Abstract
OBJECTIVE:
The objective of this study was to evaluate the outcome of management in eyes with intraocular retinoblastoma (RB) that had received inadequate initial therapy (chemotherapy without focal therapy) before eventually receiving necessary consolidation therapy at a tertiary referral center.
METHODS:
A retrospective observational case series of 30 eyes from 26 RB patients who had initially received systemic chemotherapy as a sole therapy. The main outcome measures were demographics, laterality, International Classification of RB (ICRB), treatments, tumor control, and survival.
RESULTS:
The median age at diagnosis was 24 months and the median delay between time at diagnosis and time at referral to a tertiary center that has adequate focal therapy for RB was 9.5 months (range 5–20 months). Sixteen (62%) patients were monocular from enucleation of the contralateral eye. Features of ICRB Group A tumors were seen in 3 (10%) eyes, Group B in 7 (23%) eyes, Group C in 2 (7%) eyes, Group D in 16 (53%) eyes, and Group E in 2 (7%) eyes. Eighteen (69%) patients required more systemic chemotherapy (median, 4.4 cycles; range, 2–8 cycles), and 8 (26%) eyes received local chemotherapy (subtenon, intravitreal, or intra-arterial). All treated eyes received consolidation therapy as transpupillary thermotherapy and/or cryotherapy. Radioactive plaque therapy was used in 1 (3%) eye and external beam radiation therapy in 3 (10%) eyes. At a mean follow-up of 13 months (median, 11.5 months; range, 9–27 months), enucleation was avoided in 25 (83%) eyes. Two (7%) eyes were enucleated initially, and 3 (10%) were enucleated after failure of additional therapy. Twenty-three (77%) eyes did not show any viable tumor after a median of 11.5 months of follow-up after the last treatment, and 2 (7%) eyes still have residual tumor recurrences that need more consolidation focal therapy.
CONCLUSION:
Chemotherapy alone cannot eradicate RB cells in effected eyes without combination with consolidation therapy by a multidisciplinary team to salvage the affected eye as well as its vision. Nonetheless, chemotherapy can be initiated (to keep the tumor at a less invasive stage) for patients from centers or countries where combination therapy is not available until they gain access to adequate management of RB.
Keywords: Chemotherapy, focal therapy, retinoblastoma, vitreous seeds
Introduction
Retinoblastoma (RB) is the most common primary intraocular malignancy in childhood and infancy. The incidence is about 1 in 15,000–20,000 live births.[1,2]
Systemic chemotherapy alone failed to cure patients with intra ocular Retinoblastoma,[3] while combination therapy (chemotherapy combined with focal therapy) has become the treatment of choice for intraocular RB since the 1990s[3,4] and achieved high globe salvage rates (100% for International Classification of RB [ICRB] Group A, 93% for Group B, 90% for Group C, and 47% for Group D eyes).[5]
Overexpression of the multidrug-resistant P-glycoprotein has been observed in 30% of untreated RB, and this may account for the poor response of some RB cases to systemic chemotherapy.[6,7,8] Therefore, we expect tumors that had received chemotherapy alone then progressed, to be resistant to chemotherapy. Therefore, systemic treatment of RB should be combined with focal therapy to eradicate the resistant cells from the beginning to avoid recurrence of the more aggressive resistant cells.[4] This needs a multidisciplinary approach, which is not available in every center in the world, and that leads patients to seek care at other centers or even travel abroad to receive the required treatment. The resulting delay causes more growth of the tumor and possibly a less favorable outcome. On the other hand, systemic chemotherapy is widely available in most medical centers in the world; therefore, in this study, we are evaluating the outcome of initiating chemotherapy alone (when consolidation therapy is not available) before referral to a tertiary center where adequate therapy (including focal therapy) can be applied.
Methods
This study was approved by the Institutional Review Board. It was a retrospective case series of 30 eyes from 26 consecutive patients who were clinically diagnosed with intraocular RB and had initially received inadequate therapy, i.e., chemotherapy alone, before referral to our center. Selection required access to patients' medical records and Ret-Cam images.
Data included patient's age and gender, tumor laterality, age at diagnosis, initial ICRB group,[5] type of seeds (subretinal or vitreal), type of systemic and/or local chemotherapy, type of focal therapy (laser or cryotherapy), radiation therapy, complications, eye salvage, metastasis, second malignancy, and mortality.
Inclusion and exclusion criteria
The eligibility criteria for inclusion were being a patient with intraocular RB (clinically diagnosed by the presence of one or more retinal tumors detected on funduscopic examination using indirect ophthalmoscopy and scleral depression) and having had been initially treated with systemic chemotherapy as a single modality of treatment (without focal or radiation therapy). Exclusion criteria included extraocular disease, having had received focal therapy, local chemotherapy, or radiation therapy. Eyes followed for <6 months were also excluded from the study.
Clinical characteristics and definitions
Tumor staging by the ICRB[5] staging system should be at diagnosis, and since no clinical data was available regarding tumor features or stage at time of diagnosis, tumors in this series were staged according to tumor features at time of initiating therapy in our referral cancer center. The new evaluation and treatment plan were according to tumor and patient status at the time of referral and not at diagnosis.
Tumors with IRCB Group B features were divided into two groups: (1) tumors <3 mm (but close to the fovea or the optic disc) and (2) tumors >3 mm with no seeds. Tumors with IRCB Group C and D features were divided into two groups: (1) tumors with subretinal seeds and (2) tumors with vitreous seeds. In this study, local chemotherapy refers to periocular chemotherapy injection, intra-arterial chemotherapy, and intravitreal chemotherapy.
Tumor control was defined as the absence of tumor activity or recurrence at the last follow-up visit (with no metastasis). All eyes with ICRB Group E features were enucleated within 1 week of diagnosis, and the decision for enucleation was approved by two ocular oncologists and after consultation with an external reviewer.
Treatment methods
We performed a combination regimen of chemotherapy that consisted of carboplatin, vincristine, and etoposide (CVE). Each CVE cycle was repeated every 4 weeks for a total of 6–8 cycles according to patient's condition and tumor status. Ocular oncology follow-up was provided with examination under anesthesia before every cycle of chemotherapy and every 4–8 weeks thereafter. Fundus photos were taken using a RetCam II (Clarity Medical System, Pleasanton, CA, USA), and focal therapy was provided using thermotherapy or cryotherapy until tumor control was achieved.
When external beam radiation therapy (EBRT) was used, the dose prescribed for the retinal target volume was 45 Gy in 25 fractions of 1.8 Gy. Statistical analysis of tumor control was correlated to the tumor's features on the first presentation to our center.
Results
Between January 2011 and September 2014, 30 eyes from 26 patients with RB who had received systemic chemotherapy as the only treatment modality before referral for the management in our tertiary cancer center (King Hussein Cancer Center, Amman, Jordan).
Demographics
The mean age at the time of referral was 25 months (median, 24 months; range, 5–72 months) while the median age at diagnosis was 11 months (median, 6.5 months; range, 2–65 months). The mean delay in referral to the tertiary cancer center was 10 months (median, 9.5 months; range, 5–20 months). The patients were 13 males (50%) and 13 females (50%) and the cases included 4 (15%) unilateral and 22 (85%) bilateral RB. The affected eye was the right in 13 (43%) cases and the left in 17 (57%) cases.
Sixteen patients (62%) were monocular from enucleation of the contralateral eye, 5 (19%) patients harbored RB in both eyes, and 4 (15%) patients had unilateral disease with normal contralateral eye.
All the patients had received systemic chemotherapy only (no laser or cryotherapy) before referral to our center. The mean number of chemotherapy cycles was 6.4 cycles (median, 6 cycles; range, 2–12 cycles). All the patients (100%) received CVE with or without cyclosporine, 21 (81%) patients received VP-16, and 4 (15%) patients received doxorubicin. Not a single patient received focal therapy (cryotherapy or laser therapy), periocular chemotherapy, or radiation therapy before referral.
Tumors' status at presentation
Since these cases had received previous therapy, no initial staging was available; therefore, we described them clinically according to the clinical features of the ICRB at time of presentation to our tertiary center. Three (10%) eyes in this series had features of ICRB Group A tumors, 7 (23%) eyes had features of ICRB Group B tumors, 2 (7%) eyes had features of ICRB Group C tumors, 16 (53%) eyes had features of ICRB Group D tumors (7 [23%] eyes had extensive subretinal seeds and 9 [30%] eyes had extensive vitreous seeds), and 2 (7%) eyes had features of ICRB Group E tumors (one had anterior chamber invasion and the other had massive hemorrhage filling all the eye associated with ciliary body invasion).
Treatment modalities
The three eyes with ICRB Group A features were treated by focal therapy (laser or cryotherapy) with no need for more systemic therapy. Eighteen (69%) patients received systemic CVE with a mean of 4 cycles (median, 4.4 cycles; range, 2–8 cycles), 4 (13%) eyes received 3 subtenon carboplatin injections per eye, 2 (7%) eyes received intravitreal melphalan injections (3 injections per eye), and 2 (7%) eyes received intra-arterial melphalan injections (3 injections per eye). Consolidation therapy was applied as postchemoreduction transpupillary thermotherapy in 26 (87%) eyes (mean, 5 sessions, median, 5 sessions; range, 2–10 sessions) and as cryotherapy in 19 (63%) eyes (mean, 1.6 sessions, median, 2 sessions; range, 1–4 sessions). Radioactive plaque therapy was used in 1 (3%) eye and EBRT in 3 (10%) eyes (standard dose of 45 Gy in 25 fractions of 1.8 GY) [Table 1].
Table 1.
Management and outcome of 16 eyes with retinoblastoma presented with international classification of retinoblastoma Group D features after extensive treatment by systemic chemotherapy without focal consolidation therapy
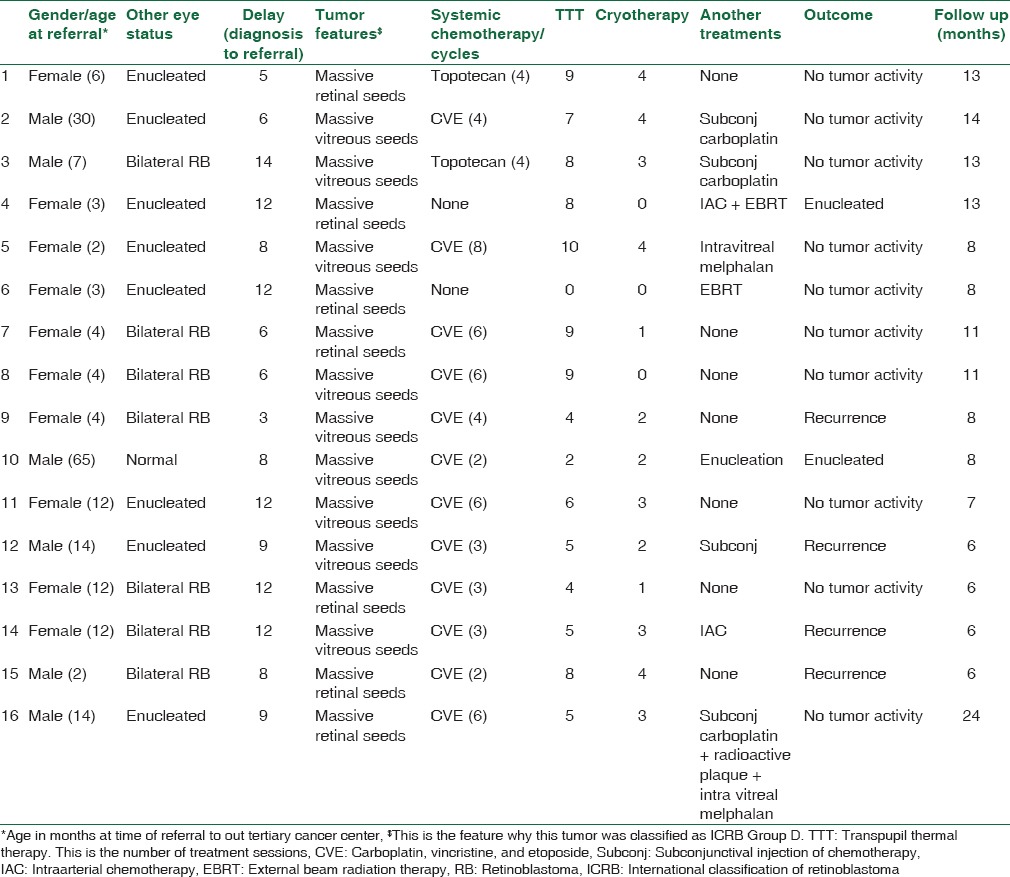
Management outcome
At a mean follow-up of 13 months (median, 11.5 months; range, 9–27 months), enucleation was avoided in 25 (83%) eyes. Two eyes that harbored features of ICRB Group E tumors were enucleated at presentation. Additional therapy failed in 3 of the treated eyes which ended with enucleation (1 eye in a unilateral RB patient who had massive vitreous seeds after completion of chemotherapy, 1 eye in a monocular patient who had massive recurrence postintra-arterial melphalan and EBRT, and the third eye in a monocular patient who had massive vitreous hemorrhage after EBRT). Twenty-three (77%) eyes did not show any viable tumor after a median of 9 months of follow-up after the last treatment and 2 (7%) still had residual tumor recurrences that needed more consolidation focal therapy. Correlation between tumor features, treatments, and outcome is presented in Tables 1 and 2.
Table 2.
Tumor features and management outcome(s) in eyes with retinoblastoma previously inadequately treated with systemic chemotherapy alone without focal therapy
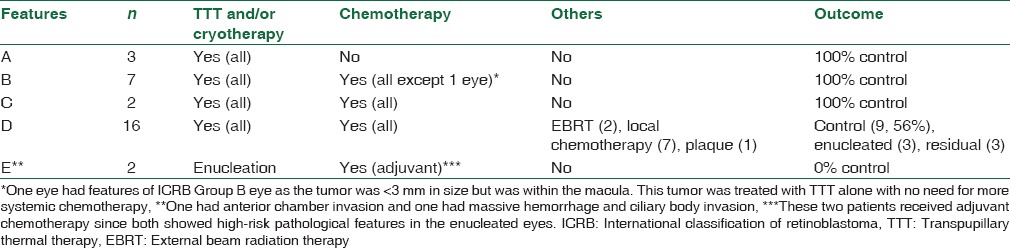
Both of the eyes enucleated at presentation which had ICRB Group E features harbored high-risk pathologic features, and therefore received adjuvant chemotherapy, while the 3 other enucleated eyes did not show high pathologic risk features. None of the patients had a second malignancy at the last follow-up visit. Ocular complications were seen in 3 (10%) eyes; rhegmatogenous retinal detachment postcryotherapy that was repaired by nondrainage scleral buckling, exudative retinal detachment post-EBRT that resolved spontaneously, and finally, ophthalmic artery occlusion postintra-arterial melphalan injection.
Discussion
Systemic chemotherapy combined with consolidation focal therapy has become the standard treatment for RB in most of the developed countries. Although systemic chemotherapy alone cannot cure RB, it may be used as a transient treatment to prevent or delay tumor progression to a more advanced stage. This might prevent or delay extraocular tumor extension and then metastasis during the delay until the patient gains access to a health-care center that provides the needed treatment.
Systemic chemotherapy for RB was first introduced in 1953 by Kupfer[9] who used systemically administered nitrogen mustard, and then, vincristine, cyclophosphamide, and doxorubicin were used during the 1970s. Unfortunately, however, it was soon discovered that even though they had a dramatic response, the tumors regrew after the treatment was stopped. That led ophthalmologists to start using this systemic chemotherapy solely to shrink tumors and to add focal treatment in the form of photocoagulation, cryotherapy, and brachytherapy.[10] In the 1990s, systemic chemotherapy (chemoreduction) with focal therapy became the standard approach in the management of eyes with intraocular RB by a trained physicians as per protocols in specialized centers.[10,11]
There is a significant influence of the treatment facility and the use of research protocols on RB mortality rate. In one study, the 4-year disease-free survival was 58% when the treatment takes place in a pediatric cancer center using a treatment protocol while it was as low as 19% when the treatment was provided at a nonpediatric cancer center and without the use of a treatment protocol.[12] Therefore, in most developed countries, RB is managed only in a specialized tertiary center by a multidisciplinary team. Unfortunately, this facility is not available at all occasions, and sometimes, it takes months for patients to get access to adequate health care. That delay will allow the tumor to grow to a more advanced stage and will probably result in a worse prognosis.[5] In our series at the King Hussein Cancer Center, we found that even with the delay in referral and getting adequate health care (9.5 months and sometimes as long as 20 months), enucleation was avoided in 83% of cases, and tumor control was achieved in 100% of tumors with Groups A, B, and C features, and 56% of tumors with Group D features. This eye salvage rate is promising and it is very close to eye salvage rate for eyes received adequate therapy from the beginning.[5]
RB patients in countries and centers where chemotherapy is available but consolidation therapy is not available have used to have two options. They can be referred to another center or abroad; however, in this case, the resulting delay due to logistics, visa, distance of traveling, and financial issues may extend to more than 6 months and will probably result in more tumor growth, more advanced stage, less eye salvage rate, and a higher mortality rate. The second option is enucleation which is not a favorable option, especially when the disease is bilateral. Our results suggest a third alternative, which is initiating chemotherapy for the patient by following one of the worldwide known protocols of chemotherapy for RB for few cycles. This cannot eradicate the active tumor, but it gives more time for the patient to gain access to a center where full therapy can be offered and therefore increases the chance for saving his life and his eye as well as salvaging his vision. This is an option as long as the intraocular tumor does not harbor features of ICRB Group E eyes which are clinical features that suggest risk of extraocular spread since 18.5%–50% of enucleated Group E eyes harbor high-risk histopathologic features predisposing to an increased risk of systemic metastatic.[12,13,14,15,16,17,18,19] Of interest, for eyes with Groups A, B, C, and D features, in our series (even with the delay of 9.5 months), not a single case had metastasis. This could be logically expected as these eyes did not harbor clinical features of tumor invasion to the vital structures such as the anterior chamber, ciliary body, choroid, or the optic nerve, and this also means that their management was justified mainly when the other eye was enucleated.[14]
Although the ICRB classification system describes tumors at presentation, and not after treatment, we found that tumor features per se (even if the tumor received previous chemotherapy) play a major role in predicting the outcome of management. All (100%) eyes in our series with features of ICRB Groups A, B, and C were salvaged by combined focal therapy and chemotherapy, which is almost equal to the reported 90%–100% salvage rate for eyes in the same groups that were treated with combined chemotherapy and focal therapy adequately from the beginning.[5,15,16]
Conclusion
Although chemotherapy alone is not a curative treatment for intraocular RB; it is recommended for patients in centers or countries where no adequate consolidation therapy is available. Chemotherapy can keep the tumor at a less invasive stage for a while giving the patient time to gain access to adequate management of RB. This study is retrospective and of limited size; larger and more comprehensive studies are still needed to analyze better the outcome, efficacy, and safety of this approach.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kivelä T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–31. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 2.Jaradat I, Yousef YA, Mehyar M, Sultan I, Khurma S, Al-Rawashded K, et al. Retinoblastoma in Jordan: An epidemiological study (2006-2010) Hematol Oncol Stem Cell Ther. 2011;4:126–31. doi: 10.5144/1658-3876.2011.126. [DOI] [PubMed] [Google Scholar]
- 3.White L. Chemotherapy in retinoblastoma: Current status and future directions. Am J Pediatr Hematol Oncol. 1991;13:189–201. [PubMed] [Google Scholar]
- 4.Fabian ID, Stacey AW, Johnson KP, Onadim Z, Chowdhury T, Duncan C, et al. Primary intravenous chemotherapy for group D retinoblastoma: A 13-year retrospective analysis. Br J Ophthalmol. 2017;101:82–8. doi: 10.1136/bjophthalmol-2016-309710. [DOI] [PubMed] [Google Scholar]
- 5.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Derenzini M, Brighenti E, Donati G, Vici M, Ceccarelli C, Santini D, et al. The p53-mediated sensitivity of cancer cells to chemotherapeutic agents is conditioned by the status of the retinoblastoma protein. J Pathol. 2009;219:373–82. doi: 10.1002/path.2612. [DOI] [PubMed] [Google Scholar]
- 7.Chan HS, Thorner PS, Haddad G, Gallie BL. Multidrug-resistant phenotype in retinoblastoma correlates with P-glycoprotein expression. Ophthalmology. 1991;98:1425–31. doi: 10.1016/s0161-6420(91)32134-1. [DOI] [PubMed] [Google Scholar]
- 8.Chan HS, Thorner P, Haddad G, Gallie BL. Effect of chemotherapy on intraocular retinoblastoma. Int J Pediatr Hematol Oncol. 1995;2:269–81. [Google Scholar]
- 9.Kupfer C. Retinoblastoma treated with intravenous nitrogen mustard. Am J Ophthalmol. 1953;36:1721–3. doi: 10.1016/0002-9394(53)90009-9. [DOI] [PubMed] [Google Scholar]
- 10.Chawla B, Jain A, Azad R. Conservative treatment modalities in retinoblastoma. Indian J Ophthalmol. 2013;61:479–85. doi: 10.4103/0301-4738.119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramson DH, Schefler AC. Update on retinoblastoma. Retina. 2004;24:828–48. doi: 10.1097/00006982-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SB. The national impact of clinical cooperative group trials for pediatric cancer. Med Pediatr Oncol. 1995;24:279–80. doi: 10.1002/mpo.2950240502. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Dimaras H, Massey C, Xu X, Huang D, Li B, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29:845–51. doi: 10.1200/JCO.2010.32.5332. [DOI] [PubMed] [Google Scholar]
- 14.Yousef YA, Hajja Y, Nawaiseh I, Mehyar M, Sultan I, Deebajah R, et al. A histopathologic analysis of 50 eyes primarily enucleated for retinoblastoma in a tertiary cancer center in Jordan. Turk Patoloji Derg. 2014;30:171–7. doi: 10.5146/tjpath.2014.01260. [DOI] [PubMed] [Google Scholar]
- 15.Chan HS, Gallie BL, Munier FL, Beck Popovic M. Chemotherapy for retinoblastoma. Ophthalmol Clin North Am. 2005;18:55–63, viii. doi: 10.1016/j.ohc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Qian J, Xue K, Gao YJ, Yuan YF, Shan HD, Bi YW. Clinical therapeutic efficiency of chemoreduction and local therapy for children with retinoblastoma. Zhonghua Yan Ke Za Zhi. 2010;46:312–6. [PubMed] [Google Scholar]
- 17.Wilson MW, Qaddoumi I, Billups C, Haik BG, Rodriguez-Galindo C. A clinicopathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95:553–8. doi: 10.1136/bjo.2009.177444. [DOI] [PubMed] [Google Scholar]
- 18.Yousef YA, Al-Hussaini M, Mehyar M, Sultan I, Jaradat I, AlRawashdeh K, et al. Predictive value of TNM classification, international classification, and Reese-Ellsworth staging of retinoblastoma for the likelihood of high-risk pathologic features. Retina. 2015;35:1883–9. doi: 10.1097/IAE.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 19.Eagle RC., Jr High-risk features and tumor differentiation in retinoblastoma: A retrospective histopathologic study. Arch Pathol Lab Med. 2009;133:1203–9. doi: 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]


