Abstract
Context:
Dexmedetomidine is being increasingly used in nerve blocks. However, there are only a few dose determination studies.
Aims:
To compare two doses of dexmedetomidine, in femoral nerve block, for postoperative analgesia after total knee arthroplasty (TKA).
Settings and Design:
A prospective, randomized, controlled trial was conducted in the Department of Anesthesia at AIIMS, a Tertiary Care Hospital.
Materials and Methods:
Sixty American Society of Anesthesiologists I–II patients undergoing TKA under subarachnoid block were randomized to three Groups A, B, and C. Control Group A received 20 ml (0.25%) of bupivacaine in femoral nerve block. Groups B and C received 1 and 2 μg/kg dexmedetomidine along with bupivacaine for the block, respectively. Outcomes measured were analgesic efficacy measured in terms of visual analog scale (VAS) score at rest and passive motion, duration of postoperative analgesia, and postoperative morphine consumption. Adverse effects of dexmedetomidine were also studied.
Statistical Analysis Used:
All qualitative data were analyzed using Chi-square test and VAS scores using Kruskal–Wallis test. Comparison of patient-controlled analgesia (PCA) morphine consumption and time to first use of PCA were done using ANOVA followed by Least Significant Difference test. A P < 0.05 was considered statistically significant.
Results:
The VAS score at rest was significantly lower in Group C compared to Groups A and B (P < 0.05). There was no difference in VAS score at motion between Groups B and C. The mean duration of analgesia was significantly longer in Group C (6.66 h) compared to Groups A (4.55 h) and B (5.70 h). Postoperative mean morphine consumption was significantly lower in Group C (22.85 mg) compared to Group A (32.15 mg) but was comparable to Group B (27.05 mg). There was no significant difference in adverse effects between the groups.
Conclusion:
The use of dexmedetomidine at 2 μg/kg dose in femoral nerve block is superior to 1 μg/kg for providing analgesia after TKA, although its role in facilitating early ambulation needs further evaluation.
Keywords: Dexmedetomidine for total knee replacement, femoral nerve block for total knee replacement, perineural dexmedetomidine dose
Introduction
Severe pain after total knee arthroplasty (TKA) imposes limitations on early postoperative mobilization, which is crucial in regaining joint function.[1] Inadequately managed postoperative pain can also lead to increased incidence of chronic pain.[2] Peripheral nerve blocks are being used as effective methods of postoperative pain management after knee arthroplasty. Many agents such as epinephrine, opioids, sodium bicarbonate, and clonidine have been used as additives to local anesthetic (LA) in nerve blocks to prolong the duration but with limited success.
Dexmedetomidine, a novel α2-agonist, has been found to significantly prolong the duration of peripheral nerve blocks, with minimal systemic side effects. Yet, the ideal dose of dexmedetomidine to be used in peripheral nerve blocks stands to be determined as there are very few dose comparison studies.
In our study, we compared two different doses of dexmedetomidine along with bupivacaine for the purpose of femoral nerve block for postoperative pain relief after TKA. The postoperative analgesic duration, postoperative opioid consumption, and incidence of any systemic adverse effects were studied.
Materials and Methods
After obtaining permission from the drugs controller general of India for the use of dexmedetomidine in peripheral nerve blocks (F. No. 12-87/12-DC), and the approval of the Institutional Ethics Committee Review Board of the All India Institute of Medical Sciences (RT-31/15.06.2013), the trial was registered in the clinical trials registry of India (CTRI/2013/11/004168). An informed and written consent was obtained from all the participants participating in this randomized, controlled, prospective, double-blinded trial conducted in the Department of Anesthesia, AIIMS, New Delhi. Sixty adult patients, 18–75 years of age, American Society of Anesthesiologists I–II, scheduled to undergo primary unilateral TKA were included in the study.
Exclusion criteria included bilateral or revision TKA, focal neurological deficits, inability to understand the use of visual analog scale (VAS), inability to use the patient-controlled analgesia (PCA) device, morbid obesity (body mass index >40), coagulation disorders, severe spinal deformities, infection at the site of subarachnoid block (SAB) or at the site of femoral nerve block, preexisting peripheral neuropathy, uncontrolled diabetes mellitus, and known hypersensitivity to LA drugs.
A computer-generated random numbers list was used to randomize the participants into one of the three Groups A, B, and C. Thus, the anesthesia provider administering the block, the participant receiving the block, as well as the investigator collecting and analyzing the data were effectively blinded.
In the operating room, standard monitoring such as electrocardiography (ECG), noninvasive blood pressure (NIBP), and pulse oximetry were attached, and intravenous (IV) access was secured. Lactated Ringer's solution was used as the maintenance fluid. Baseline hemodynamic parameters were recorded. Injection midazolam 1 mg was administered IV.
With the patient in sitting position, SAB was given with a 25-gauge spinal needle inserted at the L2–L3 or L3–L4 intervertebral space using 2.5 ml (0.5%) of heavy bupivacaine. The patient was made to lie supine immediately after the spinal block.
Femoral nerve block was administered as per the technique described by Winnie et al.[3] With the patient in the supine position, the ipsilateral extremity was abducted 10°–20°. Under sterile conditions, the pulse of the femoral artery was identified, the nerve stimulator needle, which was set to deliver 2 mA, was inserted cephalad, 45° to skin and at the level of femoral crease, 1–1.5 cm lateral to the femoral artery pulse. The femoral nerve was identified by quadriceps muscle contractions causing ascension of the patella (“dancing patella”). The current was gradually reduced to achieve twitches of the quadriceps muscle at 0.2–0.5 mA, the needle was stabilized at this position and total volume of 20 ml the study drug solution was injected after negative aspiration. Participants in Group A received 0.25% of bupivacaine for femoral nerve block. Participants in Group B received 0.25% of bupivacaine with dexmedetomidine 1 μg/kg. Participants in Group C received 0.25% of bupivacaine with dexmedetomidine 2 μg/kg. A constant volume of LA solution 20 ml was used in all the groups.
Intraoperative hemodynamics and blood loss were recorded. Hypotension (systolic blood pressure <80 mmHg) was managed with IV boluses of 3 mg ephedrine. Bradycardia (pulse rate <50/min) was managed with IV atropine 0.5 mg. All the patients were observed for side effects such as nausea, vomiting, block site hematoma, local anesthetic toxicity (lightheadedness, dizziness, tinnitus, disorientation, drowsiness, generalized muscle twitching, convulsions, respiratory depression, and cardiovascular depression), and postblock neuropathy. All the patients were given injection paracetamol 1 g IV before the end of the procedure.
Postoperatively, the participants were shifted to the recovery room. ECG, NIBP, and SpO2 were monitored every 15 min for the first 2 h and then hourly for the next 8 h. Paracetamol 1 g was infused IV every 6th hourly for the first 24 h. VAS score at rest and during movement was assessed at 0, 4, 8, 12, 24, and 48 h after surgery. A baseline VAS of 4 was aimed in all patients. All patients enrolled in the study were provided with PCA for 24 h postoperatively, capable of delivering boluses of 1 mg morphine, with a lockout interval of 10 min, allowing a maximum dose of 20 mg in 4 h, with no background opioid infusion. PCA morphine consumption was assessed at the end of 24 h. If the patient complained pain (VAS >4) despite the use of PCA, IV morphine 3 mg bolus was given for rescue and was repeated every 20 min till the VAS reached 4.
Quality of postoperative analgesia was assessed using VAS (0–10 scale, 0 - no pain, and 10 - worst possible pain). Pain during rest at 0, 4, 8, 12, 24, and 48 h after surgery and pain on motion (passive knee flexion) at 12, 24, and 48 h after surgery were recorded on VAS scale. Postoperative opioid consumption in terms of total dose of PCA morphine and dose of morphine used for rescue analgesia used were recorded. Duration of analgesia was recorded as the time to first use of PCA from the time of administering the femoral nerve block.
Adverse effects of dexmedetomidine in peripheral nerve block such as nausea, vomiting, pruritus, sedation, hypotension, bradycardia, and neuropathy in the perioperative period were noted.
Statistical analysis
Assuming a significance level (α) of 0.05 and power of 0.9, 15 participants were required in each group to detect a difference between the groups in mean time for the first use of PCA as small as 1.5 times its standard deviation (SD). The sample size was increased to 20 per group to make up for dropouts and for a probable skewed distribution of time for the first PCA use.
All the statistical analyses were carried out using IBM SPSS Statistics for Windows, Version 20.0, (Armonk, NY: IBM Corp. Released 2011). Data were summarized as mean ± SD, median and range, or number and percentage as appropriate. All qualitative data were analyzed using Chi-square test. Comparison of VAS score at rest and at motion and rescue morphine consumption was done using Kruskal–Wallis test since the data followed a nonnormal distribution. Comparison of PCA morphine consumption and time to first use of the PCA pump were done using one-way ANOVA followed by Least Significant Difference test for intergroup comparisons. The P < 0.05 was considered statistically significant.
Results
Sixty patients were included in the study, twenty patients in three groups. The demographic variables were comparable between the groups [Table 1].
Table 1.
Patient demographics
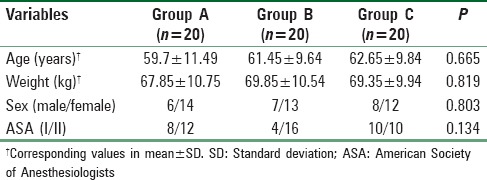
The duration of analgesia as studied by the time to first use of the PCA was significantly longer in Group B (5.70 ± 1.30 h) compared to Group A (4.55 ± 1.47 h). The time was significantly longer in Group C (6.66 ± 1.27 h) compared to both Group A and Group B. The differences were statistically significant. The duration and the mean difference are tabulated in Table 2.
Table 2.
Duration of analgesia
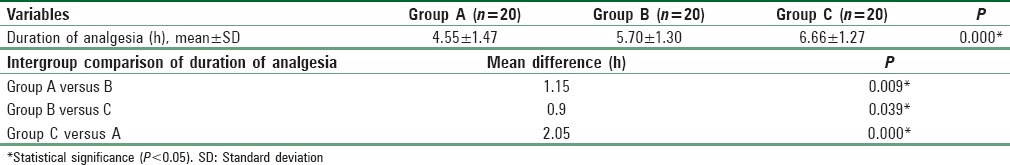
The mean dose of PCA morphine consumed and the mean difference between the groups are tabulated in Table 3. Mean dose of PCA morphine consumed by Group C (22.85 ± 10.26 mg) was significantly lower than Group A (32.15 ± 8.73 mg). There was no statistical difference in the PCA morphine dose between Group A and Group B (27.05 ± 10.6 mg), as well as between Group B and Group C.
Table 3.
Patient-controlled analgesia morphine consumption
The mean dose of rescue morphine received was 3.75 mg in Group A, 2.25 mg in Group B, and 1.5 mg in Group C. The difference between Groups A and C was found to be statistically significant (P = 0.012). There was no significant difference in other intergroup comparisons.
The median VAS score at rest [Chart 1] was significantly lower in Group B compared to Group A at 4 and 8 h. The median VAS score at rest was significantly lower in Group C compared to Group B at 8 and 24 h. The median VAS score at rest was significantly lower in Group C compared to Group A at 4, 8, 12, 24, and 48 h. The above-mentioned differences were statistically significant.
Chart 1.
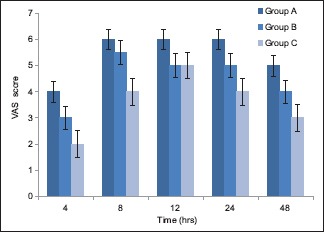
Visual analog scale at rest
The median VAS score at passive motion of knee [Chart 2] was significantly lower in Group B compared to Group A at 48 h. There was no significant difference in VAS scores at motion between Groups B and C at any point of time. The median VAS score at motion was significantly lower in Group C compared to Group A at 48 h.
Chart 2.
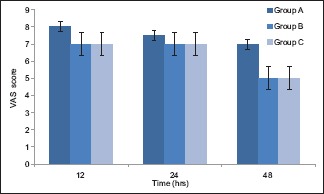
Visual analog scale at motion
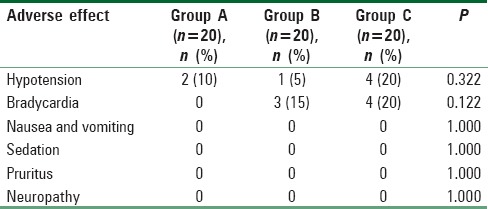
The incidence of intraoperative and postoperative hypotension and bradycardia were not significantly different between the groups [Table 4]. No patients enrolled had clinically significant nausea, vomiting, sedation, pruritus, or neuropathy.
Table 4.
Incidence of adverse effects
Discussion
Dexmedetomidine has been used clinically for sedation and anxiolysis in the Intensive Care Unit and procedural sedation. It has been used perioperatively for premedication to decrease emergence delirium, to attenuate the stress response of anesthesia and surgery, and treatment of postoperative pain.[4] The idea of perineural administration of dexmedetomidine was conceived in the last decade, following the successful use of clonidine in nerve blocks. The perineural action of clonidine is mediated through inhibition of hyperpolarization-activated cation current [I (H)] rather than through α2-mediated mechanism.[5] Dexmedetomidine is a newer α2 adrenergic agonist. Its affinity for binding to the α2/α1 receptor is 1600:1 compared with 220:1 for clonidine. Dexmedetomidine has also been proposed to act by inhibiting I (H) hyperpolarization-activated cation current like clonidine.[6]
The initial animal studies carried out by Brummett et al. showed a significant increase in the duration of analgesia achieved by perineural dexmedetomidine, without significant neurotoxicity.[7,8] This was followed by studies of perineural dexmedetomidine on healthy human volunteers,[9,10] and on surgical patients, most of which consistently demonstrated a prolonged analgesic effect without significant adverse effects.
In our study, we compared two doses of dexmedetomidine, 1 and 2 μg/kg, against a control group receiving plain bupivacaine in femoral nerve block. The duration of postoperative analgesia (mean ± SD) (hours) was 4.55 ± 1.47 h in the control group, 5.7 ± 1.3 h in 1 μg/kg group, and 6.66 ± 1.27 h in 2 μg/kg group. The differences between the groups were statistically significant.
In a similar dose comparison study,[11] authors performed femoral nerve block for knee arthroscopies using 25, 50, and 75 μg of dexmedetomidine with bupivacaine. They found a significant prolongation in duration of analgesia, especially at 50 and 75 μg doses. The durations were 10.8, 11, 21.8, and 28.6 h for control, 25, 50, and 75 μg groups, respectively. Morphine consumption was also significantly lower with dexmedetomidine use, especially at higher doses. However, higher doses were associated with hypotension. The prolonged duration of action can be explained by the less painful nature of arthroscopy compared to joint replacement.
There are many studies regarding the use of dexmedetomidine with various LAs in brachial plexus blocks for various upper limb procedures. Doses ranging from 0.75 to 1.5 μg/kg have been used, and a significant prolongation in the duration of block and analgesia ranging from 400 to over 1000 min have been observed in these studies.[12,13,14,15]
In a study where 1 μg/kg of dexmedetomidine was used in greater palatine nerve block, in children undergoing cleft palate repair, postoperative analgesia of 22 h duration was noted, as against 14 h with plain bupivacaine.[16]
There are other studies that have used dexmedetomidine in epidural,[17,18] caudal analgesia,[19,20] all demonstrating the prolonged analgesic efficacy of perineural dexmedetomidine.
In a study, which used 100 μg dexmedetomidine with bupivacaine for combined femoral-sciatic block for below knee surgeries, a prolonged duration of analgesia of 807 min was observed, significantly longer than plain bupivacaine. However, dexmedetomidine was associated with bradycardia and hypotension.[21]
We were not able to observe such a prolonged duration of analgesia as seen in above studies. This wide variation in the duration of analgesia provided by dexmedetomidine could be attributed to the difference in the location of the block, different doses of LA used, the differences in the nature of surgeries performed, and differences in the methods of pain evaluation. Further, though femoral nerve is the major sensory supply of the knee joint, the contributions of obturator and sciatic nerves remain significant. Since only femoral nerve block was used in our study, this could be another factor for relatively shorter duration of analgesia observed in our study.
In a study similar to ours, femoral perineural catheter was inserted for analgesia following TKA performed under SAB. A bolus of ropivacaine with 1.5 μg/kg dexmedetomidine was injected into the catheter and the time for the first demand bolus of LA through the catheter was counted as the duration of analgesia, which was found to be 346 min, which closely resembles the duration noted in our study.[22]
The postoperative PCA morphine consumption in the first 24 h was 32.15 ± 8.73 mg, 27.05 ± 10.69 mg, and 22.85 ± 10.26 mg in the control group, 1 and 2 μg/kg dexmedetomidine groups, respectively. The difference between 2 μg/kg dexmedetomidine group and the control group was statistically different. Although the difference is statistically significant, the clinical significance of a difference in morphine dose of about 10 mg over 24 h stands to be clarified. The difference in morphine consumption was not statistically significant in other intergroup comparisons. In a similar study which studied perineural dexmedetomidine in femoral nerve block post-TKA,[22] tramadol was used for rescue analgesia and it was found that the tramadol consumption was comparable over 24 h between the control (116 mg) and the dexmedetomidine groups (100 mg).
The VAS scores at rest were significantly lesser in the 2 μg/kg dexmedetomidine group compared to the other two groups. However, VAS score during passive movement of the knee joint did not show any significant difference among the groups. This introduces further doubt on the usefulness of dexmedetomidine since joint mobilization after replacement is the primary purpose of analgesia; hence, more studies are warranted in this regard.
The incidence of adverse effects related to dexmedetomidine was not significantly different between the groups. There are mixed reports on the incidence of systemic adverse effects with perineural dexmedetomidine.[12,13,16] A few of the above-mentioned studies have observed significant bradycardia and hypotension after dexmedetomidine use,[11,14,21] which we did not notice in our study. Considering the low incidence of systemic adverse effects associated with perineural dexmedetomidine, our study was not powered to find any significant difference in the incidence of adverse effects between the groups. A large, adequately powered study might be required to demonstrate the same.
Conclusion
Thus, we conclude that the use of 2 μg/kg dexmedetomidine as an adjuvant to bupivacaine in femoral nerve block is superior to 1 μg/kg dexmedetomidine for providing postoperative analgesia after TKA. At a dose of 2 μg/kg, dexmedetomidine yields a significantly longer duration of postoperative analgesia and also a statistically significant opioid sparing, without any significant adverse effects. However, the efficacy of dexmedetomidine in aiding joint mobilization is still unclear and needs further evaluation. In addition, the centrally mediated analgesic action of high doses of dexmedetomidine such as 2 μg/kg needs to be ruled out by pharmacokinetic studies on its perineural administration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I would like to mention with gratitude the support provided by the Department of Anesthesia AIIMS and my guide Dr. Lokesh and also acknowledge the help provided by Dr. Puneet, Dr. Souvik and all my seniors.
References
- 1.Horlocker TT. Pain management in total joint arthroplasty: A historical review. Orthopedics. 2010;33(9 Suppl):14–9. doi: 10.3928/01477447-20100722-65. [DOI] [PubMed] [Google Scholar]
- 2.McCartney CJ, Nelligan K. Postoperative pain management after total knee arthroplasty in elderly patients: Treatment options. Drugs Aging. 2014;31:83–91. doi: 10.1007/s40266-013-0148-y. [DOI] [PubMed] [Google Scholar]
- 3.Winnie AP, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: The “3-in-1 block”. Anesth Analg. 1973;52:989–96. [PubMed] [Google Scholar]
- 4.Halaszynski TM. Dexmedetomidine: A look at a promising new avenue of use. Saudi J Anaesth. 2012;6:104–6. doi: 10.4103/1658-354X.97019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroin JS, Buvanendran A, Beck DR, Topic JE, Watts DE, Tuman KJ. Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology. 2004;101:488–94. doi: 10.1097/00000542-200408000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med. 2010;35:427–31. doi: 10.1097/AAP.0b013e3181ef4cf0. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 10.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 11.Abdulatif M, Fawzy M, Nassar H, Hasanin A, Ollaek M, Mohamed H. The effects of perineural dexmedetomidine on the pharmacodynamic profile of femoral nerve block: A dose-finding randomised, controlled, double-blind study. Anaesthesia. 2016;71:1177–85. doi: 10.1111/anae.13603. [DOI] [PubMed] [Google Scholar]
- 12.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:109–14. doi: 10.4103/1658-354X.97021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 15.Kathuria S, Gupta S, Dhawan I. Dexmedetomidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block. Saudi J Anaesth. 2015;9:148–54. doi: 10.4103/1658-354X.152841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–4. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 17.Saravana Babu M, Verma AK, Agarwal A, Tyagi CM, Upadhyay M, Tripathi S. A comparative study in the post-operative spine surgeries: Epidural ropivacaine with dexmedetomidine and ropivacaine with clonidine for post-operative analgesia. Indian J Anaesth. 2013;57:371–6. doi: 10.4103/0019-5049.118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng XZ, Xu YM, Cui XG, Guo YP, Li WZ. Low-dose epidural dexmedetomidine improves thoracic epidural anaesthesia for nephrectomy. Anaesth Intensive Care. 2014;42:185–90. doi: 10.1177/0310057X1404200204. [DOI] [PubMed] [Google Scholar]
- 19.El Shamaa HA, Ibrahim M. A comparative study of the effect of caudal dexmedetomidine versus morphine added to bupivacaine in pediatric infra-umbilical surgery. Saudi J Anaesth. 2014;8:155–60. doi: 10.4103/1658-354X.130677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong Y, Ren H, Ding X, Jin S, Chen Z, Li Q. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: A meta-analysis. Paediatr Anaesth. 2014;24:1224–30. doi: 10.1111/pan.12519. [DOI] [PubMed] [Google Scholar]
- 21.Helal SM, Eskandr AM, Gaballah KM, Gaarour IS. Effects of perineural administration of dexmedetomidine in combination with bupivacaine in a femoral-sciatic nerve block. Saudi J Anaesth. 2016;10:18–24. doi: 10.4103/1658-354X.169469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma B, Rupal S, Swami AC, Lata S. Effect of addition of dexmedetomidine to ropivacaine 0.2% for femoral nerve block in patients undergoing unilateral total knee replacement: A randomised double-blind study. Indian J Anaesth. 2016;60:403–8. doi: 10.4103/0019-5049.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]


