Abstract
Development of novel therapies for CNS tumors requires reliable assessment of response and progression. This requirement has been particularly challenging in neuro-oncology for which contrast enhancement serves as an imperfect surrogate for tumor volume and is influenced by agents that affect vascular permeability, such as antiangiogenic therapies. In addition, most tumors have a nonenhancing component that can be difficult to accurately quantify. To improve the response assessment in neuro-oncology and to standardize the criteria that are used for different CNS tumors, the Response Assessment in Neuro-Oncology (RANO) working group was established. This multidisciplinary international working group consists of neuro-oncologists, medical oncologists, neuroradiologists, neurosurgeons, radiation oncologists, neuropsychologists, and experts in clinical outcomes assessments, working in collaboration with government and industry to enhance the interpretation of clinical trials. The RANO working group was originally created to update response criteria for high- and low-grade gliomas and to address such issues as pseudoresponse and nonenhancing tumor progression from antiangiogenic therapies, and pseudoprogression from radiochemotherapy. RANO has expanded to include working groups that are focused on other tumors, including brain metastases, leptomeningeal metastases, spine tumors, pediatric brain tumors, and meningiomas, as well as other clinical trial end points, such as clinical outcomes assessments, seizures, corticosteroid use, and positron emission tomography imaging. In an effort to standardize the measurement of neurologic function for clinical assessment, the Neurologic Assessment in Neuro-Oncology scale was drafted. Born out of a workshop conducted by the Jumpstarting Brain Tumor Drug Development Coalition and the US Food and Drug Administration, a standardized brain tumor imaging protocol now exists to reduce variability and improve reliability. Efforts by RANO have been widely accepted and are increasingly being used in neuro-oncology trials, although additional refinements will be needed.
INTRODUCTION
Despite extensive efforts, progress in developing more effective therapies for tumors of the CNS has been disappointingly slow. There are many reasons for this lack of progress, including tumor characteristics, such as intrinsic resistance to most available therapies, molecular heterogeneity, and redundant signaling pathways; lack of predictive preclinical models; poor passage of most therapeutic agents across the blood–tumor barrier; and a relative lack of interest from pharmaceutical companies because of the relatively small patient population and the poor record of success to date.1 Over the past decade, there has also been a growing consensus that the lack of reliable and widely accepted response criteria and clinical trial end points is another limiting factor in identifying more effective therapies in neuro-oncology.2,3 This review summarizes the current efforts to improve response assessment in neuro-oncology clinical trials, including those led by the international Response Assessment in Neuro-Oncology (RANO) working group,4,5 as well as some of the remaining challenges.
DEVELOPMENT OF GUIDELINES AND RESPONSE CRITERIA
The process of developing guidelines and response criteria within each RANO working group is detailed in their respective publications.4,6-9 In brief, each working group is an international, multidisciplinary effort that consists of neuro-oncologists, medical oncologists, neurosurgeons, radiation oncologists, neuropsychologists, neuropathologists, and experts in quality-of-life measures who work in collaboration with government and industry. After the completion of literature review and critique, each working group convened to formulate consensus proposals for response criteria through an iterative process. Subsequent efforts are focused on incorporating the response criteria into clinical trials and validating them.
HIGH-GRADE GLIOMAS
High-grade gliomas (HGGs) account for the majority of malignant primary brain tumors.10 Although response assessments in clinical trials in oncology are based on one-dimensional tumor measurements as defined by Response Evaluation Criteria in Solid Tumors (RECIST) criteria,11 until recently, tumor measurements in neuro-oncology were based on two-dimensional measurements that used the criteria proposed in 1990 by Macdonald et al.12 These criteria determined response by using enhancing tumor area—the product of the maximal cross-sectional enhancing diameters—together with changes in the neurologic status of patients and corticosteroid use.12 A number of studies have compared RECIST criteria with two-dimensional, three-dimensional, and volumetric measurements and have found generally good concordance between methods.4,13-15 Nonetheless, because of concerns regarding the irregular shapes of such tumors as glioblastoma, the Macdonald Criteria remained the most widely used response criteria.
Over time, it became apparent that the Macdonald Criteria had a number of important limitations.4,16 It did not provide guidance on the definition of progression that was necessary for entry into clinical trials, measurability of disease, definition of target lesions, or the handling of patients who were suspected of experiencing pseudoprogression—the transient increase in contrast enhancement observed in 10% to 30% of patients with glioblastoma immediately after treatment with chemoradiotherapy17 (Fig 1A). The Macdonald Criteria also did not take into account the pseudoresponse observed with antiangiogenic therapies, such as bevacizumab, that rapidly reduce vascular permeability and contrast enhancement.4 These agents can produce a marked decrease in contrast enhancement as early as 1 to 2 days after the initiation of therapy that does not necessary reflect a true reduction of tumor burden. Of most importance, the Macdonald Criteria measured only contrast-enhancing tumor and did not take into account nonenhancing disease. This is especially problematic for WHO grade II and III gliomas. In addition, up to 40% of patients who were treated with agents, such as bevacizumab, that reduce vascular permeability develop progressive increase only in T2/fluid-attenuated inversion recovery (FLAIR) signal and not contrast enhancement as the initial manifestation of progression, a phenomenon termed nonenhancing tumor progression18 (Fig 1B).
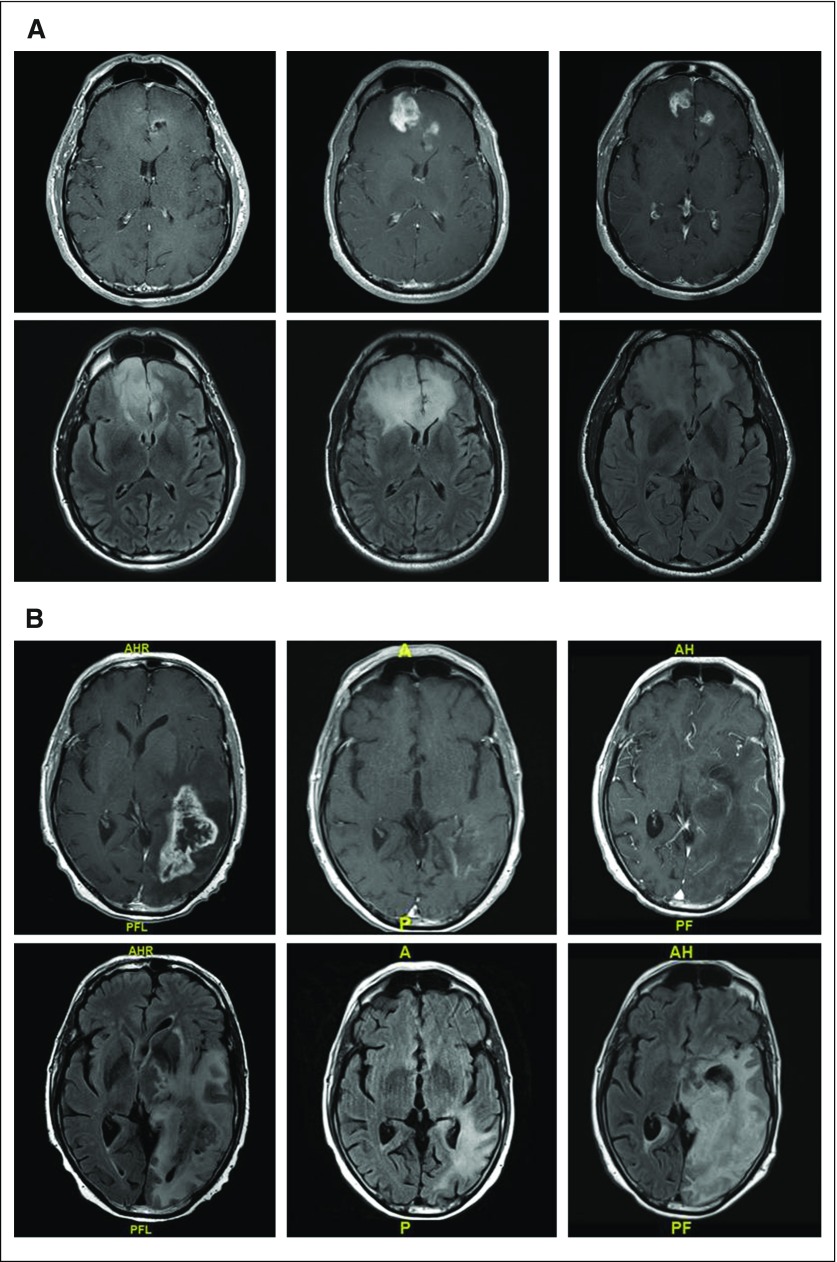
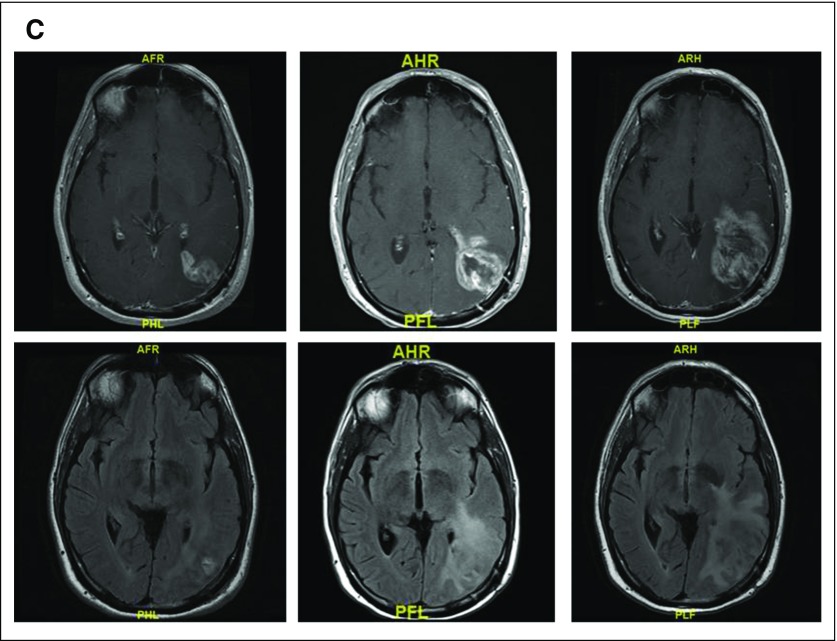
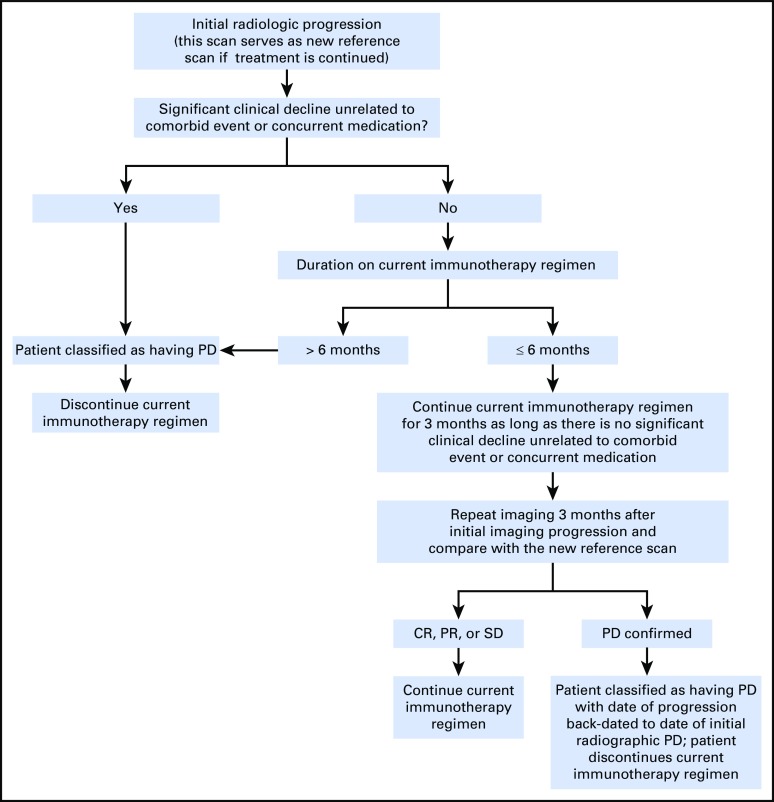
Fig 1.
(A) T1 postcontrast and fluid-attenuated inversion recovery (FLAIR) images before (left), 2 months after (middle), and 3 months (right) after completing radiation and concurrent temozoloimde in a patient with a newly diagnosed glioblastoma (GBM). The extent of enhancement initially increases, but then improves with time to suggest that the initial post-treatment changes represented pseudoprogression. (B) T1 postcontrast and FLAIR images before (left), after one cycle (middle), and after five cycles (right) of bevacizumab in a patient with recurrent GBM. Although enhancement remains nonmeasurable after five cycles of treatment, the extent of FLAIR only continues to expand in this case of nonenhancing disease progression. (C) T1 postcontrast and FLAIR images before (left), 1 month after (middle), and 3 months (right) after completing radiation and concurrent nivolumab in a patient with a newly diagnosed GBM. Tumor resection pathology revealed almost all treatment effect.
To address the limitations of the Macdonald Criteria, the RANO working group proposed updated response criteria for HGGs in 2010.4 These RANO Criteria are summarized in Table 1. The RANO criteria built on the Macdonald Criteria by using two-dimensional tumor measurements, but included a definition of progression for patients being considered for enrollment in clinical trials (≥ 25% increase in the product of perpendicular diameters compared with baseline or best response); definitions for measurable disease (two perpendicular diameters of at least 10 mm, visible on two or more axial slices that are preferably, at most, 5 mm apart with 0-mm skip); and allowance of up to five target lesions. To address pseudoprogression, the RANO criteria recommended that within the first 12 weeks after irradiation, patients should be excluded from clinical trials for recurrent disease unless progression is clearly outside the radiation field or there was clear histologic documentation of progression. To account for pseudoresponse after antiangiogenic therapies, patients who achieved partial or complete responses required a confirmatory scan at least 4 weeks later to ensure that responses were sustained. In addition, the RANO criteria defined progression not only as a 25% increase in contrast-enhancing area over baseline or best response, but also included any significant enlargement of nonenhancing T2/FLAIR signal on magnetic resonance imaging (MRI) that was attributed to tumor growth. Given the difficulty of measuring nonenhancing disease progression, no objective criteria were proposed—a significant limitation of the RANO criteria. Of importance, there were also recommendations for dealing with equivocal imaging changes, which allowed patients to stay on study with a repeat scan in ≥ 4 weeks. If progression is subsequently confirmed, the time of progression is backdated to the time point at which the issue was first suspected. This prevents patients from being discontinued prematurely from studies when imaging findings are equivocal. Since its introduction, the RANO criteria have been adopted in the majority of clinical trials of HGG.
Table 1.
Criteria for Response Assessment Incorporating Magnetic Resonance Imaging and Clinical Factors
In 2014, the Jumpstarting Brain Tumor Drug Development Coalition and the US Food and Drug Administration conducted a workshop to evaluate clinical trial neuroimaging end points.2,19-21 There was general consensus that the RANO criteria was reasonably reproducible and accurate, despite some limitations. Further work was deemed necessary to define the value of including T2/FLAIR progression in the definition of progression as well as the role of advanced MRI techniques. Of most importance, it highlighted the need, in the field, to standardize neuroimaging to reduce variability and increase reliability. This led to the development of a standardized brain tumor imaging protocol that is increasingly being used in neuro-oncology clinical trials, especially in Europe and North America.22,23
A number of outstanding issues regarding response assessment in HGG remain to be addressed. These include the optimal management of pseudoprogression from chemoradiation and immunotherapies, determination of the value of T2/FLAIR assessment and volumetric imaging and T1 subtraction maps, and establishment of a more objective score for the assessment of clinical deterioration.
Pseudoprogression
The ability to differentiate pseudoprogression as a result of radiochemotherapy from true tumor progression remains a significant challenge.24,25 Advanced MRI techniques, such as dynamic susceptibility contrast (perfusion) and dynamic contrast-enhanced MRI,26-28 as well as amino acid positron emission tomography,29,30 have shown promise but additional work is necessary to standardize these approaches and improve their sensitivity and specificity. In addition, issues of cost and accessibility will need to be addressed before they can be widely adopted in clinical trials. Given the difficulty in determining pseudoprogression from progression on the first MRI scan after chemoradiotherapy, Ellingson et al31 have proposed that, instead of using the postoperative MRI as the baseline scan, the baseline scan should be the first postradiation MRI. If the next scan shows progression and the patient is clinically stable, then the patient will remain on treatment and only be taken off study if the subsequent scan confirms progression. This approach reduces the challenges posed by pseudoprogression on the first postradiation scan but requires validation.
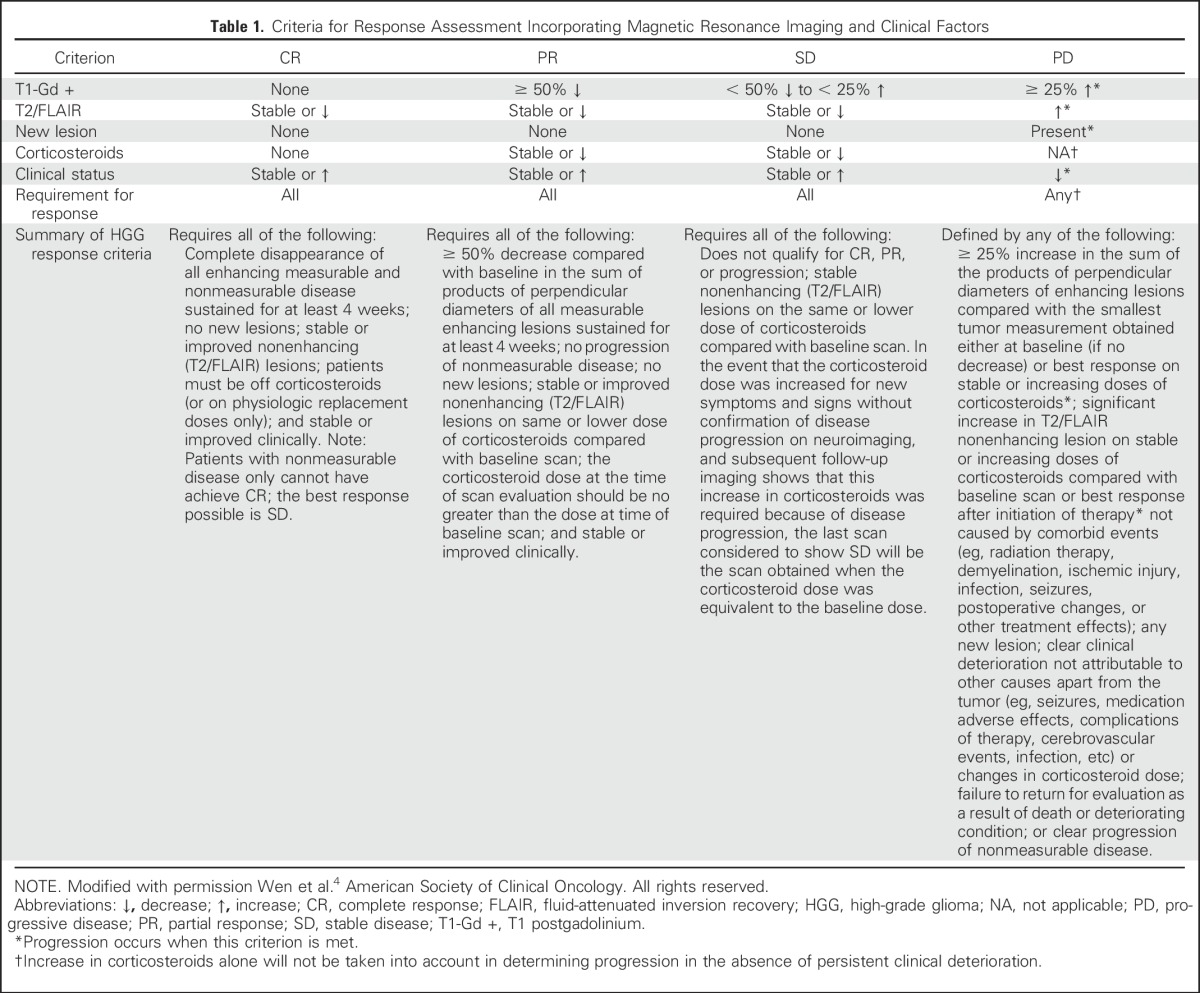
The recent introduction of immunotherapies in neuro-oncology has led to the realization that a subset of patients who receive these therapies can also develop pseudoprogression, which is characterized by transient enlargement of the tumor or the appearance of new lesions that ultimately regress32 (Fig 1C). To address this phenomenon in neuro-oncology, the immunotherapy Response Assessment for Neuro-Oncology (iRANO) criteria were recently proposed33 (Appendix Fig A1, online only). These criteria were based on the immune-related response criteria that were proposed for systemic cancers as well as on attempts to prevent patients in clinical trials from being discontinued prematurely as a result of the experience of pseudoprogression by requiring that progression be confirmed.32 The iRANO criteria suggest that within the first 6 months of initiating an immunotherapy—when pseudoprogression is most likely to occur—if MRI scans show radiologic progression (a 25% increase in area or appearance of new lesions), provided the patient is clinically stable, the patient can stay on treatment and be observed closely with repeat MRI scans in 3 months or sooner. If the subsequent scans confirm progression, the date of progression will be backdated to the date of initial progressive disease. The iRANO criteria have been incorporated into a number of ongoing immunotherapy trials of patients with brain tumor, which has allowed its value to be assessed.
T2/FLAIR
Incorporation of T2/FLAIR changes in the determination of progression was one of the major changes proposed by the RANO criteria for HGG; however, because of the difficulty of measuring T2/FLAIR accurately, no objective criteria were proposed. Instead, worsening of T2/FLAIR was a subjective assessment that is characterized by a significant increase in T2/FLAIR nonenhancing lesion that is felt to be a result of tumor growth and not because of comorbid events—for example, radiation therapy, demyelination, ischemic injury, infection, seizures, postoperative changes, or other treatment effects.4 As it is often difficult to differentiate T2/FLAIR changes that result from tumor growth from other causes, there is significant interobserver variability and unclear benefit, which has led to some proposals to eliminate this aspect of the RANO criteria.31,34 For agents that generally do not affect vascular permeability, such as most cytotoxic agents and many targeted agents, nonenhancing tumor progression is unlikely and the measurement of T2/FLAIR is likely unnecessary. With immunotherapies for which an inflammatory component that results in increased T2/FLAIR is likely, using T2/FLAIR to determine tumor progression is probably inappropriate; however, when antiangiogenic agents, such as bevacizumab, are being evaluated, up to 40% of patients experience progression initially with nonenhancing disease,18 and determination of progression using the RANO criteria results in significantly shorter progression-free survival compared with the Macdonald Criteria.35 T2/FLAIR may potentially still have a role for this class of agents that affect vascular permeability and for grade III gliomas. A number of studies are ongoing that attempt to better quantify T2/FLAIR36 as well as to assess its value in response determination with respect to survival outcomes and its ease of use and reproducibility.
Volumetric MRI and T1 Subtraction Maps
Given the irregular shape of brain tumors and the difficulties in evaluating tumors using two-dimensional measurements, there has been longstanding interest in using volumetric imaging, and, more recently, T1-subtraction maps, to more accurately determine T1 contrast-enhancing tumor37,38; however, whereas some studies have failed to show a benefit of volumetric imaging, these were not performed with standard protocols, and additional studies will be required to determine whether the additional cost and complexity of volumetric measurements will enhance response assessment.13,14,39
NEUROLOGIC ASSESSMENT
Although response rate, progression-free survival, and overall survival are well-established end points, there is increasing recognition of the importance of clinical outcome assessments that help measure the benefits of treatments on the basis of patients’ quality of life, neurocognitive status, or function. Clinical status is a criterion for the proposed response assessment in several RANO efforts, including those for HGG,4 low-grade glioma (LGG),6 brain metastases,7 and leptomeningeal disease.8 Whereas clinician-reported measures, such as Karnofsky performance status, are commonly used in clinical trials and correlate with overall survival, Karnofsky performance status is not a sensitive or specific measure of response to treatment40 and may not accurately capture the neurologic status of a patient.
To this end, the Neurologic Assessment in Neuro-Oncology (NANO) working group developed a more objective, quantifiable proxy for clinical status in patients with brain tumors.9 NANO is a simple neurologic assessment conducted and reported by a trained health care professional who evaluates the patient in nine domains: gait, strength, upper extremity ataxia, sensation, visual fields, facial strength, language, level of consciousness, and behavior. Each domain contains a score from 0 to 3 or 0 to 2, depending on the domain, with higher scores indicating worse neurologic function (Table 2). The cumulative score can be compared over time to determine whether clinical status is stable, better, or worse as part of the response assessment. In pilot studies, NANO is associated with high interobserver agreement.9 The value of NANO is being determined in a number of trials that have incorporated this tool.
Table 2.
Neurologic Assessment in Neuro-Oncology Scale to Assess Neurologic Function for Integration Into Response Assessment in Neuro-Oncology Criteria
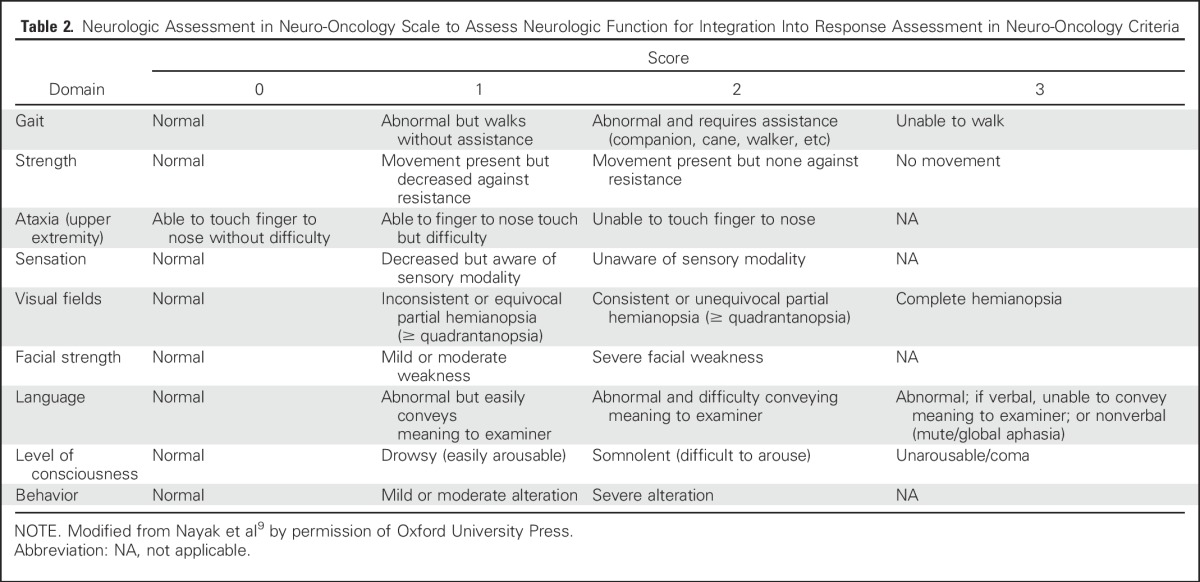
In conjunction with the RANO neurologic assessment working group, the RANO leptomeningeal metastases (RANO-LM) working group has modified NANO for patients with LM to be used as part of the response assessment.8 Ten domains are included: gait, strength, sensation, vision, eye movements, facial strength, hearing, swallowing, level of consciousness, and behavior. Similarly, each domain is scored from 0 to 3 or 0 to 2 on the basis of the level of function. RANO-LM is discussed in further detail in the LM section.
LGGs
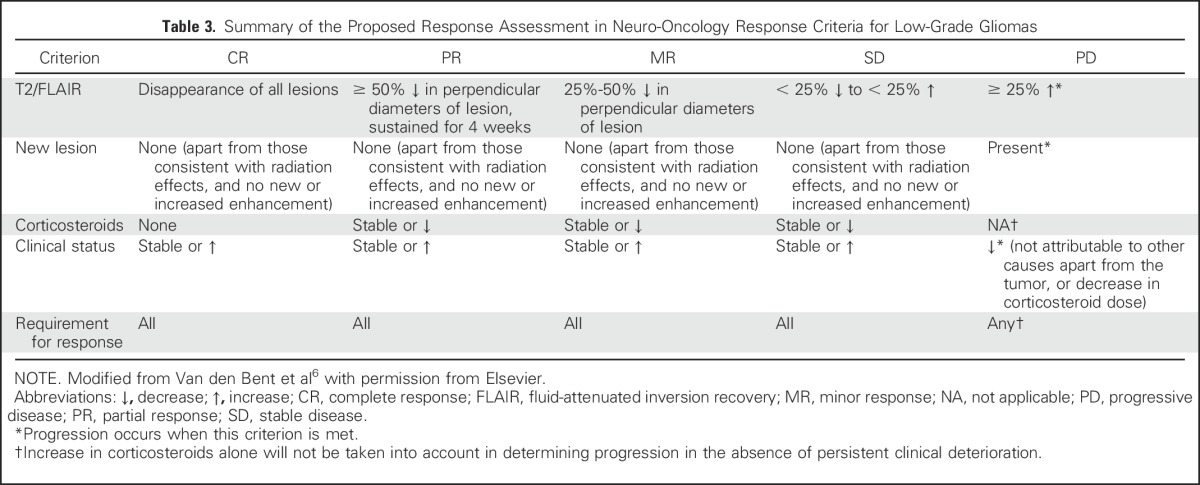
Recent advances in the understanding of the molecular pathogenesis of LGGs41 has led to renewed interest in developing novel therapies for patients with these tumors. The RANO working group proposed response criteria for LGG (Table 3) that are similar to that for HGG but that measures T2/FLAIR rather than contrast enhancement as these tumors rarely enhance. In addition, because responses are often relatively modest, minor response criteria that are characterized by a decrease in T2/FLAIR tumor of 25% to 50% was introduced. Although T2/FLAIR provides the clearest and most reproducible definition of LGG, distinguishing tumor from radiation-induced changes, postsurgical changes, demyelination, ischemic injury, and other comorbid events can be difficult. As validated imaging modalities that more accurately reflect tumor burden are developed, these criteria can be revised. With LGGs, clinical outcome assessments, such as neurocognitive function, quality of life, and seizure control, assume greater importance for the determination of the response to treatment. The RANO group recently proposed guidelines for using seizure control as a metric to assess the efficacy of tumor treatment in LGG trials by using a composite score of seizure classification, frequency, outcome, and severity.42
Table 3.
Summary of the Proposed Response Assessment in Neuro-Oncology Response Criteria for Low-Grade Gliomas
BRAIN METASTASES
Before the RANO brain metastases (RANO-BM) working group convened in 2011, clinical trials of patients with brain metastases were notable for their heterogeneity with respect to patient population, imaging modality, frequency of assessment, definitions of progression, and response (WHO43 v modified Macdonald Criteria12 v RECIST11 v ad hoc radiographic assessment criteria), among other features.44,45 Even though BMs are the most common malignant brain tumor, affecting up to one third of adults with cancer,46 the field lacked the common definitions and guidelines for response assessment and clinical trial design that are needed for quality and consistency in trial reporting. RANO-BM proposed response assessment on the basis of literature review and consensus opinion.7 For clinical trials of systemic agents, the group recommended the use of RECIST 1.111 for non-CNS response assessment and RANO-BM for CNS response assessment. With features that were adopted from RECIST and RANO-HGG to meet the particular needs of patients with solid tumor brain metastases, RANO-BM response assessment is based on the sum diameter of one-dimensional measurements, corticosteroid dosing, and clinical status7 (Table 4). Guidance is also provided for cases in which patients were treated with stereotactic radiosurgery or immunotherapy to avoid equating treatment effect with tumor progression.
Table 4.
Summary of the Proposed Response Assessment in Neuro-Oncology Response Criteria for Brain Metastases

Several controversies arose during the development of these criteria. As a result of concerns over reproducibility and the interpretation of changes in small lesions as well as to maintain consistency with RECIST 1.1, measurable disease was defined in RANO-BM as a contrast-enhancing lesion that can be accurately measured in at least one dimension with a minimum size of 10 mm. The working group acknowledged that many patients present with subcentimeter brain metastases and, therefore, RANO-BM provides guidance for investigators who choose to lower the minimum size limit of measurable disease to 5 mm. Similarly, the use of one-dimensional versus volumetric assessments was debated within the working group. Ultimately, one-dimensional measurements formed the basis of RANO-BM criteria as the group felt that the existing data on volumetric assessments were not strong enough to justify the real-time cost and complexity as discussed previously with respect to RANO-HGG. However, RANO-BM does provide guidance for those investigators who wish to include volumetric assessment within their trials.
LM
Response assessment in LM is particularly difficult for a variety of reasons. The clinical presentation and neuroimaging features in patients with LM are varied. Although CSF cytology, when positive for malignant cells, is definitive for disease confirmation, false negatives are common in as much as 50% of patients.47,48 Imaging changes often do not correlate with the clinical status of the patient, and LM can be difficult to measure quantitatively.48
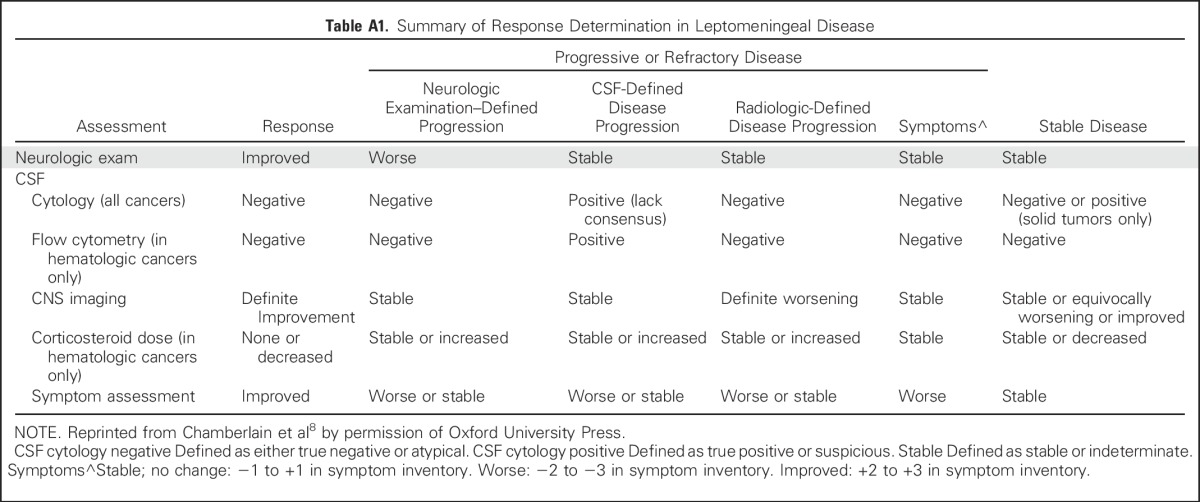
With these challenges in mind, the proposed response criteria from the LM working group recommended assessment using three elements: standardized neurologic examination, CSF cytology or flow cytometry, and radiographic evaluation8 (Appendix Table A1, online only). Steroid dose contributed to assessment only in hematologic cancers. Although cytologic responses can be clinically relevant, RANO-LM also recognized that the sensitivity of CSF cytology is poor. As the neuroimaging features of LM varies from patient to patient, many radiographic features contribute to radiographic assessment on the basis of change in size and extent of disease, including nodular lesions (defined as measurable when greater than 5 mm × 10 mm), leptomeningeal enhancement, cranial nerve enhancement, hydrocephalus, and nerve root enhancement. Progressive disease is defined by worsening neurologic examination as a result of LM or worsening neuroradiographic assessment. The working group lacked consensus on how to define progression or refractory disease on the basis of CSF cytology or flow cytometry. The current criteria suggest defining progression/refractory disease by conversion of negative to positive cytology or failure to convert positive cytology to negative after induction therapy—although it is unclear how to define response assessment in a patient with persistently positive CSF cytology or flow cytometry who are otherwise clinically and radiographically stable to improved.
OTHER RANO EFFORTS
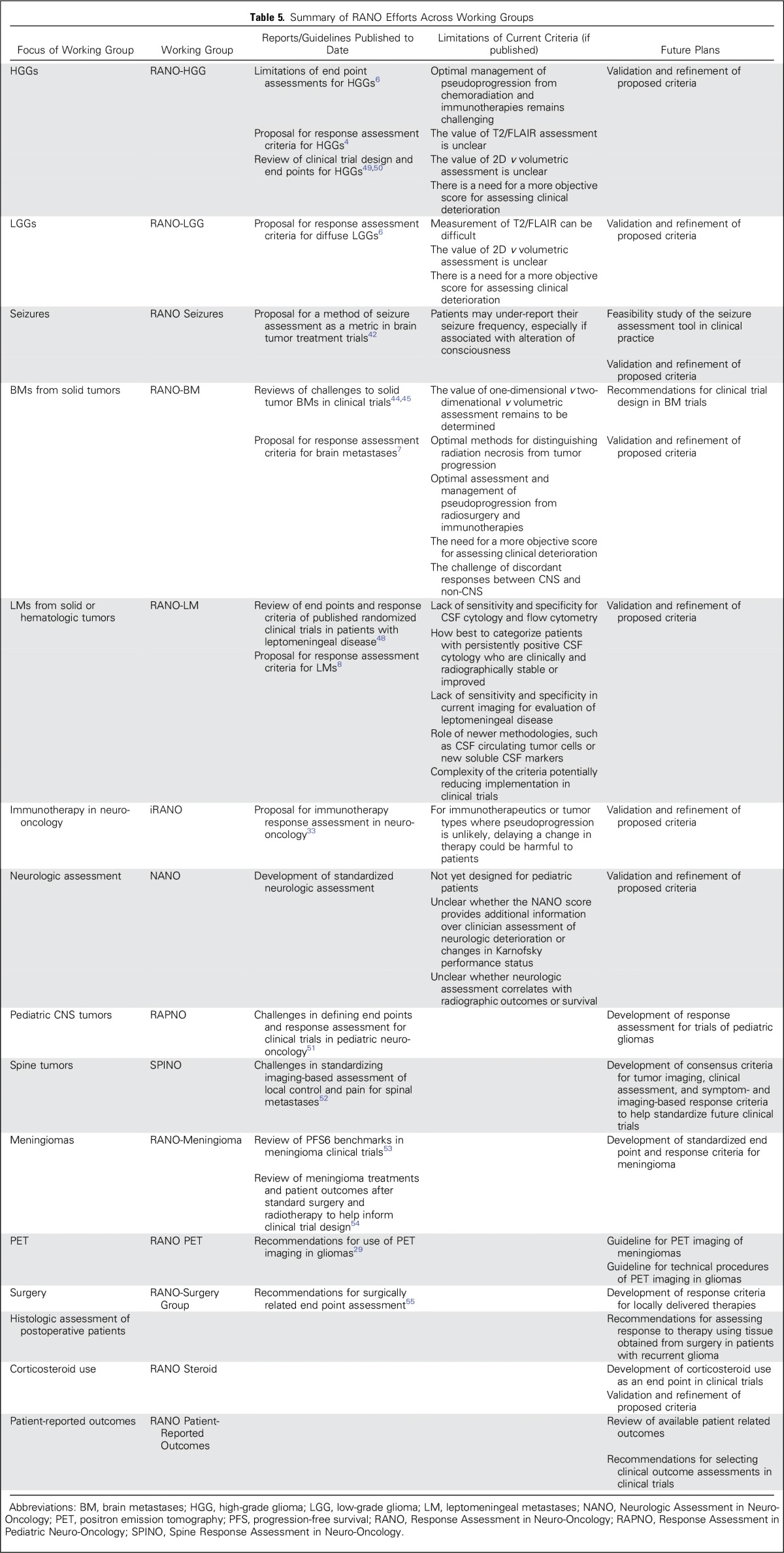
Several other RANO working groups are developing response criteria and clinical trial guidelines for use in neuro-oncology clinical trials.5 These efforts are summarized in Table 5.
Table 5.
Summary of RANO Efforts Across Working Groups
VALIDATION OF RESPONSE CRITERIA
By using RECIST as a model for validating and, ultimately, updating response criteria, each working group will monitor the implementation of their respective guidelines. Thus far, RANO-HGG and RANO-LGG are the standard response criteria used in glioma studies. Increasingly, clinical trials are implementing RANO-BM and NANO criteria. Each working group will use data from these prospective clinical trials to evaluate the usefulness, relevance, and applicability of their criteria, including correlation of response with progression-free survival and overall survival. RANO-BM is currently working with RECIST to analyze historical data sets as a means of validating these criteria.7
FUTURE DIRECTIONS
During the past decade, the efforts of the RANO working group and others to develop consensus criteria for assessing response and progression in CNS tumors, together with the development of the standardized brain tumor imaging protocol, have improved reliability and reduced variability in determining outcomes in neuro-oncology clinical trials. Nonetheless, there are important challenges still to be addressed. The role of advanced imaging, including volumetric measurements and digital subtraction maps, in defining contrast-enhancing tumor need to be determined. The value of dynamic susceptibility contrast and dynamic contrast-enhanced MRI and amino acid positron emission tomography in diagnosing pseudoprogression also requires additional studies, and for these techniques to be incorporated routinely into clinical trials, standardization will be necessary. The value of including T2/FLAIR progression for agents that affect vascular permeability remains to be determined. A number of proposals, such as the iRANO, RANO-BM, NANO, and RANO-LM criteria, require validation in clinical trials, as will newer proposals that address the use of seizures, corticosteroid use, and patient-reported outcomes as potential trial end points. For slow-growing tumors that are unlikely to decrease significantly in size in response to therapy, studies are underway to evaluate whether a reduction in the rate of growth after the initiation of treatment may indicate benefit. These advances in response assessment will hopefully help accelerate the identification of more effective therapies for patients with CNS tumors.
ACKNOWLEDGMENT
We acknowledge the large number of collaborators in the Response Assessment in Neuro-Oncology effort.
Appendix
Fig A1.
Response Assessment in Neuro-Oncology treatment algorithm for the assessment of progressive imaging findings in neuro-oncology patients treated with immunotherapy. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease. Reprinted from Okada et al33 with permission from Elsevier.
Table A1.
Summary of Response Determination in Leptomeningeal Disease
Footnotes
Supported by US National Institutes of Health, National Cancer Institute Grants. No. UM1-CA137443 and P50-CA165962, and US Food and Drug Administration Grant No. R01-FD004400 (to P.Y.W.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: All authors
Administrative support: All authors
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Response Assessment in Neuro-Oncology Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Patrick Y. Wen
Consulting or Advisory Role: Agios, AstraZeneca, Alexion Pharmaceuticals, Genentech, Insys Therapeutics, Monteris Medical, Novartis, Novogen, Vascular Biogenics, VBI Vaccines
Speakers' Bureau: Merck
Research Funding: Agios (Inst), Angiochem (Inst), AstraZeneca (Inst), Genentech (Inst), Novartis (Inst), ImmunoCellular Therapeutics (Inst), Kadmon (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Eli Lilly (Inst), Oncoceutics (Inst), Sanofi (Inst), Vascular Biogenics (Inst)
Susan M. Chang
Consulting or Advisory Role: Agios, Blaize, Neuro-Oncology, Tocagen, Edge
Research Funding: Agios (Inst), Tocagen (Inst), Novartis (Inst), Quest Diagnostics (Inst), Roche (Inst)
Martin J. Van den Bent
Consulting or Advisory Role: AbbVie, Actelion, Blue Earth Diagnostics, Cavion, Roche, Celldex, Bristol-Myers Squibb, Novartis, MSD
Research Funding: AbbVie (Inst)
Michael A. Vogelbaum
Leadership: Infuseon Therapeutics
Stock or Other Ownership: Infuseon Therapeutics
Honoraria: Tocagen, Neuralstem, Medicenna
Research Funding: Tocagen (Inst)
Patents, Royalties, Other Intellectual Property: Patents on a drug delivery device (Inst)
David R. Macdonald
No relationship to disclose
Eudocia Q. Lee
Consulting or Advisory Role: Genentech
REFERENCES
- 1.Miller JJ, Wen PY: Emerging targeted therapies for glioma. Expert Opin Emerg Drugs 21:441-452, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Cloughesy TF, Ellingson BM, et al. : Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials neuroimaging endpoint workshop (January 30, 2014, Bethesda MD). Neuro-oncol 16:vii36-vii47, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Bent MJ, Vogelbaum MA, Wen PY, et al. : End point assessment in gliomas: Novel treatments limit usefulness of classical Macdonald’s Criteria. J Clin Oncol 27:2905-2908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen PY, Macdonald DR, Reardon DA, et al. : Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963-1972, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Chang S, Wen P, Vogelbaum M, et al. : Response Assessment in Neuro-Oncology (RANO): More than imaging criteria for malignant glioma. Neuro-Oncol Pract 2:205-209, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Bent MJ, Wefel JS, Schiff D, et al. : Response Assessment in Neuro-Oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583-593, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Lin NU, Lee EQ, Aoyama H, et al. : Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 16:e270-e278, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain M, Junck L, Brandsma D, et al. : Leptomeningeal metastases: A RANO proposal for response criteria. Neuro-oncol 19:484-492, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayak L, DeAngelis LM, Brandes AA, et al. : The Neurologic Assessment in Neuro-Oncology (NANO) scale: A tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro-oncol 19:625-635, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrom QT, Gittleman H, Xu J, et al. doi: 10.1093/neuonc/now207. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-Oncol 18:v1-v75, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Macdonald DR, Cascino TL, Schold SC, Jr, et al. : Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277-1280, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Shah GD, Kesari S, Xu R, et al. : Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro-oncol 8:38-46, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galanis E, Buckner JC, Maurer MJ, et al. : Validation of neuroradiologic response assessment in gliomas: Measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro-oncol 8:156-165, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren KE, Patronas N, Aikin AA, et al. : Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst 93:1401-1405, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Van den Bent M, Volgelbaum M, Wen P, et al. : End point assessment in gliomas: Novel treatments limit the usefulness of the classical Macdonald’s Criteria. J Clin Oncol 27:2905-2908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandsma D, Stalpers L, Taal W, et al. : Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453-461, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Nowosielski M, Wiestler B, Goebel G, et al. : Progression types after anti-angiogenic therapy are related to outcome in recurrent glioblastoma. Neurology 82:1684-1692, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Ellingson BM, Wen PY, van den Bent MJ, et al. : Pros and cons of current brain tumor imaging. Neuro-oncol 16:vii2-vii11, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reardon DA, Ballman KV, Buckner JC, et al. : Impact of imaging measurements on response assessment in glioblastoma clinical trials. Neuro-oncol 16:vii24-vii35, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellingson BM, Bendszus M, Sorensen AG, et al. : Emerging techniques and technologies in brain tumor imaging. Neuro-oncol 16:vii12-vii23, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellingson BM, Bendszus M, Boxerman J, et al. : Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro-oncol 17:1188-1198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sul J, Krainak DM: Brain tumor clinical trials imaging: A (well-standardized) picture is worth a thousand words. Neuro-oncol 17:1179-1180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandsma D, van den Bent MJ: Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22:633-638, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Radbruch A, Fladt J, Kickingereder P, et al. : Pseudoprogression in patients with glioblastoma: Clinical relevance despite low incidence. Neuro-oncol 17:151-159, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel P, Baradaran H, Delgado D, et al. : MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro-oncol 19:118-127, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boxerman JL, Shiroishi MS, Ellingson BM, et al. : Dynamic susceptibility contrast MR imaging in glioma: Review of current clinical practice. Magn Reson Imaging Clin N Am 24:649-670, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Shiroishi MS, Boxerman JL, Pope WB: Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro-oncol 18:467-478, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert NL, Weller M, Suchorska B, et al. : Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-oncol 18:1199-1208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galldiks N, Law I, Pope WB, et al. : The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. Neuroimage Clin 13:386-394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellingson BM, Wen PY, Cloughesy TF: Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14:307-320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolchok JD, Hoos A, O’Day S, et al. : Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 15:7412-7420, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Okada H, Weller M, Huang R, et al. : Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol 16:e534-e542, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boxerman JL, Ellingson BM: Response assessment and magnetic resonance imaging issues for clinical trials involving high-grade gliomas. Top Magn Reson Imaging 24:127-136, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Huang RY, Rahman R, Ballman KV, et al. : The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res 22:575-581, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Ellingson BM, Lai A, Nguyen HN, et al. : Quantification of nonenhancing tumor burden in gliomas using effective T2 maps derived from dual-echo turbo spin-echo MRI. Clin Cancer Res 21:4373-4383, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorensen AG, Patel S, Harmath C, et al. : Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol 19:551-557, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Ellingson BM, Kim HJ, Woodworth DC, et al. : Recurrent glioblastoma treated with bevacizumab: Contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology 271:200-210, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gahrmann R, van den Bent M, van der Holt B, et al. : NIMG-13. Radiological response assessment in the era of bevacizumab: RANO or volumetry? A report from the BELOB trial. Neuro Oncol 18:vi126, 2016. (suppl 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blakeley JO, Coons SJ, Corboy JR, et al. : Clinical outcome assessment in malignant glioma trials: Measuring signs, symptoms, and functional limitations. Neuro-oncol 18:ii13-ii20, 2016. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cancer Genome Atlas Research Network. Brat DJ, Verhaak RG, et al. : Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481-2498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avila EK, Chamberlain M, Schiff D, et al. : Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro-oncol 19:12-21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AB, Hoogstraten B, Staquet M, et al. : Reporting results of cancer treatment. Cancer 47:207-214, 1981 [DOI] [PubMed] [Google Scholar]
- 44.Lin NU, Lee EQ, Aoyama H, et al. : Challenges relating to solid tumour brain metastases in clinical trials, part 1: Patient population, response, and progression. A report from the RANO group. Lancet Oncol 14:e396-e406, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Lin NU, Wefel JS, Lee EQ, et al. : Challenges relating to solid tumour brain metastases in clinical trials, part 2: Neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol 14:e407-e416, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Arvold ND, Lee EQ, Mehta MP, et al. : Updates in the management of brain metastases. Neuro-oncol 18:1043-1065, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glass JP, Melamed M, Chernik NL, et al. : Malignant cells in cerebrospinal fluid (CSF): The meaning of a positive CSF cytology. Neurology 29:1369-1375, 1979 [DOI] [PubMed] [Google Scholar]
- 48.Chamberlain M, Soffietti R, Raizer J, et al. : Leptomeningeal metastasis: A Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro-oncol 16:1176-1185, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reardon DA, Galanis E, DeGroot JF, et al. : Clinical trial end points for high-grade glioma: The evolving landscape. Neuro-oncol 13:353-361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galanis E, Wu W, Cloughesy T, et al. : Phase 2 trial design in neuro-oncology revisited: A report from the RANO group. Lancet Oncol 13:e196-e204, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Warren KE, Poussaint TY, Vezina G, et al. : Challenges with defining response to antitumor agents in pediatric neuro-oncology: A report from the response assessment in pediatric neuro-oncology (RAPNO) working group. Pediatr Blood Cancer 60:1397-1401, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thibault I, Chang EL, Sheehan J, et al. : Response assessment after stereotactic body radiotherapy for spinal metastasis: A report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol 16:e595-e603, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Kaley T, Barani I, Chamberlain M, et al. : Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro-oncol 16:829-840, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers L, Barani I, Chamberlain M, et al. : Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 122:4-23, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogelbaum MA, Jost S, Aghi MK, et al. : Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) working group. Neurosurgery 70:234-243, discussion 243-244, 2012 [DOI] [PubMed] [Google Scholar]