Abstract
Primary CNS lymphoma (PCNSL) is a rare form of extranodal non-Hodgkin lymphoma that is typically confined to the brain, eyes, and cerebrospinal fluid without evidence of systemic spread. The prognosis of patients with PCNSL has improved during the last decades with the introduction of high-dose methotrexate. However, despite recent progress, results after treatment are durable in half of patients, and therapy can be associated with late neurotoxicity. PCNSL is an uncommon tumor, and only four randomized trials and one phase III trial have been completed so far, all in the first-line setting. To our knowledge, no randomized trial has been conducted for recurrent/refractory disease, leaving many questions unanswered about optimal first-line and salvage treatments. This review will give an overview of the presentation, evaluation, and treatment of immunocompetent patients with PCNSL.
INTRODUCTION
Primary CNS lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma confined to the CNS, including the brain, spine, cerebrospinal fluid (CSF), and eyes. Unlike other brain tumors, it often has a favorable response to both chemotherapy and radiation therapy, but compared with lymphomas outside the CNS, survival is usually inferior. Moreover, the prognosis for PCNSL that has failed first-line therapy remains poor. Although new therapeutic approaches have improved survival, the management of this disease still poses a challenge in neuro-oncology.
EPIDEMIOLOGY
PCNSL can develop in immunosuppressed (HIV/AIDS, organ transplant, immunosuppressive agents) or immunocompetent patients. In this review, we focus on the latter. PCNSL in immunocompetent patients is rare and represents 4% of all intracranial neoplasms and 4% to 6% of all extranodal lymphomas.1 However, in recent years, a rising incidence has been recognized, particularly in patients older than 60 years, with an incidence rate of 0.5 per 100,000 per year.2 Approximately 1,500 new patients are diagnosed each year in the United States.
CLINICAL PRESENTATION AND DIAGNOSTICS
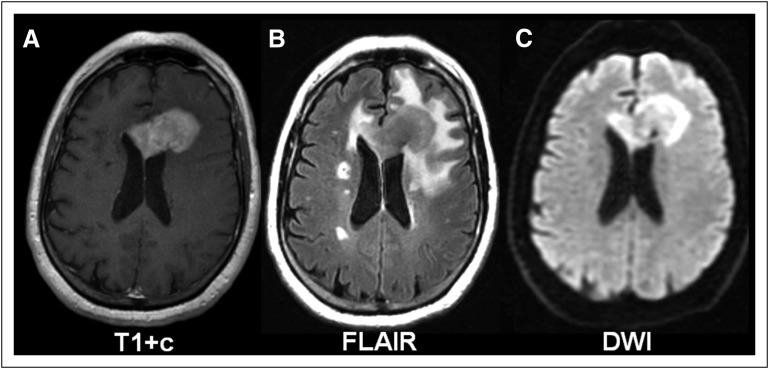
Patients with PCNSL develop neurologic signs over weeks, including focal neurologic deficits (56% to 70%), mental status and behavioral changes (32% to 43%), symptoms of increased intracranial pressure (headaches, nausea, vomiting, papilledema, 32% to 33%), and seizures (11% to 14%),3 depending on the site of CNS involvement. Imaging usually reveals a homogenously enhancing mass lesion (Fig 1), most often a single brain lesion (66%), with a supratentorial location (87%) and involvement of the frontoparietal lobes (39%).3 Less frequently, eyes (15% to 25%),4 CSF (7% to 42%),5-7 and only in rare cases, the spinal cord are involved. To assess the extent of disease, the International PCNSL Collaborative Group recommends baseline staging, including magnetic resonance imaging of the brain (and spine, if spinal symptoms are present), ophthalmologic evaluation, and CSF evaluation.8 To detect the presence of non-CNS disease, a body positron emission tomography/computed tomography scan and bone marrow biopsy should be performed. The diagnostic procedure of choice to establish the diagnosis of PCNSL is a stereotactic biopsy, or, if ocular or CSF involvement is evident, vitrectomy or CSF cytology might be sufficient.
Fig 1.
Characteristic primary CNS lymphoma imaging pattern on magnetic resonance imaging. (A) T1 sequence with gadolinium contrast (T1+c) demonstrates a single, frontal, homogenously enhancing brain lesion. (B) Fluid-attenuated inversion recovery (FLAIR) sequence visualizes a comparatively small area of edema surrounding the mass lesion. (C) Diffusion-weighted imaging (DWI) demonstrates restricted diffusion within the tumor.
PATHOLOGY AND PATHOPHYSIOLOGY
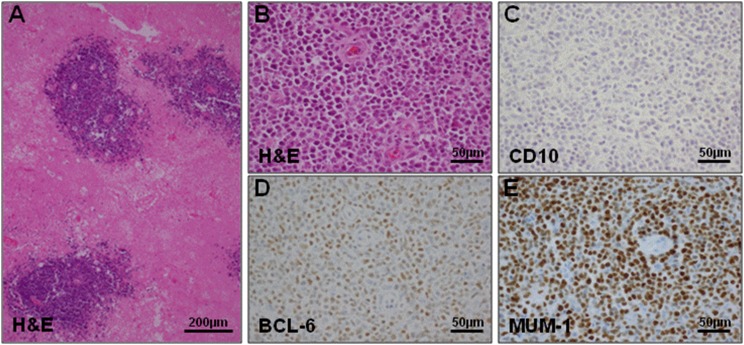
Pathology reveals highly proliferative tumor cells in an angiocentric growth pattern, diffusely infiltrating the CNS (Fig 2A-2B). Most PCNSLs are diffuse large B-cell lymphoma (DLBCL; 90%) and, rarely, Burkitt, low-grade, or T-cell lymphoma.9 Gene-expression profiling has identified three molecular subgroups of non-CNS DLBCL, including the germinal center B-cell–like, activated B-cell–like, and type 3 subgroups.10 Staining of PCNSL biopsies with antibodies that distinguish these DLBCL subgroups11 showed that the vast majority of PCNSLs were nongerminal center subtype12 (Figs 2C-2E). Outside the CNS, this DLBCL subgroup is associated with worse outcome and frequent mutations in the B-cell receptor pathway.13 In PCNSL cohorts,14-18 the B-cell receptor signaling axis, with its downstream target, NFκB, is affected by frequent recurrent mutations, mainly in MYD88 and CD79B. This suggests a central role of this pathway in PCNSL maintenance. Recently, copy number gains at chromosome 9p24.1, the programmed death ligand 1/programmed death ligand 2 locus, have been described, suggesting that immune evasion might play a role in PCNSL.18
Fig 2.
Histologic features of primary CNS lymphoma (PCNSL). (A) Hematoxylin/eosin (H&E) staining of a PCNSL biopsy sample demonstrating the angiocentric growth pattern of PCNSL. (B) Higher magnification H&E shows that the blood vessels are surrounded by infiltrative PCNSL cells. (C, D, and E) Cell-of-origin determination using three immunohistochemical markers (CD10, BCL-6, MUM-1, respectively) and the Hans algorithm.11 The majority of PCNSL are of the nongerminal center subtype and display a similar staining pattern, as shown (CD10 negative [C], BCL-6 positive [D], and MUM-1 positive [E]).
PROGNOSIS
To predict outcome and better stratify patients in clinical trials, two scoring systems are used: the International Extranodal Lymphoma Study Group (IELSG) score6 and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic score.19 The IELSG score uses five parameters (age, Eastern Cooperative Oncology Group performance score, lactate dehydrogenase level, CSF protein concentration, and deep brain involvement). The presence of 0 to 1, 2 to 3, or 4 to 5 adverse risk factors correlates with 2-year survival rates of 80%, 48%, or 15%, respectively. The MSKCC score distinguishes three groups on the basis of age and Karnofsky performance status (KPS)—age ≤ 50 years, age older than 50 years plus KPS ≥ 70, or age older than 50 years plus KPS less than 70—which correlate with median overall survival (OS) of 8.5, 3.2, and 1.1 years in an MSKCC population and 5.2, 2.1, and 0.9 years, respectively, in a validation cohort.
INDUCTION THERAPY
Treatment of PCNSL has evolved over the last decades, but no uniform consensus on the optimal treatment regimen exists currently. Experts in the field agree that high-dose methotrexate (HD-MTX) is the backbone of multimodal therapy, including other chemotherapeutic agents with and without radiation. Current controversies include the role of surgery, the optimal upfront chemotherapy regimen, the role of radiation, and treatment of the CSF space.
Surgery
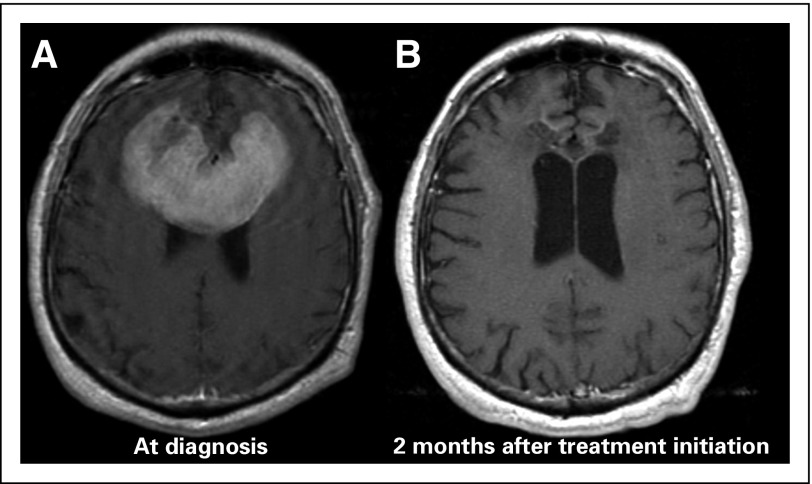
The role of surgery in PCNSL is generally restricted to stereotactic biopsy due to widespread and diffusely infiltrative tumor growth. A surgical resection increases the risk of permanent neurologic deficits in a disease that often involves deep structures and is highly chemosensitive (Fig 3). No survival benefit from subtotal or gross total resection has been observed in retrospective studies,3,20,21 but recently, this view has been challenged by a subset analysis of the German PCNSL Study Group-1 trial,22 which reported improved clinical outcomes for patients undergoing subtotal or gross total resection. The survival benefit was lost when adjusted for the total number of lesions. Currently, there is insufficient evidence to recommend an aggressive surgical approach, including resection, to PCNSL.
Fig 3.
PCNSL is highly chemosensitive. (A) Magnetic resonance imaging (T1+gadolinium) demonstrates a large, frontal-enhancing brain lesion. (B) Follow-up magnetic resonance imaging demonstrates resolution of the large lesion 2 months after treatment initiation with a high-dose methotrexate-based regimen.
Upfront Regimen
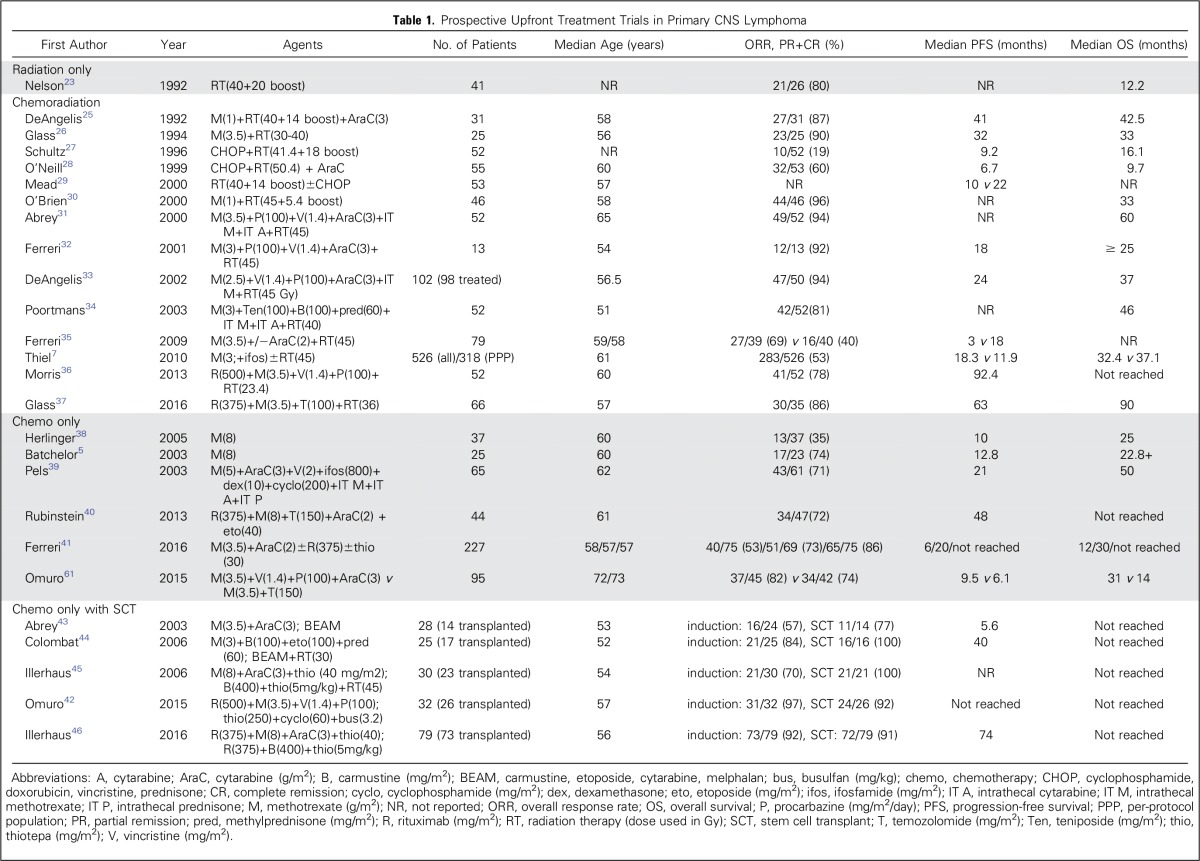
Until the early 1980s, whole-brain radiotherapy (WBRT) was used to treat newly diagnosed PCNSL. Overall response rates (ORRs) reached 90%, but OS was limited to 12 to 18 months23,24 (Table 1). Focal radiation resulted in increased relapses in regions excluded from the radiation port, confirming the need for WBRT in PCNSL47 when radiotherapy is used. In the 1980s to 1990s, chemotherapy was added to WBRT, and it became apparent that regimens used in non-CNS DLBCL, such as cyclophosphamide, doxorubicin, vincristine, and prednisone, were ineffective,27,29,48 partly due to inadequate penetration of the blood-brain barrier (BBB). Methotrexate (MTX) penetrates the BBB when administered at high doses (> 1.5 g/m2) as a rapid infusion.49,50 HD-MTX was effective in patients with CNS metastases from lymphoma or lymphoid leukemias, and when added to WBRT, it enhanced response and prolonged survival in PCNSL. High doses of MTX are possible with the concomitant use of leucovorin, which prevents bone marrow and systemic organ damage while limiting the rescue of lymphoma cells in the CNS because of its poor BBB penetration. With the introduction of HD-MTX in combination with WBRT, ORR remained high (71% to 94%) but outcomes improved, with a median OS of 30 to 60 months and 5-year survival rates of 30% to 50%.25,26,30,31,32,33,34 Most studies were single-arm, phase II trials, with the exception of Ferreri et al,35 who demonstrated that the addition of cytarabine to HD-MTX and WBRT improved ORR from 40% to 69% and prolonged progression-free survival (PFS) from 3 to 18 months, suggesting that polychemotherapy is more effective than single agent HD-MTX.
Table 1.
Prospective Upfront Treatment Trials in Primary CNS Lymphoma
With prolonged survival times, patients treated with chemoradiation developed neurotoxicity. Clinically, patients presented with psychomotor slowing, executive and memory dysfunction, behavioral changes, gait ataxia, and incontinence. Imaging demonstrated diffuse white matter disease and cortical-subcortical atrophy. Autopsy data revealed white matter damage with gliosis, thickening of small vessels, and demyelination.52 These changes are mainly attributed to the synergistic toxicity of HD-MTX and WBRT, particularly at WBRT doses > 42 Gy, and are found in up to 40% of all patients with PCNSL treated with chemoradiation and 75% of those ≥ 60 years of age.53
In an effort to reduce neurotoxicity, chemotherapy-only trials were conducted using single-agent HD-MTX5,54 and polychemotherapy regimens,39 demonstrating ORRs of 35% to 74%, with a median OS of 25 to 50 months. The only phase III randomized study conducted in PCNSL examined whether the omission of WBRT affected survival. All patients received HD-MTX with or without ifosfamide, and those who achieved a complete response were randomly assigned to receive 45 Gy WBRT or observation; those patients who failed to achieve a complete response were randomly assigned to receive 45 Gy WBRT or high-dose cytarabine.7 The study failed to meet its predetermined noninferiority end point despite 551 patients being enrolled. There was 34% noncompliance in the WBRT arm contrasting with complete compliance seen in the chemotherapy-alone arm, making the comparison difficult. However, the data demonstrated that patients who received WBRT had a significantly longer PFS of 18 months compared with those who did not receive WBRT (12 months), but there was no difference in OS (32.4 months with WBRT v 37.1 months without WBRT). On the basis of these data and the high risk of neurotoxicity, most physicians eliminate WBRT as part of routine care of patients with PCNSL.
Rituximab, a monoclonal antibody directed against the B-cell surface antigen CD20, dramatically improved response and clinical outcome in DLBCL55 and was incorporated into first-line PCNSL treatment regimens. Rituximab is a large protein, but it can be detected in the CSF at a low level after systemic administration in patients with PCNSL56 and at the tumor site where the BBB is disrupted.57 The IELSG32 trial randomly assigned patients with PCNSLs to receive HD-MTX and cytarabine with or without thiotepa and with or without rituximab first-line treatment followed by WBRT (45 Gy) or high-dose chemotherapy with stem-cell rescue (HDC-ASCT) as consolidation. The results of the first randomization demonstrated that the addition of rituximab to HD-MTX/cytarabine improved ORR (73% v 53%) and median PFS (20 v 6 months).41 Moreover, the addition of thiotepa to rituximab and HD-MTX/cytarabine (MATRix regimen) further improved ORR to 86%, and median PFS has not been reached. Another ongoing randomized trial by the Hemato-Oncologie voor Volwassenen Nederland/Australasian Leukaemia and Lymphoma Group (HOVON/ALLG; EudraCT, No. 2009-014722-42) is also addressing the role of rituximab in PCNSL by randomly assigning patients to receive HD-MTX, teniposide, carmustine (BCNU), and prednisone (MVBP) with or without rituximab, followed by cytarabine and WBRT consolidation. This trial has not yet reported outcome data, but retrospective data58 and the MATRix trial highly suggest that the addition of rituximab to induction therapy is beneficial in patients with PCNSL.
To address whether reduced-dose WBRT for consolidation leads to less neurotoxicity with durable disease control, we used rituximab, HD-MTX, vincristine, and procarbazine (R-MVP) followed by reduced-dose WBRT (23.4 Gy) in a phase II single-institution study. We demonstrated no cognitive impairment clinically or on formal psychometric testing, with an ORR of 78% and a median PFS of 7.7 years.36 This approach is being tested by the RTOG (Radiation Therapy Oncology Group)/NRG (NCT01399372) in a randomized phase II setting in which all patients will continue to receive psychometric testing to assess the cognitive consequences of relapse as well as treatment.
The multicenter Cancer and Leukemia Group B (CALGB) study 50202 used rituximab, HD-MTX, and temozolomide (R-MT), followed by consolidation with high-dose etoposide and cytarabine. An ORR of 72% with a median PFS of 48 months was observed, comparable to results achieved with combined chemoradiation.59 Interestingly, RTOG 0227 used a similar R-MT regimen followed by WBRT consolidation. Only 66% of patients were assessable for radiographic response, but an ORR of 86% was observed, with a median PFS of 90 months.37 The R-MT regimen is currently being used in a randomized phase II CALGB trial (NCT01399372) in which all patients will receive R-MT followed by consolidation with etoposide/cytarabine or HDC-ASCT.
To further intensify consolidation, particularly in patients with complete or significant partial response, HDC-ASCT may improve disease control by higher CNS drug concentrations, circumventing chemoresistance mediated by the BBB. Different conditioning regimens have led to varied outcomes, although thiotepa-based treatments have demonstrated better clinical results43,44,60 compared with the more commonly used BCNU-based regimens (BCNU, etoposide, cytarabine, melphalan [BEAM] or cyclophosphamide, etoposide, BCNU [CBV]).43 Recently, two studies—one using rituximab, HD-MTX, thiotepa, and cytarabine46 and the other using R-MVP61 as induction regimens, and HDC-ASCT as consolidation (both with thiotepa-based conditioning)—demonstrated high ORR (> 90%) and prolonged PFS (> 74 months), suggesting that HDC-ASCT is a promising consolidative strategy, but this approach is limited to patients with adequate organ function and might exclude elderly patients.
Currently, HD-MTX (> 3 g/m2) and rituximab should be part of any induction treatment. Regimens currently used for induction are R-MVP, R-MT, MATRix, or R-MVBP, depending on geographic region and physician preference. No comparison study has been conducted thus far. The only comparison study compared HD-MTX and temozolomide with HD-MTX, vincristine, and procarbazine (MVP) in an elderly population (age ≥ 60 years) in a multicenter phase II trial. Toxicity profiles were similar between the groups. ORR was 82% in the MVP group and 71% in the HD-MTX and temozolomide group, and median OS was 31 and 14 months, respectively. Although these trends were not statistically significant, the results favor the MVP regimen.61 For consolidation, radiation (23.4 or 45 Gy), conventional chemotherapy (cytarabine, etoposide plus cytarabine), HDC-ASCT (in younger patients and patients with adequate organ function), or observation (in elderly patients or those unable to tolerate additional treatment) is used. Ongoing trials that randomly assign patients to different consolidation treatments will hopefully shed more light on the optimal consolidation regimen. In addition, age and response to induction therapy should be used to guide the choice of consolidation.
Treatment of CSF Space
No consensus exists regarding the role of intraventricular or intrathecal (IT) chemotherapy. The CSF can be a sanctuary for lymphoma cells and potentially contribute to treatment failure and early relapses. Longer exposure to cytotoxic concentrations in the CSF can be achieved with IT drug administration, but it can enhance neurotoxicity.62 No benefits in response rates or survival by adding IT chemotherapy have been demonstrated in retrospective studies,63,64 and a high Ommaya infection rate has been observed in at least one study. IT chemotherapy is not a routine part of any induction regimen at this time. IT rituximab alone or in combination with MTX was associated with response in both recurrent leptomeningeal and subependymal disease.65,66 There are currently no data to suggest that newly diagnosed patients with PCNSL with or without CSF or ocular disease should be treated differently.
TREATMENT OF RECURRENT PCNSL
Disease recurrence is commonly observed in patients with PCNSL and rarely occurs outside the CNS. Despite advances in initial treatment, up to half of patients relapse and 10% to 15% have primary refractory disease.67 Patients with primary refractory or relapsed PCNSL have a poor prognosis, with median survival of 2 months without additional treatment.68 Median time to relapse is 10 to 18 months, and most relapses occur within the first 2 years of initial diagnosis.67 Moreover, relapsing disease has been observed more than 5 years after initial diagnosis.69 The optimal salvage regimen for patients with recurrent or refractory PCNSL has not been established. No randomized trials have been conducted so far in this patient population, in part because of (1) limited insights into the pathophysiology of this disease pointing to specific drug targets and (2) the heterogeneous sites of recurrence (brain, CSF, eyes, or a combination thereof), number of recurrences, and age at recurrence.
Numerous small retrospective studies have been conducted (Table 2). WBRT and HD-MTX rechallenge seem to be effective. Rechallenging patients with MTX led to an ORR of 85% to 91%,74,80 with median OS of 41 to 62 months. WBRT was associated with an ORR of 74% to 79% and median OS of 10 to 16 months,75,76 and might be considered in patients who have not received it as a part of initial therapy. The efficacy of HD-MTX rechallenge or WBRT has not been evaluated in prospective studies so far, but HD-MTX rechallenge can be considered as the most frequently used treatment regimen in patients with recurrent PCNSL, especially when there was a long period of remission after initial HD-MTX therapy and the patient has responded to HD-MTX before.
Table 2.
Salvage Regimen in Primary CNS Lymphoma
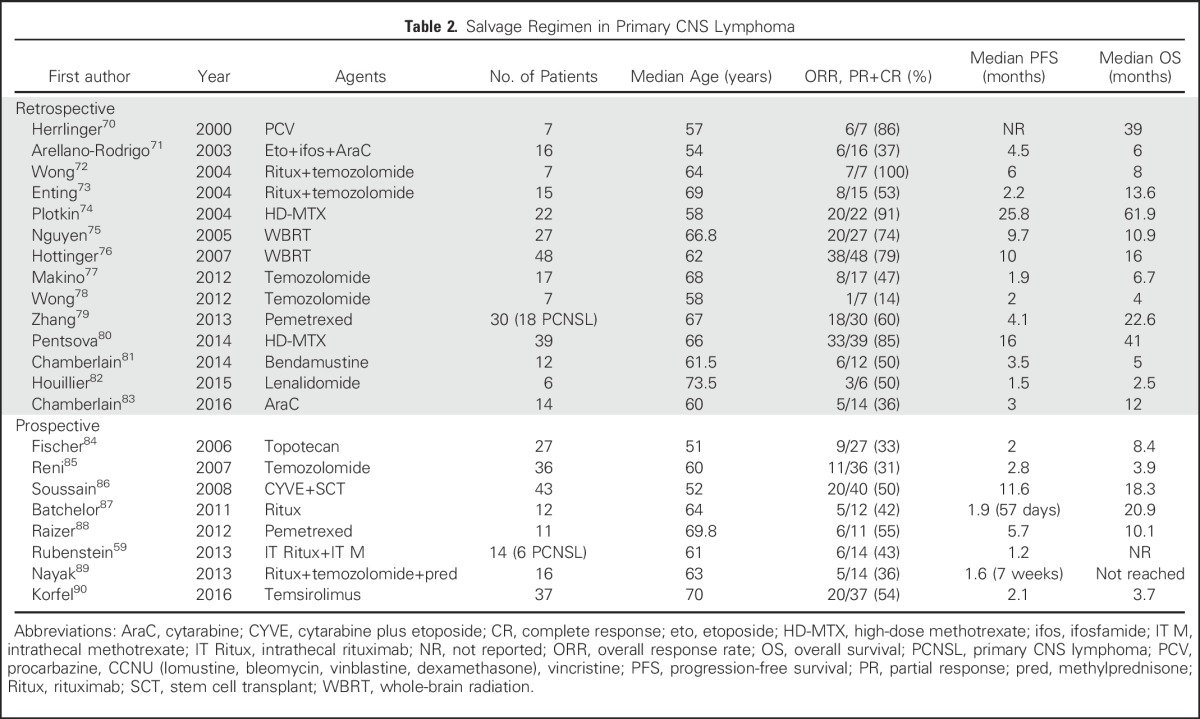
Prospective trials using single agents such as pemetrexed,88 topotecan,84 and temozolomide,85 as well as rituximab,87,89 have demonstrated ORRs of 31% to 55% with limited median PFS of 1.6 to 5.7 months (Table 2). Moreover, promising outcomes observed in retrospective reports were not confirmed in prospective trials (eg, rituximab in combination with temozolomide). Rubenstein et al66 investigated an IT regimen of rituximab (10 or 25 mg) twice a week in combination with once-weekly MTX (12 mg) in patients with recurrent PCNSL. Toxicities were limited to lymphopenia, paresthesias, chills, hypertension, and rigors. A response rate of 43% was observed. This treatment regimen can be considered in patients who can no longer tolerate systemic options. A French prospective multicenter trial of high-dose etoposide/cytarabine followed by HDC-ASCT demonstrated a median PFS of 11.6 months and 2-year OS of 45%.86 The age of participants was limited to those younger than 65 years of age. HDC-ASCT is not feasible in most elderly patients, limiting this treatment approach to younger patients with recurrent PCNSL.
Increased insight into the pathophysiology of PCNSL has led to the introduction of targeted agents in the treatment of disease recurrence. The first targeted agent was the mammalian target of rapamycin inhibitor temsirolimus in a German multicenter phase II trial.90 Treatment was associated with an ORR of 54%, but median PFS was only 2.1 months. Additional targeted agents are being investigated currently. At the American Society of Hematology 2016 meeting, an entire section was dedicated to the results of targeted agents in this patient population. Two studies used the Bruton tyrosine kinase inhibitor, ibrutinib, at 560 and 840 mg daily. In the 560-mg trial, patients with recurrent PCNSL or ocular lymphoma were enrolled, and the first 18 patients had three complete and seven partial responses after 2 months of treatment.91 In the 840-mg trial, 20 patients with recurrent PCNSL and secondary CNS lymphoma achieved an ORR (complete and partial) of 75% (77% in PCNSL and 71% in secondary CNS lymphoma) and a median PFS of 5.4 months at a median follow-up of 255 days.92 In both trials, as well as in an additional trial combining ibrutinib with temozolomide, doxorubicin, etoposide, dexamethasone, and rituximab (DA-TEDDI-R; NCT02203526), pulmonary and cerebral aspergillosis were observed. A trial using lenalidomide in combination with rituximab maintenance had an ORR of 67% and median PFS of 8.1 months (45 of 50 patients were evaluable for response).93 These high response rates are encouraging, and additional trials with targeted inhibitors (NCT02669511, NCT01722305), combining targeted agents with conventional chemotherapy (NCT02315326) and checkpoint inhibitors (NCT02779101, NCT02857426) are ongoing.
As with the upfront regimens, age, performance status, previous therapies, and duration of prior response are guides to the choice of salvage treatment until data from randomized clinical trials will identify the optimal treatment regimen for patients with recurrent CNS lymphoma.
OUTLOOK
Significant progress has been made in the treatment of PCNSL over the last decades. We now anticipate that up to half of the newly diagnosed patients with PCNSL will have long-term control, even though rare relapses more than 10 years after diagnosis69 have been reported. The current focus is to optimize upfront treatment to reduce the number of refractory patients, to prolong remission, and to increase treatment options for patients with recurrent PCNSL. In addition, the growth of the elderly population and increase in elderly patients with PCNSL demands trials targeting this patient population in particular.
ACKNOWLEDGMENT
The authors thank Judith Lampron for her editorial assistance.
Footnotes
Supported by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant No. P30 CA008748, Lymphoma Research Foundation Career Development Award, Memorial Sloan Kettering Brain Tumor Center, Susan and Peter Solomon Divisional Fund, Geoffrey Beene Cancer Research Center, and Cycle for Survival Equinox Innovation Award.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Primary CNS Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Christian Grommes
No relationship to disclose
Lisa M. DeAngelis
Consulting or Advisory Role: Juno Therapeutics, Sapience, Roche, CarThera
REFERENCES
- 1.Villano JL, Koshy M, Shaikh H, et al. : Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 105:1414-1418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill BP, Decker PA, Tieu C, et al. : The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol 88:997-1000, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataille B, Delwail V, Menet E, et al. : Primary intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg 92:261-266, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Grimm SA, Pulido JS, Jahnke K, et al. : Primary intraocular lymphoma: An International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol 18:1851-1855, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Batchelor T, Carson K, O’Neill A, et al. : Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07. J Clin Oncol 21:1044-1049, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Ferreri AJ, Blay JY, Reni M, et al. : Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J Clin Oncol 21:266-272, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Thiel E, Korfel A, Martus P, et al. : High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol 11:1036-1047, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Abrey LE, Batchelor TT, Ferreri AJ, et al. : Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034-5043, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Camilleri-Broët S, Martin A, Moreau A, et al. : Primary central nervous system lymphomas in 72 immunocompetent patients: Pathologic findings and clinical correlations. Groupe Ouest Est d’étude des Leucénies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol 110:607-612, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Alizadeh AA, Eisen MB, Davis RE, et al. : Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Hans CP, Weisenburger DD, Greiner TC, et al. : Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275-282, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Camilleri-Broët S, Crinière E, Broët P, et al. : A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood 107:190-196, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Pasqualucci L, Dalla-Favera R: The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol 52:67-76, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruno A, Boisselier B, Labreche K, et al. : Mutational analysis of primary central nervous system lymphoma. Oncotarget 5:5065-5075, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Tateishi K, Niwa T, et al. : Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 42:279-290, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Braggio E, Van Wier S, Ojha J, et al. : Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res 21:3986-3994, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vater I, Montesinos-Rongen M, Schlesner M, et al. : The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia 29:677-685, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Chapuy B, Roemer MG, Stewart C, et al. : Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 127:869-881, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrey LE, Ben-Porat L, Panageas KS, et al. : Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 24:5711-5715, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Reni M, Ferreri AJ, Garancini MP, et al. : Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: Results of a critical review of the literature. Ann Oncol 8:227-234, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Bellinzona M, Roser F, Ostertag H, et al. : Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: A series of 33 cases. Eur J Surg Oncol 31:100-105, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Weller M, Martus P, Roth P, et al. : Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro-oncol 14:1481-1484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson DF, Martz KL, Bonner H, et al. : Non-Hodgkin’s lymphoma of the brain: Can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys 23:9-17, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Shibamoto Y, Ogino H, Hasegawa M, et al. : Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys 62:809-813, 2005 [DOI] [PubMed] [Google Scholar]
- 25.DeAngelis LM, Yahalom J, Thaler HT, et al. : Combined modality therapy for primary CNS lymphoma. J Clin Oncol 10:635-643, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Glass J, Gruber ML, Cher L, et al. : Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: Long-term outcome. J Neurosurg 81:188-195, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Schultz C, Scott C, Sherman W, et al. : Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: Initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol 14:556-564, 1996 [DOI] [PubMed] [Google Scholar]
- 28.O'Neill BP, Wang CH, O'Fallon JR, et al. Primary central nervous system non-Hodgkin's lymphoma (PCNSL): Survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86-72-52. Int J Radiat Oncol Biol Phys. 1999;43:559–63. doi: 10.1016/s0360-3016(98)00450-7. [DOI] [PubMed] [Google Scholar]
- 29.Mead GM, Bleehen NM, Gregor A, et al. : A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: Cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer 89:1359-1370, 2000 [PubMed] [Google Scholar]
- 30.O’Brien P, Roos D, Pratt G, et al. : Phase II multicenter study of brief single-agent methotrexate followed by irradiation in primary CNS lymphoma. J Clin Oncol 18:519-526, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Abrey LE, Yahalom J, DeAngelis LM: Treatment for primary CNS lymphoma: The next step. J Clin Oncol 18:3144-3150, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Ferreri AJ, Reni M, Dell’Oro S, et al. : Combined treatment with high-dose methotrexate, vincristine and procarbazine, without intrathecal chemotherapy, followed by consolidation radiotherapy for primary central nervous system lymphoma in immunocompetent patients. Oncology 60:134-140, 2001 [DOI] [PubMed] [Google Scholar]
- 33.DeAngelis LM, Seiferheld W, Schold SC, et al. : Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 20:4643-4648, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. : High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol 21:4483-4488, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Ferreri AJ, Reni M, Foppoli M, et al. : High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 374:1512-1520, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Morris PG, Correa DD, Yahalom J, et al. : Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long-term outcome. J Clin Oncol 31:3971-3979, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass J, Won M, Schultz CJ, et al. : Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG Oncology RTOG 0227. J Clin Oncol 34:1620-1625, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrlinger U, Küker W, Uhl M, et al. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: Final report. Ann Neurol. 2005;57:843–847. doi: 10.1002/ana.20495. [DOI] [PubMed] [Google Scholar]
- 39.Pels H, Schmidt-Wolf IG, Glasmacher A, et al. : Primary central nervous system lymphoma: Results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol 21:4489-4495, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013;31:3061–8. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreri AJ, Cwynarski K, Pulczynski E, et al. : Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 3:e217-e227, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Omuro A, Correa DD, DeAngelis LM, et al. : R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 125:1403-1410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrey LE, Moskowitz CH, Mason WP, et al. : Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: An intent-to-treat analysis. J Clin Oncol 21:4151-4156, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Colombat P, Lemevel A, Bertrand P, et al. : High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: A multicenter phase II study of the GOELAMS group. Bone Marrow Transplant 38:417-420, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Illerhaus G, Marks R, Ihorst G, et al. : High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol 24:3865-3870, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Illerhaus G, Kasenda B, Ihorst G, et al. : High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: A prospective, single-arm, phase 2 trial. Lancet Haematol 3:e388-e397, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Shibamoto Y, Hayabuchi N, Hiratsuka J, et al. : Is whole-brain irradiation necessary for primary central nervous system lymphoma? Patterns of recurrence after partial-brain irradiation. Cancer 97:128-133, 2003 [DOI] [PubMed] [Google Scholar]
- 48.O’Neill BP, O’Fallon JR, Earle JD, et al. : Primary central nervous system non-Hodgkin’s lymphoma: Survival advantages with combined initial therapy? Int J Radiat Oncol Biol Phys 33:663-673, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Shapiro WR, Young DF, Mehta BM: Methotrexate: Distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med 293:161-166, 1975 [DOI] [PubMed] [Google Scholar]
- 50.Borsi JD, Moe PJ: A comparative study on the pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer 60:5-13, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Glass J, Gruber ML, Cher L, et al. : Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: Long-term outcome. J Neurosurg 81:188-195, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Omuro AM, Ben-Porat LS, Panageas KS, et al. : Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 62:1595-1600, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Gavrilovic IT, Hormigo A, Yahalom J, et al. : Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol 24:4570-4574, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Reference deleted.
- 55.Coiffier B, Lepage E, Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Ruhstaller TW, Amsler U, Cerny T: Rituximab: Active treatment of central nervous system involvement by non-Hodgkin’s lymphoma? Ann Oncol 11:374-375, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Iwamoto FM, Schwartz J, Pandit-Taskar N, et al. : Study of radiolabeled indium-111 and yttrium-90 ibritumomab tiuxetan in primary central nervous system lymphoma. Cancer 110:2528-2534, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Holdhoff M, Ambady P, Abdelaziz A, et al. : High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 83:235-239, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubenstein JL, Hsi ED, Johnson JL, et al. : Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 31:3061-3068, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montemurro M, Kiefer T, Schüler F, et al. : Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: Results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol 18:665-671, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Omuro A, Chinot O, Taillandier L, et al. : Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: An intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol 2:e251-e259, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Brugnoletti F, Morris EB, Laningham FH, et al. : Recurrent intrathecal methotrexate induced neurotoxicity in an adolescent with acute lymphoblastic leukemia: Serial clinical and radiologic findings. Pediatr Blood Cancer 52:293-295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sierra Del Rio M, Ricard D, Houillier C, et al. : Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol 106:143-146, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Khan RB, Shi W, Thaler HT, et al. : Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol 58:175-178, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Rubenstein JL, Fridlyand J, Abrey L, et al. : Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 25:1350-1356, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Rubenstein JL, Li J, Chen L, et al. : Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 121:745-751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahnke K, Thiel E, Martus P, et al. : Relapse of primary central nervous system lymphoma: Clinical features, outcome and prognostic factors. J Neurooncol 80:159-165, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Reni M, Ferreri AJM, Villa E: Second-line treatment for primary central nervous system lymphoma. Br J Cancer 79:530-534, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nayak L, Hedvat C, Rosenblum MK, et al. : Late relapse in primary central nervous system lymphoma: Clonal persistence. Neuro-oncol 13:525-529, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrlinger U, Brugger W, Bamberg M, et al. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology. 2000;54:1707–8. doi: 10.1212/wnl.54.8.1707. [DOI] [PubMed] [Google Scholar]
- 71.Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–24. doi: 10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 72.Wong ET, Tishler R, Barron L, et al. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101:139–45. doi: 10.1002/cncr.20339. [DOI] [PubMed] [Google Scholar]
- 73.Enting RH, Demopoulos A, DeAngelis LM, et al. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901–3. doi: 10.1212/01.wnl.0000137050.43114.42. [DOI] [PubMed] [Google Scholar]
- 74.Plotkin SR, Betensky RA, Hochberg FH, et al. : Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 10:5643-5646, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Nguyen PL, Chakravarti A, Finkelstein DM, et al. : Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol 23:1507-1513, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Hottinger AF, DeAngelis LM, Yahalom J, et al. : Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology 69:1178-1182, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Makino K, Nakamura H, Hide T, et al. Salvage treatment with temozolomide in refractory or relapsed primary central nervous system lymphoma and assessment of the MGMT status. J Neurooncol. 2012;106:155–60. doi: 10.1007/s11060-011-0652-z. [DOI] [PubMed] [Google Scholar]
- 78.Wong SF, Gan HK, Cher L. A single centre study of the treatment of relapsed primary central nervous system lymphoma (PCNSL) with single agent temozolomide. J Clin Neurosci. 2012;19:1501–5. doi: 10.1016/j.jocn.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Zhang JP, Lee EQ, Nayak L, et al. Retrospective study of pemetrexed as salvage therapy for central nervous system lymphoma. J Neurooncol. 2013;115:71–7. doi: 10.1007/s11060-013-1196-1. [DOI] [PubMed] [Google Scholar]
- 80.Pentsova E, Deangelis LM, Omuro A: Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol 117:161-165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chamberlain MC. Salvage therapy with bendamustine for methotrexate refractory recurrent primary CNS lymphoma: A retrospective case series. J Neurooncol. 2014;118:155–62. doi: 10.1007/s11060-014-1411-8. [DOI] [PubMed] [Google Scholar]
- 82.Houillier C, Choquet S, Touitou V, et al. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology. 2015;84:325–6. doi: 10.1212/WNL.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 83.Chamberlain MC. High-dose cytarabine salvage therapy for recurrent primary CNS lymphoma. J Neurooncol. 2016;126:545–50. doi: 10.1007/s11060-015-1994-8. [DOI] [PubMed] [Google Scholar]
- 84.Fischer L, Thiel E, Klasen HA, et al. : Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol 17:1141-1145, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Reni M, Zaja F, Mason W, et al. : Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer 96:864-867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soussain C, Hoang-Xuan K, Taillandier L, et al. : Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 26:2512-2518, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Batchelor TT, Grossman SA, Mikkelsen T, et al. : Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 76:929-930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raizer JJ, Rademaker A, Evens AM, et al. : Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer 118:3743-3748, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Nayak L, Abrey LE, Drappatz J, et al. : Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma 54:58-61, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korfel A, Schlegel U, Herrlinger U, et al. : Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol 34:1757-1763, 2016 [DOI] [PubMed] [Google Scholar]
- 91. Choquet C, Bijou F, Houot R, et al: Ibrutinib monotherapy in relapse or refractory primary CNS lymphoma (PCNSL) and primary vitreo-retinal lymphoma (PVRL). Result of the interim analysis of the iLOC phase II study from the Lysa and the French LOC Network. American Society of Hematology 58th Annual Meeting, San Diego, CA, December 3-6, 2016 (abstr) [Google Scholar]
- 92. Grommes C, Gavrilovic I, Kaley T, et al: Single-agent ibrutinib in recurrent/refractory central nervous system lymphoma. American Society of Hematology 58th Annual Meeting, San Diego, CA, December 3-6, 2016 (abstr) [Google Scholar]
- 93.Ghesquieres H, Houillier C, Chinot O, et al. Rituximab-lenalidomide (REVRI) in relapse or refractory primary central nervous system (PCNSL) or vitreo retinal lymphoma (PVRL): Results of a “proof of concept” phase II study of the French LOC network. American Society of Hematology 58th Annual Meeting; San Diego, CA: December 3-6, 2016 (abstr) [Google Scholar]