google Scholar]
- 11.Plantamura J, Bigaillon C, Bousquet A, Delaune D, Larréché S, Bugier S, et al. Mycoplasma genitalium: A mycoplasma still underestimated. Ann Biol Clin (Paris) 2017;75:209–14. doi: 10.1684/abc.2017.1228. [DOI] [PubMed] [Google Scholar]
- 12.Short VL, Totten PA, Ness RB, Astete SG, Kelsey SF, Murray P, et al. The demographic, sexual health and behavioural correlates of Mycoplasma genitalium infection among women with clinically suspected pelvic inflammatory disease. Sex Transm Infect. 2010;86:29–31. doi: 10.1136/sti.2009.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]


