Abstract
Twelve benzylisoquinoline alkaloids, including pavine and phenanthroindolizidine types, were isolated from a MeOH/CH2Cl2 extract of Cryptocarya laevigata (stem bark) through bioactivity-guided fractionation for antitumor effects. Selected compounds were evaluated for antiproliferative activity against five human tumor cell lines, including a multidrug-resistant subline. Since more common 2,3,8,9-tetrasubstituted pavine alkaloids, such as crychine (3), exhibit very mild or no cytotoxicity, this compound type has not been well investigated for antitumor activity. Thus, this report is the first discovery of a 7-hydroxylated pavine alkaloid, (−)-neocaryachine (1), to demonstrate strong antiproliferative activity, with IC50 values of 0.06 to 0.41 µM against five tested tumor cell lines, including an MDR subline. Further mechanism of action studies revealed that 1 impacts the cellular S-phase by inducing DNA double-strand breaks.
Graphical Abstract
Rainforests are great treasure houses of biodiversity.1,2 Although they cover only about 6% of the earth’s land surface, almost half of all plant species live in rainforest areas.3 The diversity in plants should also support a large variety of bioactive natural products. It is known that plants were among the first sources of drugs, and they still serve as an important source of modern medicines.4 Plant-derived natural products have contributed immensely to the area of cancer chemotherapeutics, e.g., paclitaxel, vinca alkaloids (vinblastine and vincristine), podophylltoxin analogues (etoposide and teniposide), and topotecan derived from camptothecin. According to a recent report,5 83% of new chemical entities for anticancer agents have been derived from natural products per se, during the time frame of 1981 to 2014.
In the course of our research focused on the discovery of antitumor natural products from rainforest plants, a CH3OH/CH2Cl2 (1:1) extract (NCI extract ID: N025183) from the rainforest plant Cryptocarya laevigata (stem bark) showed potent antiproliferative activity against several human tumor cell lines (Table S1, Supporting Information). The genus Cryptocarya belongs to the family Lauraceae and produces multiple secondary metabolites, such as lactones, α-pyrones, flavonoids, chalcones, and alkaloids.6−25 These chemical components show diverse bioactivities, including nitric oxide inhibitory activity,7 glucose transport inhibitory effects,8 cytotoxicity,8−11 antimicrobial activity,11 and dengue virus NS5 polymerase inhibitory properties,12 as well as anti-inflammatory,13 antioxidant, antiplasmodial,25 and cholinesterase inhibitory activities.26 Among over 350 species in the genus Cryptocarya, phytochemical research on C. laevigata has not been reported, except for one article published in 1978.27
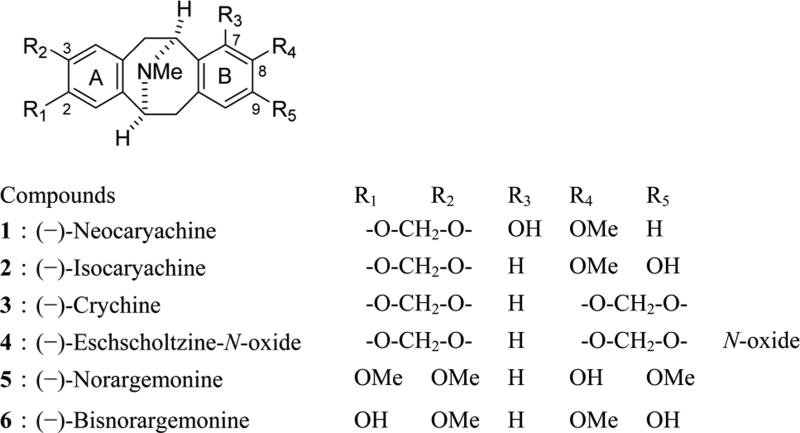
The crude extract of N025183 (9.8 g), provided by the NCI, was partitioned between EtOAc and water. The EtOAc extract was separated by silica gel column chromatography and preparative TLC techniques to give six known pavine alkaloids, (−)-neocaryachine (1),28 (−)-isocaryachine (2),29 (−)-crychine (3),30 (−)-eschscholtzine-N-oxide (4),31,32 (−)-norargemonine (5),33,34 and (−)-bisnorargemonine (6),33,35 as well as a phenanthroindolizidine alkaloid and (−)-13aα-antofine36 together with their biosynthetic intermediates, (+)-N-demethylphyllocaryptine,37 (+)-cinnamolaurine,38−40 (+)-N-methylcoculaurine,41 and (−)-reticuline.39,40 The structures of all isolated compounds were identified based on various NMR spectroscopic and HRMS data analyses, and their spectroscopic data agreed with published values. We evaluated five (1−5) of the six pavine alkaloids isolated from C. laevigata for antiproliferative activity against five human tumor cell lines, A549 (lung carcinoma), MDA-MB-231 (triple-negative breast cancer), MCF-7 (breast cancer), KB (originally isolated from epidermoid carcinoma of the nasopharynx), and vincristine-resistant KB-subline KB-VIN showing multidrug resistance (MDR) phenotype with overexpression of P-glycoprotein (Pgp) (Table 1).
Table 1.
Antiproliferative Activities of the Selected Pavine Alkaloids
| human cell linesa/IC50 (µM)b | |||||
|---|---|---|---|---|---|
|
|
|||||
| alkaloid | A549 | MDA-MB- 231 |
MCF-7 | KB | KB-VIN |
| 1 | 0.064 | 0.264 | 0.413 | 0.228 | 0.241 |
| 2 | 3.981 | 5.707 | 7.421 | 5.488 | 5.907 |
| 3 | 8.709 | 26.13 | 26.56 | 17.03 | 19.80 |
| 4 | 29.26 | 41.01 | >40 | 35.50 | >40 |
| 5 | >40 | >40 | >40 | >40 | >40 |
| PXL (nM) | 6.20 | 8.82 | 10.40 | 6.27 | 1926 |
A549 (lung carcinoma), MDA-MB-231 (triple-negative breast cancer), MCF-7 (estrogen receptor-positive & HER2-negative breast cancer), KB (epidermoid carcinoma of the nasopharynx), KB-VIN (Pgp-overexpressing MDR subline of KB).
Antiproliferative activity as IC50 values for each cell line, the concentration of compound that caused 50% reduction relative to untreated cells determined by the SRB assay.
It is generally believed that pavine alkaloids are noncytotoxic at a micromolar concentration; thus, little attention has been given to the antiproliferative activity of such alkaloids from the family Lauraceae. Instead, in this same family, phenanthroindolizidine-type alkaloids, which were isolated together with pavine alkaloids, demonstrated significant cytotoxicity with submicromolar IC50 values.42 Surprisingly, among the five pavine alkaloids tested in this study, (−)-neocaryachine (1) exhibited remarkable antiproliferative activity with IC50 values of less than 0.5 µM against all tested tumor cell lines, even the MDR phenotype. (−)-Isocaryachine (2) also potently inhibited the tumor cell growth, although its activity was lower than that of 1. The results indicated that a hydroxyl group at the C-7 position is a crucial factor for the antiproliferative activity. On the basis of a comparison of compounds 3 and 4, an N-oxide resulted in the loss of activity. Because no antiproliferative activity was observed for compound 5, a methylenedioxy moiety in ring A might be important for an antiproliferative effect. Interestingly, all compounds tested in this study were not substrates of P-gp, because the compounds suppressed chemosensitive (KB) and MDR (KB-VIN) cell growth at the same concentrations. However, many alkaloids, such as paclitaxel (PXL) and vincristine (VIN), are substrates of the ABC transporters expressed in MDR cancer cells, resulting in poor outcomes in cancer chemotherapy.
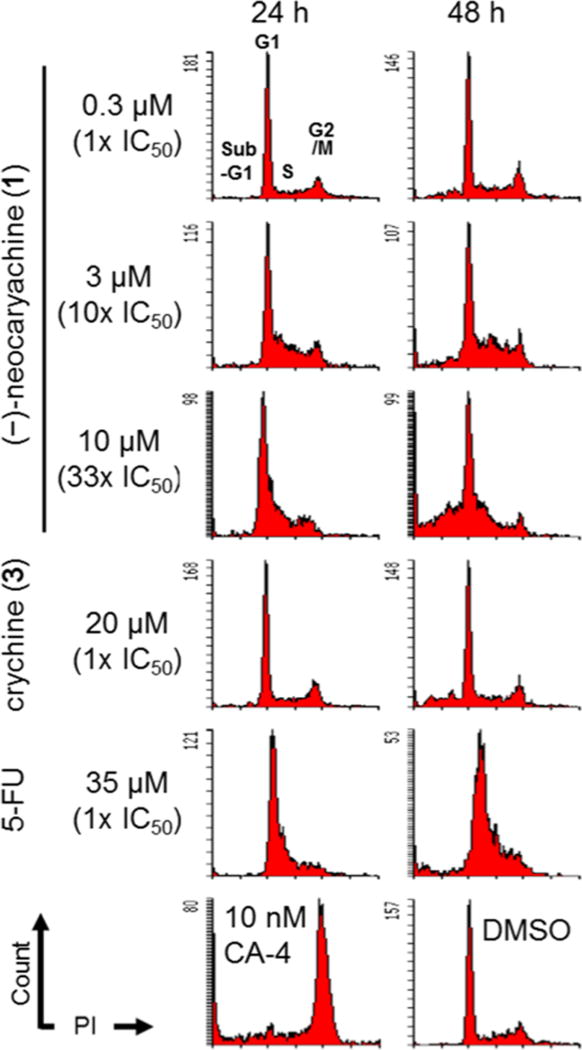
To understand the mechanism of action for the antiproliferative activity of these potent pavine alkaloids, the effects of compounds 1 and 3 on cell cycle progression in KB-VIN cells were initially examined using flow cytometry. As shown in Figure 1, at 0.3 µM, (−)-neocaryachine (1) had no effect after 24 h, while some cells accumulated in the sub-G1 phase after 48 h, suggesting apoptotic induction. In contrast, cells accumulated in the S phase in response to treatment with 3 or 10 µM 1, demonstrating that compound 1 impacted S phase progression, such as induction of DNA damage or inhibition of DNA duplication. Compound 3 showed no effects on the S phase, although sub-G1 was increased after 48 h of treatment at 20 µM. 5-Fluorouracil (5-FU) and combretastatin A-4 (CA-4) were used as controls for induction of cell cycle arrest in the S and G2/M phases, respectively.
Figure 1.
Effects of (−)-neocaryachine (1) and crychine (3) on cell cycle progression in MDR cells. KBKB-VIN cells were treated with (−)-neocaryachine (1), crychine (3), or 5-FU for 24 or 48 h or CA-4 for 24 h at the indicated concentrations. DMSO was used as a vehicle control. Cell cycle distributions of treated cells were analyzed by flow cytometry after staining with propidium iodide (PI).
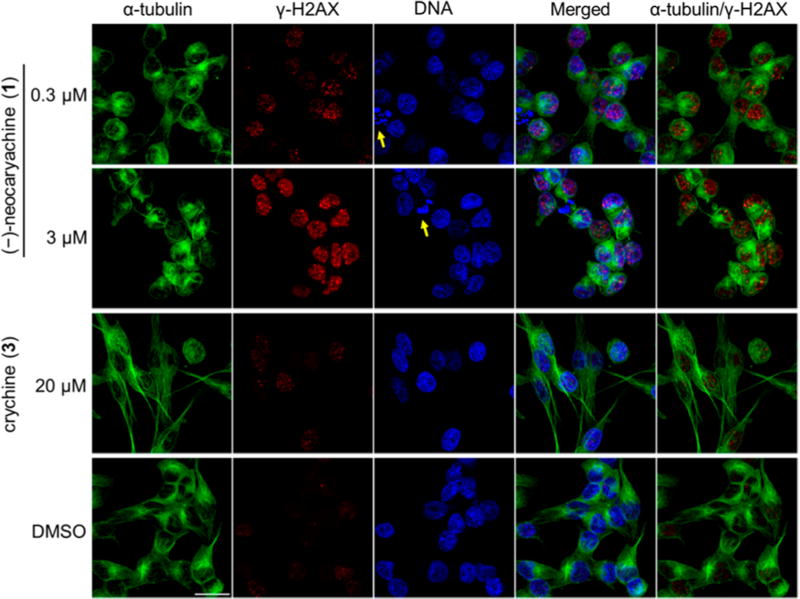
To further confirm the effects of 1 on the S phase, 1-treated KB-VIN cells were labeled with antibodies to α-tubulin together with γ-H2AX, a biomarker for DNA double-strand breaks (DSBs), and 4′,6-diamidino-2-phenylindole (DAPI) for labeling DNA (Figure 2). The γ-H2AX-positive nuclei were detectable in the cells treated with 0.3 µM 1, while they were unclear in the control cells (DMSO). The intensity of γ-H2AX signals and numbers of γ-H2AX-positive nuclei were dramatically increased when cells were treated with 1 (3 µM), suggesting that DSBs were induced by 1 in a concentration-dependent manner. Nuclear fragmentation was also observed in the compound-treated cells, suggesting apoptotic induction. In addition, abnormal morphology of microtubules was observed when cells were treated with 1 at 3 µM. No obvious γ-H2AX signal or defects in microtubule morphology were observed in the cells treated with 3. These immunostaining results were consistent with the results of the cell cycle analysis showing an impact on the S phase.
Figure 2.
Induction of γ-H2AX, a marker of DNA double-strand breaks, by (−)-neocaryachine (1) in MDR cells. KB-VIN cells were treated with (−)-neocaryachine (1) or crychine (3) for 24 h at the indicated concentrations. Cells were triple-stained with antibodies to α-tubulin (green), γ-H2AX (red), and DAPI (blue) for DNA. Stained cells were observed by confocal fluorescence microscopy, and all confocal images were stacked. Nuclear fragmentations are indicated by arrows. Bar: 25 µm.
In summary, we have shown for the first time that the antiproliferative pavine alkaloid 1 impacts the cell cycle in the S phase by inducing DSBs and resulting in the induction of apoptosis.
EXPERIMENTAL SECTION
General Experimental Procedures
Optical rotations were measured on a JASCO P-2200 digital polarimeter. NMR spectra were recorded on JEOL JMN-ECS400 and JMN-ECA600 spectrometers with tetramethylsilane as an internal standard. Chemical shifts are expressed as δ values. HRMS data were obtained on a JMS-SX102A (FAB) or JMS-T100TD (DART) mass spectrometer. Analytical and preparative TLC were carried out on precoated silica gel 60F254 and RP-18F254 plates (0.25 or 1.0 mm thickness; Merck).
Plant Material
The stem bark of Cryptocarya laevigata was collected in the Philippines by D. D. Soejarto, E. Reynoso, E. Sagcal, and R. Edrada in March 1990 and identified by D. D. Soejarto. A voucher specimen (#U44Z-7141) was deposited at the Smithsonian Institution (Washington DC, USA), and voucher extracts (N025183) are stored at the NCI (Frederick, MD, USA) and Kanazawa University (Kanazawa, Japan).
Extraction and Isolation
A CH3OH/CH2Cl2 (1:1) extract of the bark of Cryptocarya laevigata (9.8 g) was partitioned with EtOAc and H2O to yield an EtOAc-soluble (2.1 g) and H2O-soluble fractions. The EtOAc-soluble fraction was subjected to CombiFlash Rf MPLC (HPsil 120 g) eluted with an n-hexane/EtOAc gradient system [5:1/1:1/1:5/0:1], then EtOAc/MeOH [9:1/4:1/0:1], to yield six fractions A− F.
Fraction E (1.2 g) was subjected to silica gel column chromatography eluted with CH2Cl2/MeOH [50:1/30:1/10:1/5:1/1:1/0:1] to yield six fractions, E1−E6. Fraction E2 (175.2 mg) was purified by preparative TLC eluted with CH2Cl2/acetone (1:3) to yield six fractions, E2a−E2f. Fraction E2c (87.3 mg) was subjected to silica gel column chromatography eluted with CH2Cl2/MeOH [1:0/100:1/75:1/50:1/10:1/0:1] to yield (−)-crychine (3, 29.7 mg). Fraction E2d (44.8 mg) was purified by preparative TLC eluted with CH2Cl2/MeOH (5:1) to yield (−)-neocaryachine (1, 0.8 mg). Fraction E3 (100.4 mg) was subjected to silica gel column chromatography eluted with CH2Cl2/MeOH [1:0/50:1/40:1/30:1/20:1/10:1/1:1/0:1], then purified by preparative TLC eluted with n-hexane/acetone (1:3) to yield (−)-13aα-antofine (0.6 mg).
Fraction F (87.2 mg) was purified by preparative TLC eluted with CH2Cl2/MeOH (50:1) to yield six fractions, F1−F6. Fraction F2 (46.8 mg) was purified by preparative TLC eluted with CHCl3/MeOH (10:1) to obtain nine fractions, F2a−F2i. Fraction F2d (5.0 mg) was then purified by preparative TLC eluted with CHCl3/EtOH (5:1) to yield (−)-isocaryachine (2, 3.4 mg). Fraction F2f (2.4 mg) was purified by preparative TLC eluted with CHCl3/MeOH (5:1), then n-hexane/EtOAc (1:1), to yield (−)-norargemonine (5, 0.2 mg). Fraction F3 (9.5 mg) was subjected to silica gel column chromatography eluted with CH2Cl2/MeOH [40:1/30:1/20:1/5:1/2:1/1:2/1:5/0:1] to yield seven fractions, F3a−F3g. Fraction F3c was (−)-N-demethylphyllocaryptine (0.6 mg). Fraction F3d (2.5 mg) was purified by preparative TLC eluted with CH2Cl2/MeOH (5:1) followed by CHCl3/EtOH (5:1) to yield (+)-cinnamolaurine (0.5 mg) and (−)-bisnorargemonine (6, 1.6 mg). Fraction F3e (1.0 mg) was purified by preparative TLC eluted with CHCl3/MeOH (4:1) to yield four fractions, F3e1−F3e4. Fraction F3f (1.7 mg) was subjected to silica gel column chromatography eluted with EtOAc/MeOH [2:1/1:2/1:0] to yield five fractions, F3f1−F3f5. Fraction F3e2 and F3f4 were combined and purified by preparative TLC eluted with CHCl3/MeOH (4:1) to yield (−)-eschscholtzine N-oxide (4, 1.4 mg). Fraction F4 (11.5 mg) was purified by preparative TLC eluted twice with CHCl3/EtOH (50:1 then 2:1) followed by CH2Cl2/MeOH (4:1) to yield (−)-reticuline (0.8 mg) and (+)-N-methylcoculaurine (1.0 mg).
Antiproliferative Activity Assay
The antiproliferative activity assay using sulforhodamine B (SRB) was performed as described previously.43 In brief, cells were seeded in 96-well microtiter plates at densities of 4000−12 000 cells per well (based on the doubling time of the cell line) with compounds solubilized in DMSO. The highest concentration of DMSO in the cultures (0.1% v/v) had no effect on cell growth. After 72 h treatment with test compounds, attached cells were fixed with 10% trichloroacetic acid followed by staining with 0.04% SRB. After solubilization of protein-bound SRB with 10 mM Tris base, absorbance was measured at 515 nm using a microplate reader (ELx800, BioTek) with Gen5 software (BioTek). The mean IC50 is the average from at least three independent experiments with duplicate samples. The following human tumor cell lines were used in the assay: A549 (lung carcinoma), KB (originally isolated from epidermoid carcinoma of the nasopharynx), KB-VIN (VIN-resistant KB subline showing MDR phenotype by overexpressing P-gp), MCF-7 (estrogen receptor-positive, HER2-negative breast cancer), MDA-MB-231 (estrogen receptor-negative, progesterone receptor-negative, HER2-negative breast cancer). All cell lines were obtained from the Lineberger Comprehensive Cancer Center (UNC-CH) or from ATCC (Manassas, VA, USA), except KB-VIN, which was a generous gift of Professor Y.-C. Cheng (Yale University). KB-VIN cells were maintained in the presence of 100 nM VIN.
Cell Cycle Analysis
Cell cycle distribution was analyzed by measurement of cellular DNA content with propidium iodide (BD Biosciences) as described previously.43 Briefly, cells were seeded in a 12-well culture plate 24 h prior to treatment with compounds. KB-VIN cells were treated for 24 h with 0.3 and 3.0 µM 1, 20 µM 3, 10 nM CA-4, or vehicle (0.1% DMSO) as a control. Stained cells were analyzed by flow cytometry (BD FACSCalibur, BD Biosciences). Experiments were repeated a minimum of three times.
Immunofluorescence Staining
Immunostaining of KB-VIN was performed as described previously.43 Briefly, KB-VIN cells were seeded on an eight-well chamber slide (Lab-Tech) for 24 h prior to treatment with compounds. Cells were treated with compound for 24 h. Concentrations of reagents were used based on their IC50 used for cell cycle analysis as follows: 0.3 and 3.0 µM of 1, 20 µM 3, and 0.1% DMSO as a control. Cells were fixed in ice-cold 4% paraformaldehyde in phosphate-buffered saline (PBS) followed by permeabilization with 0.5% Triton X-100 in PBS. Fixed cells were labeled with mouse monoclonal antibody to α-tubulin (B5-1-2, Sigma) and rabbit polyclonal antibody to γ-H2AX (BETHYL Lab.) followed by FITC-conjugated antibody to mouse IgG (Sigma) and Alexa Fluor 546-conjugated antibody to rabbit IgG (Life Technologies). Nuclei were labeled with DAPI (Sigma). Fluorescence-labeled cells were observed using a confocal microscope (Zeiss, LSM700) controlled by ZEN software (Zeiss). Parameters (laser, beam splitter, band-pass filter) for confocal fluorescence imaging were used as follows: track 1 for DAPI (405 nm, 420 nm, 420−1000 nm), track 2 for FITC (488 nm, 544 nm, 490−555 nm), and track 3 for Alexa Fluor 546 (555 nm, 559 nm, 505−600 nm). Confocal images were reconstructed by stacking using ZEN (black edition) software (Zeiss). Final images were prepared by Photoshop CS6 (Adobe).
Supplementary Material
Chart 1. Structures of Pavine Alkaloids Isolated from C. laevigata.
Acknowledgments
We appreciate critical comments, suggestions, and editing of the manuscript by Dr. S. L. Morris-Natschke (UNC-CH). We wish to thank the Microscopy Service Laboratory (UNC-CH) for confocal microscopy. This study was supported by a Grantin-Aid from the Ministry of Education, Culture, Sports, Science and Technology (JSPS KAKENHI, Japan) awarded to K.N.G. (Grant Numbers 25293024 and 25670054) and by a grant from the University Research Council (UNC) as well as the Eshelman Institute for Innovation, Chapel Hill, North Carolina, awarded to M.G. This work was also supported by NIH grant CA177584 from the National Cancer Institute, awarded to K.H.L. We also thank the Biological Testing Branch, DTP, DCTD, NCI, for performing the NCI 60-cell cytotoxicity assay and the Natural Products Support Group, Leidos Biomedical Inc., for plant extraction.
Footnotes
ASSOCIATED CONTENT
- 1H and 13C NMR data, HRMS, and optical rotations for the isolated compounds (PDF)
The authors declare no competing financial interest.
References
- 1.Bagchi R, Gallery RE, Gripenberg S, Gurr SJ, Narayan L, Addis CE, Freckleton EP, Lewis OT. Nature. 2014;506:85–88. doi: 10.1038/nature12911. [DOI] [PubMed] [Google Scholar]
- 2.Wright SJ. Oecologia. 2002;130:1–14. doi: 10.1007/s004420100809. [DOI] [PubMed] [Google Scholar]
- 3.http://www.rainforestfoundation.org/home
- 4.Harvey AL, Edrada-Ebel R, Quinn RJ. Nat. Rev. Drug Discovery. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 6.Davis RA, Demirkiran O, Sykes ML, Avery VM, Suraweera L, Fechner GA, Quinn RJ. Bioorg. Med. Chem. Lett. 2010;20:4057–4059. doi: 10.1016/j.bmcl.2010.05.091. [DOI] [PubMed] [Google Scholar]
- 7.Yang BY, Kong LY, Wang XB, Zhang YM, Li RJ, Yang MH, Luo JG. J. Nat. Prod. 2016;79:196–203. doi: 10.1021/acs.jnatprod.5b00839. [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Yuan C, Qian Y, Chai HB, Chen X, Goetz M, Kinghorn AD. J. Nat. Prod. 2013;77:550–556. doi: 10.1021/np400809w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou TH, Chen JJ, Lee SJ, Chiang MY, Yang CW, Chen IS. J. Nat. Prod. 2010;73:1470–1475. doi: 10.1021/np100014j. [DOI] [PubMed] [Google Scholar]
- 10.Feng R, Wang T, Wei W, Tan RX, Ge HM. Phytochemistry. 2013;90:147–153. doi: 10.1016/j.phytochem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Zhang WJ, Cheng YQ, Jiang R, Wei W, Chen CJ, Wang G, Jiao RH, Tan RX, Ge HM. Planta Med. 2014;80:925–930. doi: 10.1055/s-0034-1368613. [DOI] [PubMed] [Google Scholar]
- 12.Allard PM, Dau ET, Eydoux C, Guillemot JC, Dumontet V, Poullain C, Canard B, Guéritte F, Litaudon M. J. Nat. Prod. 2011;74:2446–2453. doi: 10.1021/np200715v. [DOI] [PubMed] [Google Scholar]
- 13.Feng R, Guo ZK, Yan CM, Li EG, Tan RX, Ge HM. Phytochemistry. 2012;76:98–105. doi: 10.1016/j.phytochem.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Nehme CJ, de Moraes PLR, Tininis AG, Cavalheiro AJ. Biochem. Syst. Ecol. 2008;36:602–611. [Google Scholar]
- 15.Cave A, Leboeuf M, Moskowitz H, Ranaivo A, Bick IRC, Sinchai W, Nieto M, Sevenet T, Cabalion P. Aust. J. Chem. 1989;42:2243–2263. [Google Scholar]
- 16.Lee SS, Lin YJ, Chen CK, Liu KCS, Chen CH. J. Nat. Prod. 1993;56:1971–1976. [Google Scholar]
- 17.Chang WT, Lee SS, Chueh FS, Liu KCS. Phytochemistry. 1998;48:119–124. [Google Scholar]
- 18.Wu TS, Lin FW. J. Nat. Prod. 2001;64:1404–1407. doi: 10.1021/np010258i. [DOI] [PubMed] [Google Scholar]
- 19.Lin FW, Wu PL, Wu TS. Chem. Pharm. Bull. 2001;49:1292–1294. doi: 10.1248/cpb.49.1292. [DOI] [PubMed] [Google Scholar]
- 20.Lin FW, Wang JJ, Wu TS. Chem. Pharm. Bull. 2002;50:157–159. doi: 10.1248/cpb.50.157. [DOI] [PubMed] [Google Scholar]
- 21.Toribio A, Bonfils A, Delannay E, Prost E, Harakat D, Henon E, Richard B, Litaudon M, Nuzillard JM, Renault JH. Org. Lett. 2006;8:3825–3828. doi: 10.1021/ol061435f. [DOI] [PubMed] [Google Scholar]
- 22.Awang K, Hadi AHA, Saidi N, Mukhtar MR, Morita H, Litaudon M. Fitoterapia. 2008;79:308–310. doi: 10.1016/j.fitote.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Saidi N, Hadi AHA, Awang K, Mukhtar MR. Indian J. Chem. 2009;9:461–465. [Google Scholar]
- 24.Saidi N. Jur. Nat. 2011;11:48–51. [Google Scholar]
- 25.Nasrullah AA, Zahari A, Mohamad J, Awang K. Molecules. 2013;18:8009–8017. doi: 10.3390/molecules18078009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan Othman WN, Liew SY, Khaw KY, Murugaiyah V, Litaudon M, Awang K. Bioorg. Med. Chem. 2016;24:4464–4469. doi: 10.1016/j.bmc.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann JJ, Luzbetak DJ, Torrance SJ, Cole JR. Phytochemistry. 1978;17:1448. [Google Scholar]
- 28.Lee SS, Liu YC, Chen CH. J. Nat. Prod. 1990;53:1267–1271. [Google Scholar]
- 29.Mollataghi A, Hadi AHA, Cheah SC. Molecules. 2012;17:4197–4208. doi: 10.3390/molecules17044197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.L ST, Lan PK. Yakugaku Zasshi. 1966;86:177–184. doi: 10.1248/yakushi1947.86.3_177. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Lin F. J. Nat. Prod. 2001;64:1404–1407. doi: 10.1021/np010258i. [DOI] [PubMed] [Google Scholar]
- 32.Urzua A, Mendoza L. J. Nat. Prod. 1986;49:922–923. [Google Scholar]
- 33.Slavik J, Slavikova L. Collect. Czech. Chem. Commun. 1971;36:2067–2069. [Google Scholar]
- 34.Youte JJ, Barbie D, Al-Mourabit A, Gnecco D, Marazano C. J. Org. Chem. 2004;69:2737–2740. doi: 10.1021/jo0357869. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AP, Luke RWA, Singh G, Boa AN. J. Chem. Soc., Perkin Trans. 1. 1996;9:907–913. [Google Scholar]
- 36.Capo M, Saa JM. J. Nat. Prod. 1989;52:389–390. [Google Scholar]
- 37.Pinna GA, Cignarella G, Scolastico S, Porceddu ML. Eur. J. Med. Chem. 1994;29:55–60. [Google Scholar]
- 38.Gellert E, Summons RE. Tetrahedron Lett. 1969;58:5055–5058. [Google Scholar]
- 39.Ruiz-Olalla A, Wuerdemann MA, Wanner MJ, Ingemann S, Maarseveen JH, Hiemstra HJ. Org. Chem. 2015;80:5125–5132. doi: 10.1021/acs.joc.5b00509. [DOI] [PubMed] [Google Scholar]
- 40.Gellert E, Summons RE. Aust. J. Chem. 1970;23:2095–2059. [Google Scholar]
- 41.Arndt RR. J. Chem. Soc. 1963:2547–2549. [Google Scholar]
- 42.Pereira MF, Rochais C, Dallemagne P. Anti-Cancer Agents Med. Chem. 2015;15:1080–1091. doi: 10.2174/1871520615666150520143600. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa-Goto K, Oda A, Hamel E, Ohkoshi E, Lee KH, Goto M. J. Med. Chem. 2015;58:2378. doi: 10.1021/jm501859j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.