Abstract
The role of infection in asthma is varied in that it may exacerbate established asthma or contribute to the initial development of the clinical onset of asthma. Mounting evidence implicates both roles, with particular viral pathogens, namely human rhinovirus (HRV) and respiratory syncytial virus (RSV), among the most likely culprits in asthma inception. Once asthma is present, infection, particularly viral infections, are a common precipitant of asthma exacerbations. Bacterial infections and colonization also have been associated with exacerbation and recurrent wheeze, an effect that may be independent or a cofactor with viruses. Atypical bacterial infections such as Mycoplasma pneumoniae and Chlamydia pneumoniae and fungi in the case of allergic bronchopulmonary aspergillosis (ABPA), also play a potential role in inducing and exacerbating this disease. Additionally, certain individuals may have a genetic predisposition toward viral induced wheezing and the development of asthma. This paper will discuss host and environmental factors, common pathogens, clinical characteristic, and genetic influences associated with infection related asthma.
Keywords: infection, asthma, exacerbation, rhinovirus, respiratory syncytial virus, biomarkers
Introduction
The role of infection in asthma is varied in that it may exacerbate established asthma or be a contributing factor to the initial development of the clinical onset of asthma. Mounting evidence implicates both roles, with particular viral pathogens, namely human rhinovirus (HRV) 1,2 and respiratory syncytial virus (RSV),3 among the most likely culprits in asthma inception.1,2 Once asthma is present, infection, particularly viral infections, are a common precipitant of asthma exacerbations.4 Bacterial infections and colonization also have been associated with exacerbation and recurrent wheeze, an effect that may be independent5,6 or a cofactor with viruses.7 Atypical bacterial infections such as Mycoplasma pneumoniae8 and Chlamydia pneumonia,9 and fungi in the case of allergic bronchopulmonary aspergillosis (ABPA),10 also play a potential role in inducing and exacerbating this disease. Additionally, certain individuals may have a genetic predisposition toward viral induced wheezing and the development of asthma.11
Demographics
Host and Environmental Factors
Upper respiratory infections (URIs) are the most common cause of acute illnesses in both children and adults. Such viral infections result in increased healthcare cost and work and school absenteeism.12 The demographics of exposure to these agents is of significance in that the seasonal pattern of asthma exacerbations often mirrors the occurrence of viral infections in the community.12 Infection related asthma is more common during preschool and early childhood. Although the prevalence of asthma is more common in boys prior to adolescence, there is no evidence that the incidence or prevalence of infection related asthma differ based on gender. For viral-induced exacerbations, increases in prevalence during both fall and spring are reported.13 The administration of omalizumab virtually eliminated spring and fall exacerbations, which were primarily caused by viral upper respiratory infections.14 These observations indicate that interactions between allergic sensitization (antigen-specific IgE antibody formation) and viral respiratory illnesses play an important role in asthma control. Genetic variation at a number of different loci also contribute to the frequency and severity of the host response to viral infection.15-18 Finally, the socioeconomic environment may also contribute. 2012 studies showing comparisons of the viral etiology during infancy in children living in the inner-cities of the USA compared to a more suburban environment revealed some interesting differences. Overall, inner-city infants had lower rates of viral detection. Considering specific viruses, sick urban infants had lower rates of detectable rhinovirus or RSV infection and higher rates of adenovirus infection.19
Pathogen Related Factors
Table 1 lists the viral and bacterial pathogens most commonly associated with infectious asthma.
Table 1.
Viral and bacterial pathogens most commonly involved in infectious asthma.
| Viruses | Rhinoviral species |
| Respiratory syncytial virus | |
| Bacteria | Haemophilus influenza |
| Streptococcus pneumoniae | |
| Moraxella catarrhalis | |
| Mycoplasma pneumoniae | |
| Chlamydia pneumoniae |
Viruses
Both RSV and HRV are capable of producing significant lower respiratory tract illnesses requiring hospitalization (bronchiolitis) during infancy, with HRV-infected individuals tending to be older and presenting with atopic dermatitis and peripheral eosinophilia.20 In addition, RSV and HRV outpatient wheezing illnesses in early in life may increase the risk for subsequent wheezing episodes and the development of asthma.21,22 Evidence tends to favor a stronger propensity for these developments with HRV than with RSV.21,23-26 However, there are significant differences observed even among rhinoviral species A, B, and C. HRV-C and -A are more likely than HRV-B to produce moderate to severe lower respiratory illnesses27 while HRV-C is more frequently associated with clinically significant exacerbations.23 Furthermore, there is evidence that HRV-C may be more likely to cause viremia than HRV-A or B.28 These collective findings have led many to believe that HRV-C may be more virulent.29 One of the notable differences between HRV-C and HRV-A or B is the receptors utilized to permit cell entry and viral replication. Species A and B use primarily intercellular adhesion molecule 1 (ICAM-1) and low density lipoprotein receptor (LDLR), whereas HRV-C is thought to utilize a unique, yet thus far unidentified receptor.29 Through attempts at culturing the virus in vivo it is thought that this receptor is expressed on epithelial cells in differentiated tissues.29
RSV has one serotype that is divided into antigenic subgroups A and B, with further division of each into genetic variants.30,31 Among hospitalized infants, group A RSV infection is associated with greater severity of disease.32 There are also data showing that infection with RSV promotes T-helper 2 type cytokine production and that this may be involved in the subsequent development of asthma.33
Bacterial Pathogens
In addition to viral causes, there have been investigations into the host microbiome as it relates to asthma inception and exacerbations. Bacteria such as Lactobacillus and Helicobacter pylori are reported to be protective against asthma, while others are associated with an increased risk of asthma.34 One study demonstrates associations between neonatal hypopharyngeal colonization of Haemophilus influenza, Streptococcus pneumoniae and Moraxella catarrhalis with the subsequent increased risk of developing recurrent wheezing and childhood asthma.6,35 It is unclear from these findings whether early colonization with these organisms in some way influences the development of asthma, or if the presence of these organisms is a reflection of an altered immune system that predisposes to altered host airway responses to respiratory pathogens. Acute wheezing episodes in preschool children are associated with these bacterial pathogens with a frequency similar to that seen with viruses.5
Mycoplasma and Chlamydia
Atypical bacterial infections, e.g., Mycoplasma pneumoniae and Chlamydia pneumonia, play a potential role in inducing and exacerbating asthma.36 Early studies involving C. pneumoniae suggest a link to infection and the onset of asthma.37 Kraft et al. published data showing that in subjects with stable asthma who tested positively by polymerase chain reaction (PCR) for M. pneumoniae or C. pneumoniae improved their lung function when treated with clarithromycin.38 However, although a later trial investigating the addition of clarithromycin to fluticasone showed improvement in airway hyperresponsiveness, it failed to show improvement in lung function, asthma control, or other secondary outcomes.39 In a 15 year longitudinal study, subjects who were serologically diagnosed with acute or chronic C. pneumoniae infection had no greater chance of developing asthma than those that did not. However, subjects who did develop asthma had a more progressive decline of lung function compared to those who tested negative.40 A 2013 study by Wood, et al. involving children with and without asthma found M. pneumoniae in 61% of all subjects, with no significant difference between asthmatic and non-asthmatic subjects.8 Children with asthma have lower antibody levels to M. pneumoniae compared to healthy controls, suggesting a poor humoral response. Among asthmatic patients, those testing positive for M. pneumoniae had significantly lower scores on asthma control and quality of life questionnaires.8
Clinical Characteristics
Clinically, HRV and RSV infections are indistinguishable and may not be symptomatic.41 If symptomatic, infections in adults typically present with nasal discharge, nasal obstruction, cough, and sore throat. Fever is not typical with adult illness.42 Children present with fever, cough, nasal discharge and obstruction. The duration of symptoms is longer than in adults, often lasting more than 10 days.43 Indications of bacterial infection in children include high fever persisting for ≥4 days, accompanied by purulent nasal discharge, and ill appearance.44,45 Production of purulent sputum does not distinguish a viral from a bacterial infection. 46 Virally induced asthma exacerbations typically begin 1-2 days after symptoms of infection begin and are characterized by brief episodes of wheezing and decreased pulmonary function. Symptoms are most significant during the first 48 hours of exacerbation and return to baseline within 5 to 10 days.47 Approximately 80% of asthma exacerbations in children and 50% in adults are attributed to respiratory viral infections, with HRV accounting for nearly two thirds of them. Such exacerbations may be followed by asymptomatic periods and normal pulmonary function. This pattern of asthma differs from other forms of asthma in both adults and children, which are characterized by more chronic and persistent symptoms as well as alterations in pulmonary function that are present between infectious episodes. More chronic asthma may have superimposed increases in symptoms and reductions in pulmonary function that occur intermittently during respiratory tract illnesses. Importantly, the number of corticosteroid-requiring exacerbations most commonly related to respiratory tract illnesses, both in children48 and adults49 are associated with reductions in lung function over time. The mechanism(s) underlying these changes are unknown, but may be due to altered immune responses to repeated viral infections, increased and prolonged airway inflammation, and the potential for long term structural changes. A 2007 study by Saglani et. al involving endobronchial biopsies obtained from children ages 3 months to 5 years with a history of wheezing (which are most likely related to respiratory pathogens) suggests pathologic changes may develop as early as 1 to 3 years of age.50
The progression of asthmatic disease as it relates to infection evolves throughout life along with the microbiome. The infant gut microbiome has decreased diversity. Children up to 5 years of age are prone to episodic wheezing caused by viral pathogens, and viral wheezing within the first three years is the most important risk factor for asthma at age 6.34 As children grow into adulthood, there is an increased burden and diversity of bacteria. Those with asthma are more vulnerable to virally induced exacerbations, likely due to defects in the respiratory epithelial barrier.34 In elderly subjects concomitant diseases often contribute to decreasing lung function, making them particularly vulnerable to viral infections.34
Physiologic changes also occur in the airways of asthmatics in response to infection. More than five decades ago it was noted that significant changes in lung function occur in asthmatic patients during infection, with decreases in FEV1 and FVC, as well as evidence of increased air trapping.51 Animal models show that viral airway injury at an early age may induce small airway lesions that are associated with small airway physiological dysfunction, which may persist into maturity.52 However, such experiments are difficult to perform in human models. In 2013, Konstantinou et. al demonstrated that during viral infections, children have enhanced airway inflammation, reversible airflow limitation, and elevated fraction of exhaled nitric oxide (FENO).47 Experimental infections with RV show more pronounced airway narrowing in response to methacholine up to 15 days after infection compared to uninfected controls.53 Children with a history of preschool HRV wheezing illness have significantly lower FEV1 in later childhood (Figure 1),54 and there are additional data showing more frequent and severe exacerbations (requiring corticosteroid intervention) in children induce more persistent losses in lung function (Figure 2).48
Figure 1.
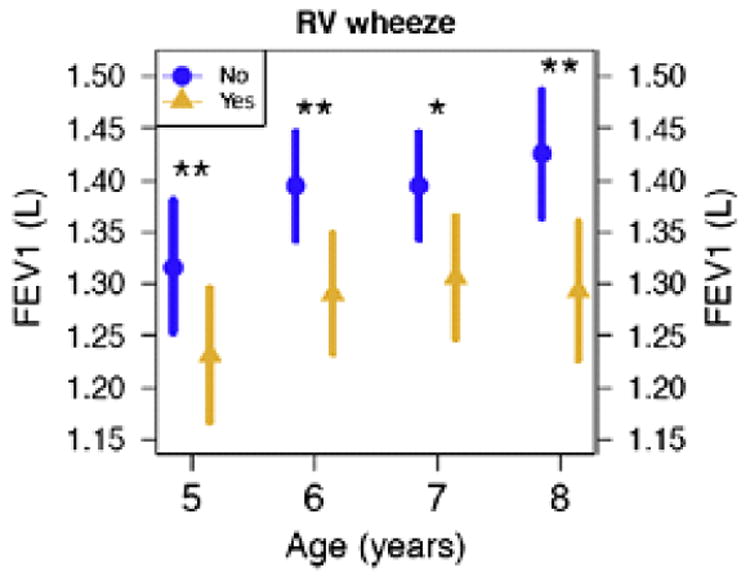
Children with preschool HRV wheezing illnesses had significantly lower FEV1 at ages 5 through 8 years. Circles and triangles represent means, and bars represent 95% CI. Significant differences between treatment groups denoted by *P < .05 and **P < .01. Adapted from Guilbert, et al. JACI 128:532, 2011
Figure 2.
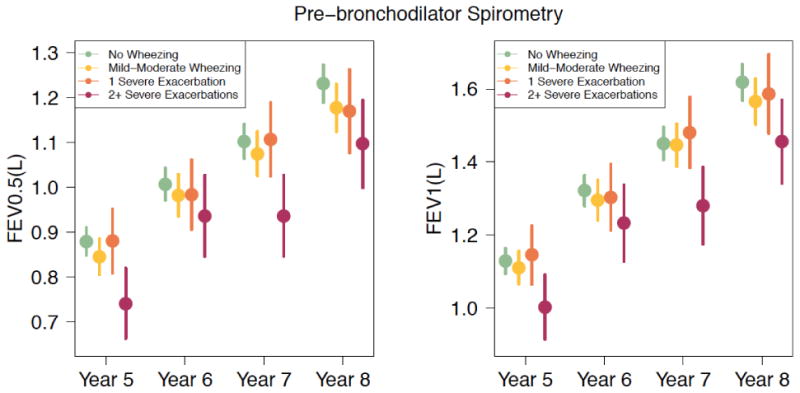
Prebronchodilator FEV0.5 and FEV1 values assessed longitudinally between 5 and 8 years of age demonstrating significant reductions in children with histories of recurrent (≥2) wheezing exacerbations treated with oral corticosteroids (OCS) compared with those seen in children with no wheezing, mild-to-moderate wheezing, or 1 severe wheezing exacerbation requiring OCSs. Adapted from O'Brian, et. al. JACI 128:4 pages 1162-1164, 2012
Comorbid Conditions
Allergic Sensitization
History of atopic disease is a common comorbid finding in asthmatics, present in up to 80% of individuals.55 In children, sensitization to aeroallergens is a clear risk factor to develop asthma and for asthma exacerbations. Studies also show increased risk of asthma development in children with food allergy56 and eczema,57 but it is not established whether these conditions influence the response to URIs. Indeed, there are data demonstrating that asthma development is influenced by both the developmental stage58 and the etiology59 of allergic sensitization. As mentioned above, trials with omalizumab raise the possibility that IgE-dependent processes are risk factors for viral respiratory infections to provoke an asthma exacerbation.14 This altered host response to viral infections in the presence of allergic sensitization may be related, at least in part, to reductions in innate immune responses in the form of reduced elaboration of interferons from antigen-processing cells.60
Other Respiratory Tract Diseases
Both RSV and HRV infections can induce more severe lower airway involvement termed “bronchiolitis” during infancy. The clinical presentation of these illnesses can include coughing and wheezing, and may be associated with the subsequent development of asthma.61,62 Croup and recurrent croup also have been reported to be associated with the development of asthma.63 Other conditions such as cystic fibrosis in patients with concomitant asthma may predispose them to infections leading to exacerbations. Allergic bronchopulmonary aspergillosis (ABPA), while not a true fungal infection, is an allergic response to a fungal organism, and is closely tied to asthma, cystic fibrosis, and atopic disease.10
Rhinosinusitis
Symptoms of acute rhinosinusitis often accompany acute viral URIs; however, chronic rhinosinusitis is not an uncommon finding among patients with infection induced asthma.64 Subjects with chronic sinus disease may have nasal polyposis65 or humoral immunodeficiencies.66 The increased frequency of sinus infections associated with these conditions may increase the frequency of infection related asthma exacerbations and their severity and duration.65 Bacteria may also play a role in asthma exacerbations; thus, further investigation about the interaction between the upper and lower airways in these conditions is warranted.
Gastroesophageal Reflux
Gastroesophageal reflux disease (GERD) is highly prevalent in children with asthma and nearly half are asymptomatic.67 The majority of studies thus far show that treatment for GERD does not consistently provide significant improvements on most asthma endpoints.67-69 In fact, some studies demonstrate an increased risk of URIs and pneumonia with treatment of GERD.68,70 Additionally, a meta-analysis of 31 trails confirmed an association of proton pump inhibitor (PPI) use and pneumonia.71
Biomarkers
Genetic
Genetic data from 2007 indicate that individuals possessing certain genotypes may be at increased asthma risk during childhood.72 This genetically-determined risk may be related to respiratory tract infections during early childhood. Specifically, Caliskan et al. demonstrated that associations between a variation in the 17q21 genetic region and asthma risk is in fact limited only to children who had HRV wheezing illnesses in early life.16 The 17q21 locus contains the genes GSDMB and ORMDL3 that code for relevant proteins in white cells, lymphoblastoid cell lines, and CD4+ T cells. Importantly, this genetically-attributable increased risk was not seen with RSV wheezing illnesses and was totally independent of the presence of allergic sensitization. Thus, it appears that HRV wheezing illnesses may be associated with increased asthma risk from at least two pathways: one involving the presence of allergic sensitization and one totally independent of it.16,73 A number of research groups are investigating mechanisms that may underlie these associations.74-76
For RSV infections, single nucleotide polymorphisms (SNPs) in IL1RL1 are associated with increased severity of bronchiolitis; however, subsequent development of asthma was not investigated.18 Polymorphisms in IL10, chemokine receptor 5, transforming growth factor β, TLR4, IL13, IL4, and IL-4RA genes are associated with RSV induced bronchiolitis severity.15
2014 studies show through genome wide association studies (GWAS) a susceptibility locus associated with early childhood asthma with severe exacerbations.17 CDHR3 is a gene encoding a transmembrane protein belonging to the cadherin family and is highly expressed in bronchial epithelial cells, although its exact function is unknown. Single nucleotide polymorphisms (SNPs) in this gene are associated with increased risk of severe asthma exacerbations and asthma related hospitalization.17 It is hypothesized that SNPs in CDHR3 lead to impaired epithelial integrity and/or repair, predisposing such individuals to infection. Finally, in a family-based association study of 134 children with bronchiolitis, a variation in the promoter region of the IL8 gene was transmitted significantly more often than expected in children who subsequently developed persistent wheezing.77
Laboratory
Other than standard laboratory tests, such as a complete blood count or chest X-rays, there are no biomarkers to indicate acute infectious asthma. Potential biomarkers of infectious asthma are the presence of peripheral blood and sputum eosinophilia. These biomarkers are linked to seasonal asthma exacerbations, often triggered by URIs. 14
If infection is present, in association with an exacerbation, as suggested by clinical evaluation, there are techniques that can be utilized to determine the causal organism. Nasopharyngeal aspiration and lavage have been used to obtain specimens for viral identification using culture or PCR technology.6,73 Sputum culture results can help to direct antimicrobial therapy for bacterial infections. There are data to suggest that macrolide antibiotics induce antiviral responses in airway epithelial cells;78 however, research is ongoing to determine if this is a clinically relevant finding.
Summary
Definition
An individual in which a respiratory tract infection influences his/her asthma can be present as follows:
Associated with new onset of disease (in both children and adults)
-
Associated with exacerbation of disease where infection is
The only mechanism of exacerbation
One mechanism of exacerbation in multi-triggered asthma, which may be more severe in nature compared to other exacerbation triggers
Recommended Information to Be Obtained To Support the Diagnosis of Infectious Asthma
-
For association with onset of disease:
Asthma first appeared after an URI or lower respiratory tract infection
-
Associated with exacerbation of disease where infection
Is the only mechanism of exacerbation
Is one of a number of exacerbating factors
Induces severe exacerbations
Research Questions and Future Direction
What are the mechanisms through which infections lead to new onset asthma?
What host factors determine the susceptibility of an individual to infection induced asthma or developing an exacerbation from asthma?
How do certain comorbidities or underlying allergic diseases influence response to infection and the resultant clinical characteristics of asthma exacerbations?
What biomarkers are most reliable to establish the susceptibility of individuals to develop infection-induced asthma or to experience asthma exacerbations or worsening of disease severity secondary to infectious agents?
Are certain strains of viruses (e.g., RSV and rhinovirus) more pathogenic for inducing or causing asthma exacerbations?
How does the host's respiratory microbiome contribute to the protection or risk for exacerbations due to infection?
Is targeting the host's immune response to infection more important than targeting the infectious organism?
How will the use of the new biological agents for asthma affect infection induced exacerbations?
Can the genetic profile of an individual help to predict asthma inception (17q21 region) or severity of exacerbations once asthma is established in the host (e.g., CDHR3)?
Conclusion
Upper respiratory infections are a common cause of acute illness in both adults and children, and are a common cause of asthma exacerbations. Several pathogens are implicated in asthma inception and exacerbations. Host factors such as allergic sensitization, genetic polymorphisms, and other comorbid conditions are associated with susceptibility to infection related asthma.
Summary.
Upper respiratory infections are the most common cause of acute illness in both adults and children, and are a common cause of asthma exacerbations. The following article will review infections and how they relate to asthma inception and exacerbations, the common organisms responsible, and host and pathogen related factors contributing to infection related asthma.
Acknowledgments
Supported by NIH grants: P01 HL070831, R01 HL097134, U10 098090
Abbreviations
- ABPA
allergic bronchopulmonary aspergillosis
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GERD
gastroesophageal reflux disease
- GWAS
genome wide association studies
- HRV
human rhinovirus
- ICAM-1
intercellular adhesion molecule 1
- LDLR
low density lipoprotein receptor
- PCR
polymerase chain reaction
- PPI
proton pump inhibitor
- RSV
respiratory syncytial virus
- SNPs
single nucleotide polymorphisms
- URI
upper respiratory infection
References
- 1.Thomas AO, Lemanske RF, Jr, Jackson DJ. Infections and their role in childhood asthma inception. Pediatr Allergy Immunol. 2013 Nov 17; doi: 10.1111/pai.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackenzie KJ, Anderton SM, Schwarze J. Viral respiratory tract infections and asthma in early life; cause and effect? Clin Exp Allergy. 2013 Apr 29; doi: 10.1111/cea.12246. [DOI] [PubMed] [Google Scholar]
- 3.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011 Sep;9(9):731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James KM, Peebles RS, Jr, Hartert TV. Response to infections in patients with asthma and atopic disease: An epiphenomenon or reflection of host susceptibility? J Allergy Clin Immunol Aug. 2012;130(2):343–351. doi: 10.1016/j.jaci.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisgaard H, Hermansen MN, Bonnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 7.Kloepfer KM, Lee WM, Pappas TE, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol May. 2014;133(5):1301–1307. 1307 e1301–1303. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood PR, Hill VL, Burks ML, et al. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol May. 2013;110(5):328–334 e321. doi: 10.1016/j.anai.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webley WC, Tilahun Y, Lay K, et al. Occurrence of Chlamydia trachomatis and Chlamydia pneumoniae in paediatric respiratory infections. Eur Respir J. 2009;33(2):360–367. doi: 10.1183/09031936.00019508. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy Aug. 2013;43(8):850–873. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 11.Smit LA, Bouzigon E, Pin I, et al. 17q21 variants modify the association between early respiratory infections and asthma. Eur Respir J. 2010;36(1):57–64. doi: 10.1183/09031936.00154509. [DOI] [PubMed] [Google Scholar]
- 12.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324(7340):763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston NW, Johnston SL, Duncan JM, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115(1):132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011 Mar 17;364(11):1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175(2):108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 16.Caliskan M, Bochkov YA, Kreiner-Moller E, et al. Rhinovirus wheezing illness and genetic risk of childhood onset asthma. N Engl J Med. 2013 Apr 11;368(15):1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet Jan. 2014;46(1):51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 18.Faber TE, Schuurhof A, Vonk A, et al. IL1RL1 gene variants and nasopharyngeal IL1RL-a levels are associated with severe RSV bronchiolitis: a multicenter cohort study. PLoS One. 2012;7(5):e34364. doi: 10.1371/journal.pone.0034364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gern JE, Pappas T, Visness CM, et al. Comparison of the etiology of viral respiratory illnesses in inner-city and suburban infants. J Infect Dis Nov. 2012;206(9):1342–1349. doi: 10.1093/infdis/jis504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korppi M, Kotaniemi-Syrjanen A, Waris M, Vainionpaa R, Reijonen TM. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23(11):995–999. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- 21.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 23.Cox DW, Bizzintino J, Ferrari G, et al. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013 Dec 1;188(11):1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010 Apr;10(2):133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jartti T, Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol Jun. 2011;22(4):350–355. doi: 10.1111/j.1399-3038.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 26.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Lee WM, Lemanske RF, Jr, Evans MD, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012 Nov 1;186(9):886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuji N, Suzuki A, Lupisan S, et al. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS One. 2011;6(11):e27247. doi: 10.1371/journal.pone.0027247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bochkov YA, Gern JE. Clinical and molecular features of human rhinovirus C. Microbes and infection / Institut Pasteur Jun. 2012;14(6):485–494. doi: 10.1016/j.micinf.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson LJ, Hierholzer JC, Tsou C, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis Apr. 1985;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- 31.Trento A, Casas I, Calderon A, et al. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol Aug. 2010;84(15):7500–7512. doi: 10.1128/JVI.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis Apr. 1997;175(4):814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 33.Zeng R, Li C, Li N, Wei L, Cui Y. The role of cytokines and chemokines in severe respiratory syncytial virus infection and subsequent asthma. Cytokine. 2011 Jan;53(1):1–7. doi: 10.1016/j.cyto.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013 Mar 9;381(9869):861–873. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 35.De Schutter I, Dreesman A, Soetens O, et al. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC Pediatr. 2012;12:83. doi: 10.1186/1471-2431-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 37.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991 Jul 10;266(2):225–230. [PubMed] [Google Scholar]
- 38.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest Journal. 2002;121(6):1782–1788. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland ER, King TS, Icitovic N, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol Oct. 2010;126(4):747–753. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasternack R, Huhtala H, Karjalainen J. Chlamydophila (Chlamydia) pneumoniae serology and asthma in adults: a longitudinal analysis. J Allergy Clin Immunol. 2005;116(5):1123–1128. doi: 10.1016/j.jaci.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Adams O, Weis J, Jasinska K, Vogel M, Tenenbaum T. Comparison of human metapneumovirus, respiratory syncytial virus and Rhinovirus respiratory tract infections in young children admitted to hospital. J Med Virol. 2014 Jul 30; doi: 10.1002/jmv.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwaltney JM, Jr, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population. II. Characteristics of illness and antibody response. JAMA. 1967;202(6):494–500. [PubMed] [Google Scholar]
- 43.Pappas DE, Hendley JO, Hayden FG, Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008 Jan;27(1):8–11. doi: 10.1097/INF.0b013e31814847d9. [DOI] [PubMed] [Google Scholar]
- 44.Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics Jul. 2013;132(1):e262–280. doi: 10.1542/peds.2013-1071. [DOI] [PubMed] [Google Scholar]
- 45.Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012 Apr;54(8):e72–e112. doi: 10.1093/cid/cir1043. [DOI] [PubMed] [Google Scholar]
- 46.Wenzel RP, Fowler AA., 3rd Clinical practice. Acute bronchitis. N Engl J Med. 2006 Nov 16;355(20):2125–2130. doi: 10.1056/NEJMcp061493. [DOI] [PubMed] [Google Scholar]
- 47.Konstantinou GN, Xepapadaki P, Manousakis E, et al. Assessment of airflow limitation, airway inflammation, and symptoms during virus-induced wheezing episodes in 4- to 6-year-old children. J Allergy Clin Immunol. 2013 Jan;131(1):87–93. e81–85. doi: 10.1016/j.jaci.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brian AL, Lemanske RF, Jr, Evans MD, Gangnon RE, Gern JE, Jackson DJ. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol. 2012 Apr;129(4):1162–1164. doi: 10.1016/j.jaci.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 50.Saglani S, Payne DN, Zhu J, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007 Nov 1;176(9):858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 51.Woolcock AJ, Read J. Lung volumes in exacerbations of asthma. Am J Med. 1966 Aug;41(2):259–273. doi: 10.1016/0002-9343(66)90021-0. [DOI] [PubMed] [Google Scholar]
- 52.Sorkness RL, Szakaly RJ, Rosenthal LA, et al. Viral bronchiolitis in young rats causes small airway lesions that correlate with reduced lung function. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2013-0096OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung D, Dick EC, Timmers MC, de Klerk EP, Spaan WJ, Sterk PJ. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995 Nov;152(5 Pt 1):1490–1496. doi: 10.1164/ajrccm.152.5.7582282. [DOI] [PubMed] [Google Scholar]
- 54.Guilbert TW, Singh AM, Danov Z, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol Sep. 2011;128(3):532–538. e531–510. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haselkorn T, Borish L, Miller DP, Weiss ST, Wong DA. High prevalence of skin test positivity in severe or difficult-to-treat asthma. J Asthma. 2006 Dec;43(10):745–752. doi: 10.1080/02770900601031540. [DOI] [PubMed] [Google Scholar]
- 56.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126(4):798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin PE, Matheson MC, Gurrin L, et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol. 2011 Jun;127(6):1473–1479. e1471. doi: 10.1016/j.jaci.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 58.Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181(11):1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 59.Stoltz DJ, Jackson DJ, Evans MD, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy Feb. 2013;43(2):233–241. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130(2):489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med May. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 63.Van Bever HP, Wieringa MH, Weyler JJ, Nelen VJ, Fortuin M, Vermeire PA. Croup and recurrent croup: their association with asthma and allergy - An epidemiological study on 5-8-year-old children. Eur J Pediatr. 1999;158(3):253–257. doi: 10.1007/s004310051062. [DOI] [PubMed] [Google Scholar]
- 64.Ledford DK, Lockey RF. Asthma and comorbidities. Curr Opin Allergy Clin Immunol. 2013 Feb;13(1):78–86. doi: 10.1097/ACI.0b013e32835c16b6. [DOI] [PubMed] [Google Scholar]
- 65.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128(4):693–707. doi: 10.1016/j.jaci.2011.08.004. quiz 708-699. [DOI] [PubMed] [Google Scholar]
- 66.Oksenhendler E, Gerard L, Fieschi C, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008 May 15;46(10):1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 67.Blake K, Teague WG. Gastroesophageal reflux disease and childhood asthma. Curr Opin Pulm Med. 2013 Jan;19(1):24–29. doi: 10.1097/MCP.0b013e32835b582b. [DOI] [PubMed] [Google Scholar]
- 68.Writing Committee for the American Lung Association Asthma Clinical Research C. Holbrook JT, Wise RA, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012 Jan 25;307(4):373–381. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gibson PG, Henry RL, Coughlan JL. Gastro-oesophageal reflux treatment for asthma in adults and children. Cochrane database of systematic reviews. 2003;(2) doi: 10.1002/14651858.CD001496. CD001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canani RB, Cirillo P, Roggero P, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics May. 2006;117(5):e817–820. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 71.Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011 Feb 22;183(3):310–319. doi: 10.1503/cmaj.092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007 Jul 26;448(7152):470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 73.Lemanske RF., Jr Early-life wheezing and respiratory syncytial virus prevention. N Engl J Med. 2013 May 9;368(19):1839–1841. doi: 10.1056/NEJMe1302063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ono JG, Worgall TS, Worgall S. 17q21 locus and ORMDL3: an increased risk for childhood asthma. Pediatr Res. 2014;75(1-2):165–170. doi: 10.1038/pr.2013.186. [DOI] [PubMed] [Google Scholar]
- 75.Miller M, Rosenthal P, Beppu A, et al. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol. 2014 Apr 15;192(8):3475–3487. doi: 10.4049/jimmunol.1303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller M, Tam AB, Cho JY, et al. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):16648–16653. doi: 10.1073/pnas.1204151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goetghebuer T, Isles K, Moore C, Thomson A, Kwiatkowski D, Hull J. Genetic predisposition to wheeze following respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34(5):801–803. doi: 10.1111/j.1365-2222.2004.1947.x. [DOI] [PubMed] [Google Scholar]
- 78.Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]


