Abstract
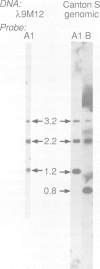
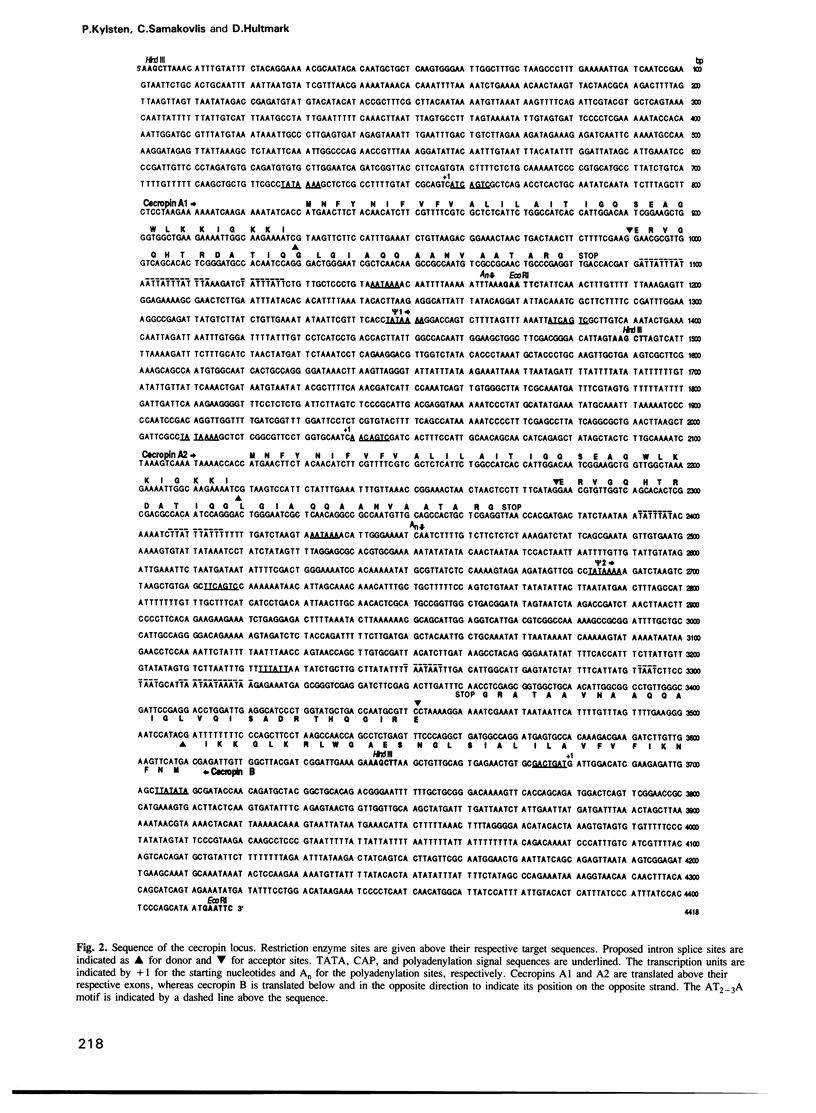
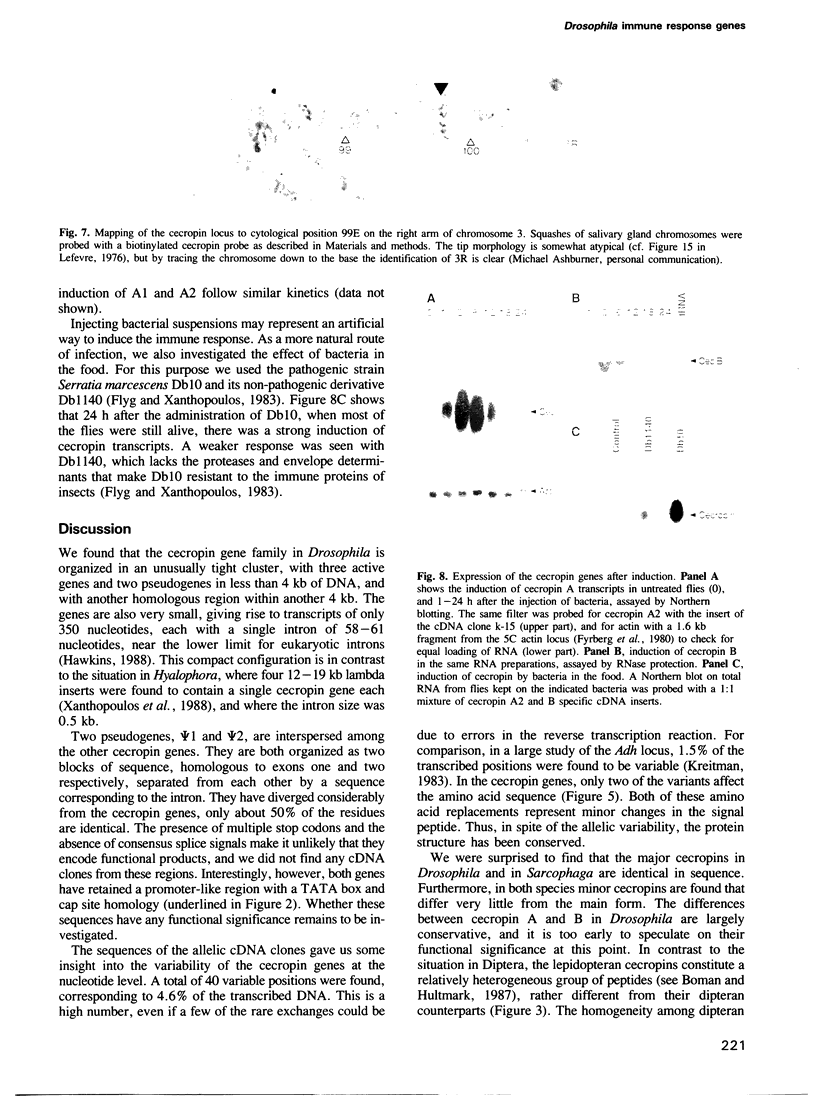
Cecropins are antibacterial peptides that are synthesized in insects as a response to infection. As a first step towards a molecular study of the induction of this response, we have isolated genomic clones that cover the cecropin locus in Drosophila melanogaster. This locus was found to be unique, and it was mapped cytologically to the chromosomal location 99E. Sequence analysis showed it to be unusually compact, with three expressed genes and two pseudogenes within less than 4 kb of DNA, and with another homologous region less than 4 kb away. Two of the genes, A1 and A2, encode a product that is identical to the major cecropin from Sarcophaga peregrina, while the cecropin encoded by the B gene differs in five positions. Cecropin transcripts appear within an hour after bacteria have been injected into the hemocoel, reach a maximum after 2-6 h, and have almost disappeared again after 24 h. The B gene is induced in parallel with the A genes, but on a lower level. The cecropin genes were also induced when the flies were kept on food with the Drosophila pathogenic bacterium Serratia marcescens Db10 or its non-pathogenic derivative Db1140.
Full text
PDF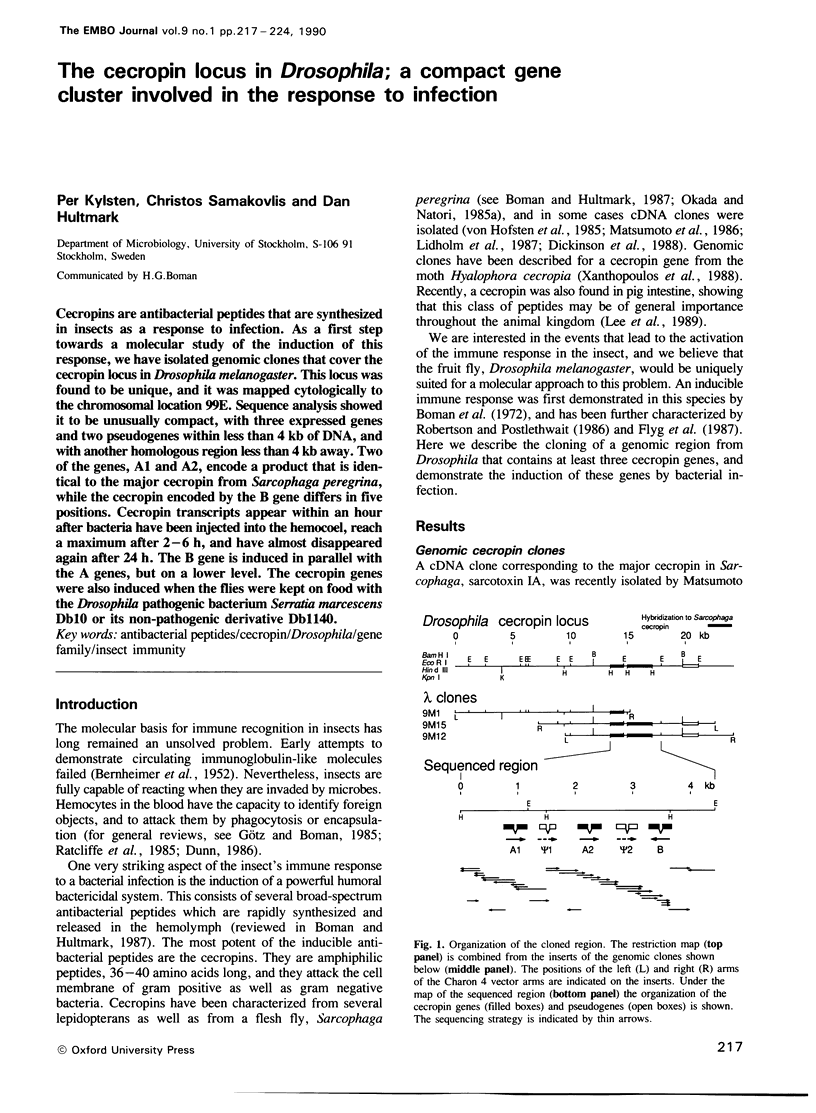





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Sequencing DNA with dideoxyribonucleotides as chain terminators: hints and strategies for big projects. Methods Enzymol. 1987;152:538–556. doi: 10.1016/0076-6879(87)52060-2. [DOI] [PubMed] [Google Scholar]
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Boman H. C., Boman I. A., Andreu D., Li Z. Q., Merrifield R. B., Schlenstedt G., Zimmermann R. Chemical synthesis and enzymic processing of precursor forms of cecropins A and B. J Biol Chem. 1989 Apr 5;264(10):5852–5860. [PubMed] [Google Scholar]
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nilsson I., Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972 May 26;237(5352):232–235. doi: 10.1038/237232a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson L., Russell V., Dunn P. E. A family of bacteria-regulated, cecropin D-like peptides from Manduca sexta. J Biol Chem. 1988 Dec 25;263(36):19424–19429. [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Gubler U. A one tube reaction for the synthesis of blunt-ended double-stranded cDNA. Nucleic Acids Res. 1988 Mar 25;16(6):2726–2726. doi: 10.1093/nar/16.6.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. D. A survey on intron and exon lengths. Nucleic Acids Res. 1988 Nov 11;16(21):9893–9908. doi: 10.1093/nar/16.21.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Hultmark D., Gehring W. J. Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J. 1985 Aug;4(8):2053–2060. doi: 10.1002/j.1460-2075.1985.tb03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Mollay C., Kaschnitz R., Haiml L., Vilas U. Prepromelittin: specific cleavage of the pre- and the propeptide in vitro. Ann N Y Acad Sci. 1980;343:338–346. doi: 10.1111/j.1749-6632.1980.tb47262.x. [DOI] [PubMed] [Google Scholar]
- Kreitman M. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature. 1983 Aug 4;304(5925):412–417. doi: 10.1038/304412a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Okada M., Takahashi H., Ming Q. X., Nakajima Y., Nakanishi Y., Komano H., Natori S. Molecular cloning of a cDNA and assignment of the C-terminal of sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina. Biochem J. 1986 Nov 1;239(3):717–722. doi: 10.1042/bj2390717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Okada M., Natori S. Ionophore activity of sarcotoxin I, a bactericidal protein of Sarcophaga peregrina. Biochem J. 1985 Jul 15;229(2):453–458. doi: 10.1042/bj2290453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Natori S. Primary structure of sarcotoxin I, an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem. 1985 Jun 25;260(12):7174–7177. [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Robertson M., Postlethwait J. H. The humoral antibacterial response of Drosophila adults. Dev Comp Immunol. 1986 Spring;10(2):167–179. doi: 10.1016/0145-305x(86)90001-7. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthopoulos K. G., Lee J. Y., Gan R., Kockum K., Faye I., Boman H. G. The structure of the gene for cecropin B, an antibacterial immune protein from Hyalophora cecropia. Eur J Biochem. 1988 Mar 1;172(2):371–376. doi: 10.1111/j.1432-1033.1988.tb13896.x. [DOI] [PubMed] [Google Scholar]
- van Hofsten P., Faye I., Kockum K., Lee J. Y., Xanthopoulos K. G., Boman I. A., Boman H. G., Engström A., Andreu D., Merrifield R. B. Molecular cloning, cDNA sequencing, and chemical synthesis of cecropin B from Hyalophora cecropia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2240–2243. doi: 10.1073/pnas.82.8.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]