Abstract
Background:
Prediction of the left ventricular remodeling (LVR) after ST-segment elevation myocardial infarction (STEMI) in patients treated with effective myocardial reperfusion is challenging.
Methods:
Forty-one consecutive patients (36 males, age 59 ± 10 years) with STEMI who underwent effective (TIMI III) primary coronary angioplasty were enrolled. All patients had an echocardiography and cardiac magnetic resonance (CMR) study within 72 h from revascularization. Three echocardiographic parameters including LV ejection fraction (EF), global longitudinal strain (GLS) and severe altered longitudinal strain (SAS) area by two-dimensional speckle-tracking echocardiography (2D-STE) and 3 CMR indices including LV global function index (LV-GFI), myocardial salvage index (MSI), and microvascular obstruction (MVO) were calculated. LVR was defined as an increase in CMR LV end-diastolic volume (EDV) >15% after 6 months.
Results:
Of 41 patients, 10 (24%) had LVR (LV-EDV from 145.1 ± 29.3 to 185.9 ± 49.8 ml, P < 0.001). A significant correlation with LV-EDV variation was found for baseline SAS area (r = 0.81), LV-GFI (r = −0.56), MVO (r = 0.55), EF (r = −0.42), GLS (r = 0.42), not for MSI (r = −0.25). At the multivariable analysis, a significant correlation remained only for the SAS area. The receiver-operating characteristic curve analysis showed that a baseline SAS area ≥15% predicts LVR with a sensitivity of 80.0% and a specificity of 90.3%.
Conclusions:
The SAS area evaluated by 2D-STE early in acute STEMI is a valuable predictor of LVR after 6 months. Further investigations are needed to verify its value in predicting patient survival.
Keywords: Myocardial infarction, speckle-tracking echocardiography, strain
INTRODUCTION
It is known that in patients who experienced acute ST-segment elevation myocardial infarction (STEMI), the acute injury may evolve in the left ventricular remodeling (LVR) despite effective coronary revascularization and optimal medical therapy. Predicting LVR in these patients can be challenging.[1] Different parameters obtained by cardiac imaging techniques have been proposed over the years,[1] including LV end-diastolic and end-systolic volumes (EDV, ESV) and ejection fraction (EF) by conventional and three-dimensional echocardiography,[2,3] global longitudinal strain (GLS) by two-dimensional speckle-tracking echocardiography (2D-STE),[4] microvascular obstruction (MVO),[5] and LV global function index (LV-GFI) by cardiac magnetic resonance (CMR) imaging.[6]
Recently, the size of the myocardial area with severely altered longitudinal strain (SAS) evaluated by 2D-STE has been shown to correlate with the transmural myocardial infarct size.[7] For this reason, this area has the potential to predict LVR if applied early in the acute phase of STEMI. Therefore, the purpose of this study was to evaluate the value of the LV SAS area in predicting LVR after STEMI and also to compare it with other echocardiographic and CMR predictors of LVR.
METHODS
Study design
We have retrospectively reviewed our Cardiology Unit database looking for all patients accessed consecutively for acute STEMI for 2 years, with CMR performed early and after 6 months. The diagnosis of STEMI was made on the basis of clinical presentation, electrocardiographic findings, and troponin elevation. Study inclusion criteria were (1) no history of cardiovascular disease and no previous coronary revascularization; (2) myocardial revascularization within 90 min from the onset of symptoms with effective coronary flow after revascularization (TIMI III); (3) echocardiography study with dedicated protocol for speckle-tracking analysis between 4 and 7 days after the acute event; (4) CMR within 7 days and after 6 months from the acute event; (5) sinus rhythm at the moment of both CMR and echocardiography. Exclusion criteria were (1) new angiographic procedures within 6 months after the first event; (2) hemodynamic instability or periprocedural MI. Forty-one patients met the inclusion criteria. Patient characteristics are reported in Table 1. The main indications for early CMR were detailed functional assessment of both ventricles, characterization of myocardial tissue, and suspect LV thrombus; the main indication for the 6-month CMR was the study of LVR. The study was approved by the local Ethics Committee.
Table 1.
Patient characteristics
| Overall patients | Patients with LVR | Patients without LVR | P | |
|---|---|---|---|---|
| n (%) | 41 (100) | 10 (24) | 31 (76) | <0.001 |
| Age (years) | 59±10 | 55.4±9.1 | 61.2±11.2 | 0.094 |
| Sex (male/female) | 36/5 | 9/1 | 27/4 | 0.81 |
| Body surface area (m2) | 1.93±0.20 | 1.84±0.16 | 1.96±0.20 | 0.061 |
| Diabetes, n (%) | 7 (17) | 1 (1) | 6 (19) | 0.49 |
| Systemic hypertension, n (%) | 15 (37) | 2 (20) | 13 (42) | 0.21 |
| Hypercholesterolemia, n (%) | 10 (24) | 0 | 10 (32) | 0.039 |
| Smoke, n (%) | 26 (63) | 6 (60) | 20 (65) | 0.80 |
| CV family history, n (%) | 14 (34) | 3 (30) | 11 (35) | 0.75 |
| Culprit coronary artery, n (%) | ||||
| LAD | 25 (61) | 6 (60) | 19 (61) | 0.94 |
| RCA | 11 (27) | 2 (20) | 9 (29) | 0.58 |
| LCx | 5 (12) | 2 (20) | 3 (10) | 0.39 |
| Number of vessel disease, n (%) | ||||
| 1-vessel disease | 34 (83) | 8 (80) | 26 (84) | 0.78 |
| 2-vessel disease | 6 (15) | 2 (20) | 4 (13) | 0.58 |
| 3-vessel disease | 1 (2) | 0 | 1 (3) | 0.57 |
| ACE-Is/ARBs, n (%) | 36 (88) | 8 (80) | 28 (90) | 0.58 |
| Beta-blockers, n (%) | 38 (93) | 9 (90) | 29 (94) | 1.000 |
| Calcium channel blockers, n (%) | 2 (5) | 0 | 2 (6) | 1.000 |
| Diuretics n (%) | 3 (7) | 1 (10) | 2 (6) | 1.000 |
ACE-Is=Angiotensin converting enzyme inhibitors, ARBs=Angiotensin receptor blockers, CV=Cardiovascular, LAD=Left anterior descending, LCx=Left circumflex, LVR=Left ventricular remodeling, RCA=Right coronary artery
Cardiac magnetic resonance imaging
All images were acquired using a 1.5-T CMR imaging system (Signa HDX, GE Medical Systems, Milwaukee, Wisconsin). An 8-channel phased-array surface coil was used. Localization was performed using breath-hold single-phase steady-state-free precession (SSFP) images of true anatomical axes of the heart. Subsequently, cine SSFP and delayed enhancement (DE) sequences were obtained. Cine SSFP images were acquired in short- and long-axis views. For the short-axis view sequences, a slice thickness of 10 mm with no gap in identical slice positions, covering the LV from the base to apex, was used. The DE images (fast-gradient-echo inversion-recovery) were acquired 7–10 min after bolus intravenous injection of 0.15 mmol/kg body weight of gadolinium-diethylenetriamine penta-acetic acid (gadobutrol, Schering, Germany), followed by saline flush. The inversion time was adapted individually to null normal myocardium. LV EDV, ESV, stroke volume (SV), mass, and EF were calculated according to standard methods. The LV-SV was calculated as LV-EDV − LV-ESV. The LV global volume was defined as the sum of the LV mean cavity volume ([LV-EDV + LV-ESV]/2) and the myocardium volume. LV myocardial volume was calculated as LV myocardial mass divided by the specific myocardial density (1.05 g/mL). The LV-GFI was defined according to the following established formula and expressed as a percentage: LV-GFI = (LV-SV/LV global volume) × 100.[8] For myocardial DE and edema analysis, CMR images were analyzed using the software Segment CMR (Medviso, Lund, Sweden).[9] Myocardial edema imaging was performed with the use of breath-hold black-blood T2-weighted, short-tau inversion recovery (T2w-STIR) time, fast spin-echo imaging in cardiac short-axis orientation. Salvaged myocardium was quantified as the difference between the area of increased T2w-STIR signal (area at risk) and the area of late gadolinium enhancement. Myocardial salvage index (MSI, in %) was calculated by normalizing salvaged myocardium for the area at risk as previously described.[10,11] The MVO was assessed approximately 15 min after gadolinium injection, again using DE images. It was defined as a dark zone within the infarcted segments, usually located in the subendocardium, and expressed in % of the LV myocardial volume.[11]
Conventional and speckle-tracking echocardiography
In all patients, a comprehensive transthoracic echocardiography examination was performed using commercially available ultrasound systems (Vivid 7 and E9, GE Medical Systems, Milwaukee, WI, USA) equipped with a 3.5 MHz phased-array transducer. Image and Doppler acquisitions were obtained at held end-expiration. LV-EF was calculated using the Simpson's biplane method.[12] For the 2D-STE image acquisition, sector size and depth were adjusted to achieve optimal visualization of all LV myocardium in the 3 standard apical views (4-, 2-, and long-axis view) with a frame rate between 60 and 100 fps. The LV longitudinal myocardial strain was assessed using a commercially available software (EchoPAC PC 112 rev. 1.3, GE Medical Systems, Milwaukee, WI, USA). End-systole was defined by the aortic valve closure in the apical long-axis view. The regions of interest were manually outlined at end-systole by marking the endocardial borders in the apical views. A manual adjustment was performed if the automated tracking was suboptimal. Peak systolic longitudinal myocardial strain was automatically calculated throughout the myocardium for each LV apical view and reported spatially - from base to apex and circumferentially - in a polar plot map using a color-coded parametric representation. The area of SAS was contoured and expressed as a percentage of the total myocardial area as described in a previous investigation.[7] The GLS was determined by averaging all peak systolic segmental strain values from the 3 standard apical views. Longitudinal peak strain values were averaged over 2 consecutive cardiac cycles.
Study end-point
LVR was defined as a 15% increase in LV-EDV evaluated by CMR after 6 months from the baseline value.[13]
Statistical analysis
Continuous variables were expressed as average values ± standard deviation, whereas categorical variables were expressed as percentages. Baseline and 6-month values were compared using the Student's t-test. Values of the subgroups of patients with and without LVR were compared using the Student's t-test or the Chi-square test when indicated. The following variables were included in the predictive analysis: Echocardiographic LV-EF, GLS, SAS area, and CMR LV-GFI, MSI, and MVO. The association between all variables and LVR was assessed first using the Pearson's r correlation coefficient (univariable analysis). Subsequently, a stepwise multivariable regression analysis was applied. The receiver-operating characteristic (ROC) curve analysis was performed to select optimal cutoff value for the variable which remained predictive at the multivariable analysis, calculating the area under the ROC curve (AUC) with the 95% confidence intervals (CIs). The statistical analysis was performed using MedCalc Statistical Software version 11.2.1.0. P < 0.05 was considered statistically significant.
RESULTS
Overall patients
Forty-one consecutive patients were studied at baseline and after 6 months. CMR parameters describing LV size and systolic function are reported in Table 2. Baseline values of parameters tested for the prediction of LVR are reported in Table 3. MVO was seen in 22 patients (54%).
Table 2.
Cardiac magnetic resonance parameters evaluated at baseline and after 6 months
| Overall patients | P* | Patients with LVR | P* | Patients without LVR | P* | P** | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=41) | 6 months (n=41) | Baseline (n=10) | 6 months (n=10) | Baseline (n=31) | 6 months (n=31) | Baseline | 6 months | ||||
| EDV (mL) | 152.5±34.5 | 160.1±44.4 | 0.063 | 145.1±29.3 | 185.9±49.8 | <0.001 | 154.9±36.2 | 151.8±39.9 | 0.216 | 0.442 | 0.033 |
| ESV (mL) | 83.5±28.0 | 84.0±38.6 | 0.876 | 90.6±26.4 | 114.7±44.3 | 0.011 | 81.2±28.5 | 74.0±31.3 | 0.001 | 0.363 | 0.003 |
| SV (mL) | 70.1±15.6 | 76.2±14.0 | <0.001 | 58.9±15.6 | 71.2±10.7 | 0.013 | 73.8±13.9 | 77.8±14.7 | 0.012 | 0.007 | 0.197 |
| EF (%) | 46.1±8.8 | 49.4±9.7 | <0.001 | 38.4±6.9 | 39.9±7.5 | 0.425 | 48.6±8.0 | 52.5±8.2 | <0.001 | <0.001 | <0.001 |
*Baseline versus 6-month evaluation, **Patients with versus without LVR at baseline and 6 months. EDV=End-diastolic volume, ESV=End-systolic volume, SV=Stroke volume, EF=Ejection fraction, LVR=Left ventricular remodeling
Table 3.
Cardiac magnetic resonance and echocardiographic parameters tested for left ventricular remodeling prediction
| Overall patients (n=41) | Patients with LVR (n=10) | Patients without LVR (n=31) | P | |
|---|---|---|---|---|
| CMR | ||||
| GFI (%) | 28.7±6.2 | 23.3±3.9 | 30.5±5.9 | 0.003 |
| MSI (%) | 42±29 | 35.8±17.3 | 44.6±32.4 | 0.419 |
| MVO (%)§ | 1.99±2.20 | 3.0±2.1 | 1.29±2.1 | 0.069 |
| Echocardiography (%) | ||||
| EF | 47.3±9.3 | 39.7±7.6 | 49.8±8.5 | 0.002 |
| GLS | −13.9±3.4 | −11.2±2.5 | −14.8±3.2 | 0.003 |
| SAS area | 10.4±9.7 | 22.3±8.9 | 6.6±6.4 | <0.001 |
§In 22 patients at baseline, 9 with LVR and 13 without LVR. GFI=Global function index, MSI=Myocardial salvage index, MVO=Microvascular obstruction, EF=Ejection fraction, GLS=Global longitudinal strain, SAS=Severe altered longitudinal strain, LVR=Left ventricular remodeling, CMR=Cardiac magnetic resonance
Left ventricular remodeling
According to the 6-month CMR LV-EDV variation, 10 patients (24%) showed LVR and 31 did not (76%) [Table 1]. No differences between patients with and without LVR were observed in terms of age, gender ratio, body surface area, cardiovascular risk factors, culprit coronary artery, and number of vessel disease [Table 1].
At baseline, LVR patients had a lower LV-EF, LV-GFI, GLS, and a higher MVO and SAS area [Tables 2 and 3]. No significant differences were observed in terms of LV volumes and MSI [Tables 2 and 3] although there was a trend for lower LV-EDV and higher LV-ESV mean values for LVR patients [Table 2].
Prediction of left ventricular remodeling
At the univariable analysis, a significant correlation was found between the 6-month LV-EDV variation and baseline SAS area [r = 0.81, P < 0.001; Figure 1], LV-GFI (r = −0.56, P < 0.001), MVO (r = 0.55, P < 0.001), LV-EF (r = −0.42, P = 0.006), and GLS (r = 0.42, P = 0.007), whereas MSI did not show a significant correlation (r = −0.25, P = 0.113). At the multivariable analysis, a significant correlation remained only for the SAS area (r = 0.80, P < 0.001). The ROC curve analysis showed that a baseline SAS area ≥15% predicted LVR at 6 months with a sensitivity and a specificity of 80.0% and 90.3%, respectively [AUC 0.934, 95% CIs 0.810–0.988, P < 0.001; Figure 2].
Figure 1.
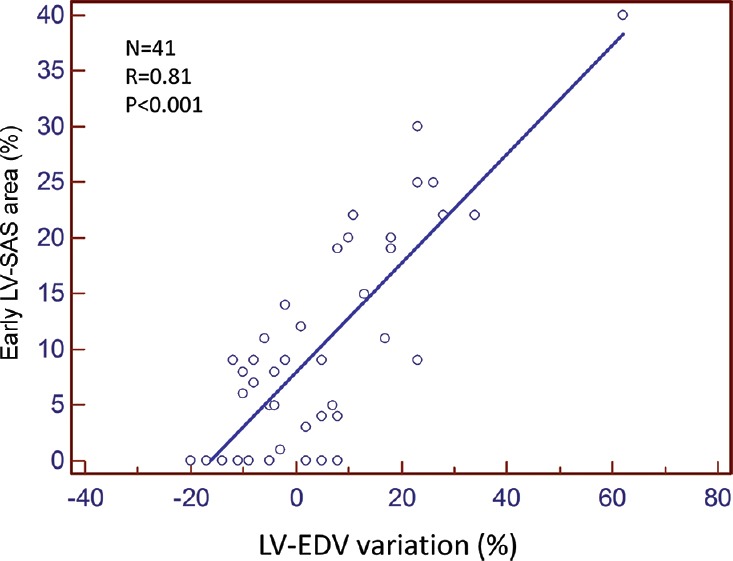
LV-SAS=Left ventricular severely altered longitudinal systolic area, LV-EDV=Left ventricular end-diastolic volume
Figure 2.
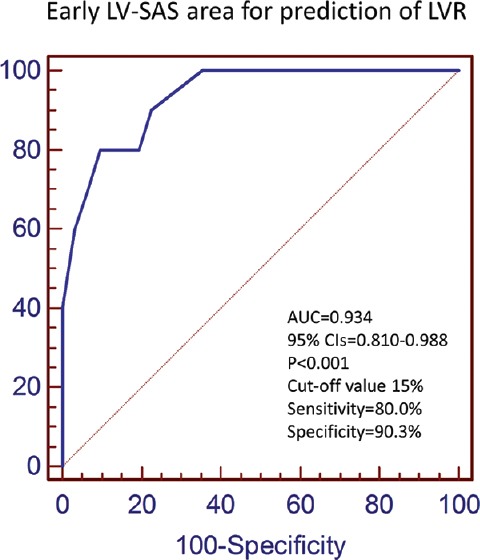
LV-SAS=Left ventricular severely altered longitudinal systolic area, LVR=Left ventricular remodeling, AUC=Area under the curve, CIs=Confidence intervals
DISCUSSION
Our data show that early evaluation of the 2D-STE SAS area after STEMI can be used to predict LVR. Assessment of other echocardiographic and CMR indices seems not to be predictive as the use of the SAS area.
Importance of predicting left ventricular remodeling
LVR is a major predictor of morbidity and mortality in overt congestive heart failure.[14] Morphologically, LVR following acute MI is characterized by chamber dilation, thinning of the infarcted segments, and compensatory hypertrophy in the noninfarcted myocardium. It is considered as an adaptive progressive process contributing to maintenance of the LV systolic function. However, this occurs only in some STEMI patients while others remain relatively stable without a significant LV cavity dilation.[15] Since LVR has been associated with the development of heart failure and a poor survival rate, its early identification is of clinical importance[16] to set up appropriate preventive strategies, especially for the high-risk patients.[1,17]
Echocardiographic predictors
Early echocardiographic evaluation of LV-EF is generally performed after an acute MI to assess the degree of myocardial damage and as a marker of early and late complications.[16] However, early assessment of LV-EF in this setting can be misleading[18] because it is affected by the presence of myocardial stunning, thus it may not distinguish viable from nonviable myocardium.[13,19]
Various studies suggested an additive value of deformation imaging parameters, and particularly of GLS, in predicting LVR. D’Andrea et al.[13] found that GLS evaluated in patients with non-ST elevated MI is a reliable predictor of LVR (defined as a ≥15% increase in LV-EDV 6 months after the acute MI) with a sensitivity of 84.8% and specificity of 87.8%. Zaliaduonyte-Peksiene et al.[20] confirmed the role of GLS in predicting LVR following acute MI (defined as a ≥15% increase in LV-EDV at the 4-month follow-up evaluation). Antoni et al.[21] reported that GLS has a strong prognostic value in terms of all-cause mortality, reinfarction, hospitalization due to heart failure, or revascularization in patients after acute MI.
Our group[7] has recently shown that the SAS area obtained acutely from 2D-STE is related to the CMR transmural scar size 6 months after the acute MI. It, therefore, has the potential to predict development of LVR. Indeed, in the present investigation, we found that the SAS area was a very good predictor of LVR and when compared with other indices, it remained the only significant predictor at the multivariable analysis. In particular, among the echocardiographic parameters, both the LV-EF and GLS did not retain their similar predictive value observed at the univariable analysis. While the weakness of the LV-EF as an LVR predictor has been explained above, the failure of GLS to maintain its predictive value at the multivariable analysis needs to be clarified. In our opinion, this mainly relies on the functional information carried by GLS, which takes into account the contribution of all myocardial segments to LV contraction, that is, it includes both normally and abnormally contracting regions. Conversely, the size of the SAS area is primarily an expression of the severely dysfunctioning nonviable myocardium.[7]
Cardiac magnetic resonance predictors
In our study, we have tested a number of CMR predictors of LVR. LV-GFI is a novel index that integrates information about LV myocardial structure and global systolic function.[8] Eitel et al.[6] recently reported that the LV-GFI strongly correlates with markers of severe myocardial and microvascular damage in patients with STEMI, offering prognostic information beyond traditional cardiac risk factors including the LV-EF. Other CMR indices, such as MVO and MSI, have also been reported to have a prognostic value in acute reperfused STEMI. For example, de Waha et al.[22] showed that MSI is a stronger predictor for adverse clinical outcome in comparison to other CMR parameters, such as MVO and infarct size. In addition, Bodi et al.[23] reported that that an MVO >2.5% can predict LVR after acute MI.
In our patients, we found significant correlations between baseline LV-GFI and MVO with LVR at the univariable analysis, whereas MSI did not correlate significantly. However, at the multivariable analysis, none of these CMR parameters was associated with LVR. We did not find a single explanation for the different predictive value of the CMR indices observed in our study and in the literature. However, there are several factors that may have played a role. First, although the LV-GFI carries additional information to LV-EF by accounting for LV mass and hypertrophy, in acute STEMI patients, it cannot be determined if such LV “hypertrophy” was related to previous long-standing arterial hypertension (true LV hypertrophy) or by acute changes induced by myocardial edema as a consequence of the ischemic event itself.[24] In this latter case, the prognostic impact of the LV-GFI could be different. Second, it is well known that, in the acute phase of MI, late-enhancement images may overestimate acute MI size by including the edematous border surrounding the infarct (peri-infarct zone): this could significantly affect the estimate of the MSI.[22] Third, as far as the MVO is concerned, in our patient population, the MVO evaluation was possible only in 54% of patients. In addition, questions have been raised regarding physiological and mechanistic assumptions underlying the T2-weighted CMR approaches for assessing the myocardium at risk.[25,26] Therefore, CMR data regarding the area at risk and myocardial salvage should be considered cautiously, as also pointed out by other authors.[6]
Study limitations
In this retrospective investigation, a limited number of STEMI patients was evaluated due to the need for a CMR performed both early and after 6 months from STEMI. However, despite this limitation, we could clearly demonstrate that the early evaluation of the SAS area size is a predictor of LVR. We recognize that there are many ways to define LVR and that results may change according to the LVR definition. However, the LVR definition applied in our investigation (that is, the LV-EDV variation) has been used in previous studies[3,13,20] and has the advantage to be clinically applicable. We recognize that the follow-up duration of our study is limited and that a longer follow-up would be necessary to confirm our results. This might be planned in a future prospective investigation. Global circumferential strain was not investigated in this study. Other authors, however, showed that this parameter may better explore the transmurality of the LV damage after STEMI and is a predictor of LVR.[27,28] Compared to strain, strain rate seems to be less load dependent, maybe better measure of contractility, and theoretically, more sensitive than strain to myocardial pathology.[29,30] Some authors showed that both longitudinal and circumferential strain rate were independent predictors of outcome after MI, whereas only circumferential strain rate was predictive of LV remodeling.[31] A certain spatial distortion occurs in representing the LV apical segments in the polar plot format, so some underestimation of the size of the SAS area may be expected at the apex.
CONCLUSIONS
Early evaluation of the SAS area by 2D-STE significantly predicts LVR 6 months after a STEMI. Further investigations are needed to verify the impact of this evaluation in predicting patients’ survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Andrea Fiorencis, MD, for assisting in the preparation of the study database.
References
- 1.Galli A, Lombardi F. Postinfarct left ventricular remodelling: A prevailing cause of heart failure. Cardiol Res Pract 2016. 2016:2579832. doi: 10.1155/2016/2579832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Springeling T, Kirschbaum SW, Rossi A, Baks T, Karamermer Y, Schulz C, et al. Late cardiac remodeling after primary percutaneous coronary intervention-five-year cardiac magnetic resonance imaging follow-up. Circ J. 2013;77:81–8. doi: 10.1253/circj.cj-12-0043. [DOI] [PubMed] [Google Scholar]
- 3.Mele D, Teoli R, Cittanti C, Pasanisi G, Guardigli G, Levine RA, et al. Assessment of left ventricular volume and function by integration of simplified 3D echocardiography, tissue harmonic imaging and automated extraction of endocardial borders. Int J Cardiovasc Imaging. 2004;20:191–202. doi: 10.1023/b:caim.0000021948.96454.3a. [DOI] [PubMed] [Google Scholar]
- 4.Shetye A, Nazir SA, Squire IB, McCann GP. Global myocardial strain assessment by different imaging modalities to predict outcomes after ST-elevation myocardial infarction: A systematic review. World J Cardiol. 2015;7:948–60. doi: 10.4330/wjc.v7.i12.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir RA, Murphy CA, Petrie CJ, Martin TN, Balmain S, Clements S, et al. Microvascular obstruction remains a portent of adverse remodeling in optimally treated patients with left ventricular systolic dysfunction after acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:360–7. doi: 10.1161/CIRCIMAGING.109.897439. [DOI] [PubMed] [Google Scholar]
- 6.Eitel I, Pöss J, Jobs A, Eitel C, de Waha S, Barkhausen J, et al. Left ventricular global function index assessed by cardiovascular magnetic resonance for the prediction of cardiovascular events in ST-elevation myocardial infarction. J Cardiovasc Magn Reson. 2015;17:62. doi: 10.1186/s12968-015-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mele D, Fiorencis A, Chiodi E, Gardini C, Benea G, Ferrari R. Polar plot maps by parametric strain echocardiography allow accurate evaluation of non-viable transmural scar tissue in ischaemic heart disease. Eur Heart J Cardiovasc Imaging. 2016;17:668–77. doi: 10.1093/ehjci/jev191. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Opdahl A, Choi EY, Almeida AL, Kawel N, Wu CO, et al. Left ventricular global function index by magnetic resonance imaging – a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: The multi-ethnic study of atherosclerosis. Hypertension. 2013;61:770–8. doi: 10.1161/HYPERTENSIONAHA.111.198028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of segment – Freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–7. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, et al. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart. 2011;97:2038–45. doi: 10.1136/heartjnl-2011-300098. [DOI] [PubMed] [Google Scholar]
- 12.Mele D, Campana M, Sclavo M, Seveso G, Aschieri D, Nesta F, et al. Impact of tissue harmonic imaging in patients with distorted left ventricles: Improvement in accuracy and reproducibility of visual, manual and automated echocardiographic assessment of left ventricular ejection fraction. Eur J Echocardiogr. 2003;4:59–67. doi: 10.1053/euje.2002.0619. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea A, Cocchia R, Caso P, Riegler L, Scarafile R, Salerno G, et al. Global longitudinal speckle-tracking strain is predictive of left ventricular remodeling after coronary angioplasty in patients with recent non-ST elevation myocardial infarction. Int J Cardiol. 2011;153:185–91. doi: 10.1016/j.ijcard.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 15.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 16.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, et al. Exercise intolerance in chronic heart failure: Mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil. 2010;17:643–8. doi: 10.1097/HJR.0b013e32833f3aa5. [DOI] [PubMed] [Google Scholar]
- 18.Mele D. Left ventricular ejection fraction: Pathophysiological aspects and intrinsic limitations. G Ital Cardiol (Rome) 2012;13:793–808. doi: 10.1714/1188.13163. [DOI] [PubMed] [Google Scholar]
- 19.Munk K, Andersen NH, Nielsen SS, Bibby BM, Bøtker HE, Nielsen TT, et al. Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr. 2011;12:156–65. doi: 10.1093/ejechocard/jeq168. [DOI] [PubMed] [Google Scholar]
- 20.Zaliaduonyte-Peksiene D, Vaskelyte JJ, Mizariene V, Jurkevicius R, Zaliunas R. Does longitudinal strain predict left ventricular remodeling after myocardial infarction? Echocardiography. 2012;29:419–27. doi: 10.1111/j.1540-8175.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- 21.Antoni ML, Mollema SA, Delgado V, Atary JZ, Borleffs CJ, Boersma E, et al. Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J. 2010;31:1640–7. doi: 10.1093/eurheartj/ehq105. [DOI] [PubMed] [Google Scholar]
- 22.de Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Stiermaier T, et al. Prognosis after ST-elevation myocardial infarction: A study on cardiac magnetic resonance imaging versus clinical routine. Trials. 2014;15:249. doi: 10.1186/1745-6215-15-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodi V, Monmeneu JV, Ortiz-Perez JT, Lopez-Lereu MP, Bonanad C, Husser O, et al. Prediction of reverse remodeling at cardiac MR imaging soon after first ST-segment-elevation myocardial infarction: Results of a large prospective registry. Radiology. 2016;278:54–63. doi: 10.1148/radiol.2015142674. [DOI] [PubMed] [Google Scholar]
- 24.Schulz-Menger J, Gross M, Messroghli D, Uhlich F, Dietz R, Friedrich MG. Cardiovascular magnetic resonance of acute myocardial infarction at a very early stage. J Am Coll Cardiol. 2003;42:513–8. doi: 10.1016/s0735-1097(03)00717-4. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Jiménez R, Sánchez-González J, Agüero J, García-Prieto J, López-Martín GJ, García-Ruiz JM, et al. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: Imaging and histological tissue characterization. J Am Coll Cardiol. 2015;65:315–23. doi: 10.1016/j.jacc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Wince WB, Kim RJ. Molecular imaging: T2-weighted CMR of the area at risk – A risky business? Nat Rev Cardiol. 2010;7:547–9. doi: 10.1038/nrcardio.2010.124. [DOI] [PubMed] [Google Scholar]
- 27.Zito C, Sengupta PP, Di Bella G, Oreto G, Cusmà -Piccione M, Longordo C, et al. Myocardial deformation and rotational mechanics in revascularized single vessel disease patients 2 years after ST-elevation myocardial infarction. J Cardiovasc Med (Hagerstown) 2011;12:635–42. doi: 10.2459/JCM.0b013e3283468130. [DOI] [PubMed] [Google Scholar]
- 28.Sugano A, Seo Y, Ishizu T, Watabe H, Yamamoto M, Machino-Ohtsuka T, et al. Value of 3-dimensional speckle tracking echocardiography in the prediction of microvascular obstruction and left ventricular remodeling in patients with ST-elevation myocardial infarction. Circ J. 2017;81:353–60. doi: 10.1253/circj.CJ-16-0944. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, et al. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 30.Mele D, Pasanisi G, Heimdal A, Cittanti C, Guardigli G, Levine RA, et al. Improved recognition of dysfunctioning myocardial segments by longitudinal strain rate versus velocity in patients with myocardial infarction. J Am Soc Echocardiogr. 2004;17:313–21. doi: 10.1016/j.echo.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812–22. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]


