Summary
Background
A new descriptive classification scheme for biomarkers used in Alzheimer's and cognitive aging research, labeled ATN, was recently proposed. One implementation of this ATN construct dichotomizes biomarkers of amyloid, tau, and neurodegeneration/neuronal injury as normal or abnormal resulting in 2 × 2× 2=8 possible biomarker profiles. We determined the clinical characteristics and prevalence of each ATN group among clinically normal individuals aged 50 and older from a population based cohort.
Methods
All individuals in this study were participants in the Mayo Clinic Study of Aging, a population-based study of cognitive aging. Potential participants were randomly selected from the Olmsted County, Minnesota population by age- and sex-stratification and invited to participate in cognitive evaluations and undergo multimodality imaging. To be eligible for inclusion in this study, participants must have been judged clinically to have no cognitive impairment and have undergone multi-modality imaging. Imaging studies were obtained from October 11, 2006 to October 5, 2016. All participants were classified as having normal (A−) or abnormal (A+) amyloid using amyloid PET, normal (T−) or abnormal (T+) tau using tau PET, and normal (−) or abnormal (N+) neurodegeneration/neuronal injury using cortical thickness. The cut points used were SUVR 1·42 (centiloid 19) for amyloid PET, 1·23 SUVR for tau PET, and 2·67 mm for MRI cortical thickness. Age- and sex- specific prevalences of the eight ATN biomarker groups were determined using 435 individuals with amyloid PET, tau PET, and MR imaging and 1113 additional clinically normal individuals who underwent amyloid PET and MR imaging, but not tau PET imaging.
Findings
There were 165 A−T−N-, 35 A−T+N-, 63 A−T−N+, 19 A−T+N+, 44 A+T−N−, 25 A+T+N−, 35 A+T−N+, and 49 A+T+N+ individuals. Age differed by ATN group (p<0 001) ranging from a median age of 57 in the A−T−N-−and A−T+N− groups to 80 in the A+T−N+ and A+T+N+ groups. The frequency of APOE ε4 carriers differed by ATN group (p=0·04) with ε4 carriers roughly twice as frequent in A+ versus A−. White matter hyperintensity volume (p<0·0001), and cognitive performance (p<0·0001) also differed by ATN group. Tau PET and neurodegeneration biomarkers were discordant in the majority of individuals who would be labeled stage 2/3 preclinical AD (86% at age 65 and 51% at age 80) or suspected non-Alzheimer's pathophysiology (SNAP) (92% at age 65 and 78% at age 80). From age 50, A−T−N− prevalence declines while A+T+N+ and A−T+N+ increase continuously with age. In both men and women, A−T−N− is the most prevalent group until their late 70s. After about age 80, A+T+N+ is the most prevalent group until their late 70s. After about age 80, A+T+N+ is the most prevalent group. The remaining ATN groups reach individual peaks in the 60–90 age range and then decline in prevalence. By age 85 over 90% of men and women have one or more biomarker abnormalities.
Interpretation
Biomarkers of fibrillar tau deposition can be included with those of Aβ and neurodegeneration/neuronal injury to more fully characterize the heterogeneous pathological profiles in the population. The prevalence of each ATN group changes substantially with age with progression toward more biomarker abnormalities even among individuals who remain clinically normal. Both abnormal amyloid and normal amyloid pathological profiles can be identified in the clinically normal population.
Introduction
Use of biomarkers as an aid in the diagnosis of Alzheimer's disease (AD) gained acceptance with the publication of the National Institute on Aging - Alzheimer's Association (NIA-AA) recommendations 1-4 and the International Working Group (IWG) criteria 5,6 for AD.1 In the NIA-AA recommendations biomarkers were divided into two classes: biomarkers of amyloid (A) and biomarkers of tau-related neurodegeneration/neuronal injury (N).1, 21, 25, 6When the NIA-AA preclinical AD staging recommendations were operationalized and applied to a cohort of 450 clinically normal (CN) individuals over age 70, roughly one third fell into stages 1–3 of preclinical AD, 40% were amyloid normal and neurodegeneration normal (A− N−), and one quarter were amyloid normal and neurodegeneration abnormal (A− N+)7.We labeled the A− N+ group suspected non- Alzheimer's pathophysiology (SNAP) on the assumption that this was a pathologically heterogeneous group with a variety of non-Alzheimer's pathologies. To reflect NIA-AA staging while accounting for SNAP and AN− groups, many research groups have adopted a 2-class biomarker construct in which individuals are assigned to one of four biomarker categories: A− N−, A+N−, A− N+ (SNAP) or A+N+. 8-13 This approach has been useful because it provided a common framework for different research groups to communicate findings in their own cohorts.14-18
In retrospect, a weakness of the NIA-AA staging plus SNAP construct was the grouping of CSF phosphorylated tau, MRI and FDG PET into one neurodegeneration/neuronal injury category. 19 1, n persons with AD it is reasonable to assume that neurodegeneration in AD-sensitive areas is most often related to tauopathy; however, neurodegeneration, even when defined based on its pattern in AD, also occurs in non-AD conditions. A solution to this problem is to separate biomarkers that are specific for fibrillar tau deposits and its associated pathophysiology from those that are nonspecific measures of neurodegeneration/neuronal injury. This refinement enables identification of tauopathy and neurodegeneration/neuronal injury that are and are not associated with each other, leading to a more precise understanding of the biological underpinnings of brain aging. To this end an international group recently proposed a new descriptive classification scheme20 for biomarkers used in AD and cognitive aging research. The construct is labeled ATN20 and is based on grouping biomarkers into three categories: fibrillary β-amyloid deposition or associated pathophysiology (A);19 paired helical filament tau or associated pathophysiology (T);14-19 and, neurodegeneration or neuronal injury (N). One possible implementation of ATN is to dichotomize each biomarker category as either normal (−) or abnormal (+) which results in 2×2×2=8 different biomarker group combinations.
The goal of the current study was to apply the ATN categorization to clinically normal (CN) individuals aged 50 and older in the population-based Mayo Clinic Study of Aging to estimate the age and sex-specific prevalences of each ATN group and to describe the clinical and demographic characteristics of the eight ATN biomarker groups. We used amyloid PET to define A, tau PET to define T, and cortical thickness to define N.
Methods
Study design and participants
All individuals in this study were participants enrolled in the Mayo Clinic Study of Aging (MCSA), a population-based study of cognitive aging among Olmsted County, Minnesota residents 21 The Rochester Epidemiology Project 22 medical records linkage system was used to enumerate all Olmsted County residents aged 50 to 89. Potential participants were randomly selected from this enumeration according to age and sex strata with equal numbers of men and women in each age category. All individuals without a medical contraindication are invited to participate in imaging studies. Since 2004, the MCSA has enrolled non-demented individuals aged 70 to 89 years, and in 2012 started to enroll subjects 50 plus years of age. 7, 8, 21 Prior to May 28, 2015 imaging included amyloid PET, FDG PET, and MRI. Beginning May 28, 2015 individuals who participated in imaging underwent each of amyloid PET, tau PET, and MR imaging. 21
Individuals from the MCSA were included in the current cross-sectional study if they were judged clinically to have no cognitive impairment and have undergone amyloid PET, tau PET (in a subset), and MR imaging between October 11, 2006 and October 5, 2016. We analyzed data from the first visit with amyloid PET, tau PET, and MRI or the more recent amyloid PET and MRI visit if no tau PET was available to estimate the age and sex-specific prevalences of each ATN group and to describe the clinical and demographic characteristics of the eight ATN biomarker groups
The MCSA and related studies were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards and written informed consent was obtained from all participants
Procedures
Amyloid PET imaging was performed with Pittsburgh Compound B19, synthesized on site with precursor purchased from ABX Biochemical Compounds, Germany. Tau PET was performed with AV1451, synthesized on site with precursor supplied by Avid Radiopharmaceuticals 17. Late uptake amyloid PET images were acquired from 40-60 minutes and tau PET from 80-100 minutes after injection. Methods of amyloid PET data analysis have been described previously. 7,23 Amyloid PET values are expressed both in SUVR units and in centiloid units.24 A tau PET composite reporter region of interest (ROI) was formed from a voxel-number weighted average of the median uptake in the entorhinal, amygdala, parahippocampal, fusiform, inferior temporal, and middle temporal ROIs normalized to the cerebellar crus grey median. 23 PET data was not partial volume corrected.
MRI was performed on one of three 3 Tesla systems from the same vendor (General Electric, Waukesha WI, USA). The primary MRI measure was a FreeSurfer (v5·3) derived temporal lobe cortical thickness composite reporter ROI of the entorhinal, inferior temporal, middle temporal, and fusiform ROIs. 23 These were consistently among the top performing ROIs across our previous ROI selection studies discriminating between A– clinically normal and A+ impaired individuals. 25, 26 As an alternative measure of neurodegeneration we used the sum of right and left hippocampal volumes from FreeSurfer adjusted for total intracranial volume (HVa) as described in 27. The MRI acquisition also included a FLAIR sequence from which white matter hyperintensity volume was measured using an algorithm developed in-house.28
We have recently conducted a thorough examination of several different methods for selecting cut-points to define abnormality on amyloid PET, tau PET and MRI thickness. 23 The optimal amyloid PET cut-point of SUVR 1·42 (centiloid 19) was based on the threshold value beyond which the rate of change in amyloid PET reliably increases. We determined cut-points for tau PET and MRI thickness by maximizing the accuracy (i.e., maximizing sensitivity plus specificity) in discriminating between amyloid positive individuals with mild cognitive impairment or dementia versus MCSA CN individuals aged 30-49. Based on this method, the cutpoint for tau PET was 1·23 SUVR and for MRI cortical thickness was 2·67 mm. Each participant in the present study was classified into one of the eight ATN states using these cut-points. As a secondary analysis, abnormal N was defined as HVa less than -1·15 cm3. This HVa cut-point was derived in the same manner and using the same samples described in. 23
Statistical methods
The MCSA sampled similar numbers of subjects within 5-year age and sex strata from age 50-90. As a result, individuals in the older age strata were overrepresented relative to the population. Therefore to summarize the overall clinical and demographic characteristics of the eight ATN groups (Fig 1, S1), it was necessary to weight our sample to reflect the actual age and sex distribution of the Olmsted County, Minnesota clinically normal population. Census Bureau estimates for 2010 along with MCI and dementia prevalence estimates from the MCSA were used to create the weights and the survey package in R was used to correct standard errors to account for strata weights (see statistical supplement).
Figure 1. Plots of ATN group characteristics.
Box plots of continuous variables and bar charts summarizing percentages of categorical variables from table 1 by ATN biomarker group. The box plots and estimated percentages reflect weighting the sample to match the age and sex distribution of Olmsted County, Minnesota residents who are clinically normal. Box and bar widths reflect relative sample sizes. As in Table 1, the 8 groups are sorted left-right hierarchically first on the basis of A- vs A+, then T- vs T+, then N- vs N+.
The estimated prevalence of each of the eight ATN groups was determined by partitioning the full 8-group model into two components: (1) a multinomial model with the 4-level AN group as the response and age and sex as covariates (n=1548) and (2) a logistic model with T+ as the response and AN, age, and sex as covariates (n=435). In this framework, the individuals without tau imaging can stabilize the overall estimates of the ATN prevalences by contributing information to part (1) of the model. Inference from the model was based on posterior simulations using the maximum likelihood estimate and the variance covariance matrix. These simulations allowed us to obtain point estimates and 95% confidence intervals for functions of the model parameters such as prevalence estimates, differences in prevalence estimates, and the age at which a prevalence curve peaks (see statistical supplement).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
The data in Table 1 represent unweighted summaries in our ATN sample (n=435). Summaries by ATN group weighted to the clinically normal Olmsted County population by age and sex are found in Fig 1. Age differed among ATN groups (p<0·0001) with individuals with worse biomarker profiles tending to be older (Table 1, Fig 1a). The group with the greatest estimated proportion of men is A−T−N+ (57%, 95% CI: 37%-77%) and the group with the greatest proportion of women is A+T−N− (78%, 95% CI: 64%-93%) however overall the sex distribution was not different among the ATN groups (p=0·21). APOE ε4 varies by ATN group (p=0·04) and is roughly twice as frequent among A+ individuals compared to A− individuals. WMH volume differed between ATN groups (Fig 1, p<0·0001) even after adjustment for age (p=0·01) (Fig S1). WMH was higher in N+ compared to N− groups (p=0·05) (Fig 1, Fig S1, table 1), although the magnitude of the differences was small. Cognitive performance also differed to some degree by group in all domains (Fig 1, p<0·0001) even after adjustment for age (Fig S1, p<0·03).
Table 1. Characteristics of participants by ATN biomarker classification.
| Characteristic | A−T−N− n = 165 | A−T+N− n = 35 | A−T−N+ n = 63 | A−T+N+ n = 19 | A+T−N− n = 44 | A+T+N− n = 25 | A+T−N+ n = 35 | A+T+N+ n = 49 |
|---|---|---|---|---|---|---|---|---|
| Age, years | 38% | 8% | 14% | 4% | 10% | 6% | 8% | 11% |
| Median (IQR) | 65 (58, 69) | 68 (62, 78) | 75 (67, 81) | 79 (73, 82) | 71 (66, 78) | 77 (70, 82) | 82 (77, 85) | 82 (76, 87) |
| Min, Max | 51, 84 | 53, 90 | 53, 90 | 63, 95 | 53, 88 | 65, 94 | 66, 91 | 64, 94 |
| Male gender, no. (%) | 83 (50%) | 19 (54%) | 45 (71%) | 9 (47%) | 16 (36%) | 15 (60%) | 22 (63%) | 27 (55%) |
| Education, years, Median (IQR) | 16 (13, 16) | 16 (14, 17) | 15 (13, 16) | 16 (13, 16) | 14 (13, 17) | 14 (14, 17) | 14 (12, 16) | 14 (12, 16) |
| APOE ε4 positive, no. (%) | 30 (19%) | 5 (15%) | 13 (22%) | 1 (5%) | 20 (49%) | 13 (52%) | 14 (41%) | 16 (33%) |
| WMH volume, Median (IQR) | 5.3 (3.4, 9.7) | 6.8 (3.7, 10.4) | 11.9 (5.6, 17.3) | 15.0 (8.2, 20.7) | 9.6 (5.2, 15.5) | 8.9 (6.4, 16.0) | 19.1 (9.7, 33.1) | 18.9 (11.1, 31.7) |
| Cognitive z-scores, Median (IQR) | ||||||||
| Memory | 0.5 (-0.2, 1.0) | -0.1 (-0.5, 0.7) | -0.2 (-0.8, 0.6) | 0.2 (-0.8, 0.9) | 0.1 (-0.5, 0.5) | 0.1 (-0.8, 0.8) | -0.6 (-1.1, -0.1) | -0.5 (-1.1, 0.3) |
| Attention | 0.4 (-0.1, 0.9) | 0.6 (0.0, 0.9) | -0.2 (-0.8, 0.3) | 0.2 (-0.2, 0.8) | 0.1 (-0.5, 0.6) | -0.2 (-0.7, 0.3) | -0.3 (-0.8, 0.2) | -0.5 (-1.4, 0.0) |
| Language | 0.3 (-0.3, 0.9 | 0.5 (-0.2, 1.1) | -0.2 (-0.7, 0.3) | -0.0 (-0.4, 0.4) | 0.2 (-0.2, 0.8) | 0.0 (-0.6, 0.4) | -0.6 (-1.0, 0.0) | -0.3 (-0.8, 0.1) |
| Visuospatial | ) 0.3 (-0.3, 0.9) | 0.3 (-0.2, 0.8) | -0.2 (-0.7, 0.4) | 0.1 (-0.4, 0.5) | 0.1 (-0.8, 0.5) | 0.2 (-0.6, 0.7) | -0.4 (-0.8, -0.0) | -0.1 (-1.3, 0.4) |
| Amyloid PET, Median (IQR)SUVR | 1.31 (1.26, 1.35) | 1.33 (1.30, 1.37) | 1.33 (1.28, 1.37) | 1.35 (1.31, 1.37) | 1.57 (1.47, 1.77) | 1.62 (1.55, 2.10) | 1.58 (1.50, 1.77) | 2.22 (1.54, 2.44) |
| Centiloid | 9 (5, 12) | 11 (8, 14) | 11 (7, 14) | 13 (9, 14) | 31 (23, 48) | 35 (29, 76) | 32 (25, 48) | 86 (28, 105) |
| Tau PET, SUVR, Median (IQR) | 1.15 (1.11, 1.19) | 1.28 (1.25, 1.30) | 1.17 (1.10, 1.20) | 1.28 (1.25, 1.30) | 1.16 (1.13, 1.20) | 1.27 (1.25, 1.34) | 1.16 (1.12, 1.20) | 1.30 (1.26, 1.36) |
| Cortical thickness, mm, Median(IQR) | 2.79 (2.73, 2.85) | 2.78 (2.75, 2.87) | 2.59 (2.53, 2.62) | 2.59 (2.52, 2.62) | 2.76 (2.72, 2.82) | 2.76 (2.72, 2.78) | 2.59 (2.47, 2.63) | 2.56 (2.51, 2.62) |
Table S1 shows demographic features of the 1548 clinically normal MCSA individuals with amyloid PET and MRI but not tau PET that were used to constrain or stabilize ATN prevalence estimates among the subset of 435 who had amyloid PET, MRI and tau PET.
For both men and women, A−T−N− prevalence declines from age 50 onward while A−T+N+ increases gradually with age starting at 60 and A+T+N+ increases more markedly with age beginning in the late 60s (Fig 2A) All of the remaining ATN groups reach individual peaks in prevalence.
Figure 2. Estimated prevalence of the ATN biomarker groups by age and sex.
Panel A shows the estimated prevalence curves by age and sex for all ATN groups. Panel B shows the same curves as panel A (except for the A−T−N−, A−T+N+, and A+T+N+) on an enlarged scale with the estimated peak for each curve shown with a square and a 95% confidence interval.
Within an ATN group, comparisons of the curves for men versus women (Fig S2) reveal slightly greater prevalence of A−T−N+ in men from age 65-75 but no other clear sex differences.
We averaged the sex-specific prevalence estimates in order to make direct age-specific prevalence comparisons between ATN groups (Fig S3). The dominant trends are: A−T−N− is the most prevalent group from age 50 to the late 70s. From the early 80s onward, A+T+N+ is the most prevalent group.
All groups except A−T−N−, A−T+N+, and A+T+N+ reach a peak prevalence. The age at which the prevalence curve peaks differs considerably among ATN groups but is similar for men and women within each group (Table 2, Fig 2B). A−T+N− is the first group to peak (age 64) followed by A+T−N− and A+T+N− (ages 71 and about 75, respectively). The N−groups (A−T+N−, A+T−N−, and A+T+N−) all peak by age 75 or earlier while the N+ groups (A−T−N+ and A+T−N+) do so at or above age 84. Table S2 shows pairwise comparisons of peak ages between the ATN groups. Differences in peak age between some ATN groups are substantial, particularly between N−and N+ groups. For example the A+T−N+ and A−T−N+ groups peak 24.9 and 21.8 years later than the A−T+N− group.
Table 2. Age (95% CI) at which the percentage of each ATN prevalence curve reaches its peak among women and men.
Differences in peaks by sex are also shown.
| Group | Women Peak Age (95% CI) | Men Peak Age (95% CI) | Men vs. Women Diff. Peak Age (95% CI) |
|---|---|---|---|
| A−T+N− | 64 (57, 68) | 64 (57, 68) | 0.1 (-1.2, 1.8) |
| A+T−N− | 71 (70, 73) | 71 (70, 72) | -0.2 (-1.0, 0.4) |
| A+T+N− | 75 (73, 79) | 74 (72, 78) | -0.8 (-2.2, 0.3) |
| A−T−N+ | 86 (81, 95) | 84 (80, 93) | -1.8 (-3.9, 0.4) |
| A+T−N+ | 88 (82, 100) | 87 (81, 100) | -1.6 (-3.8, 0.9) |
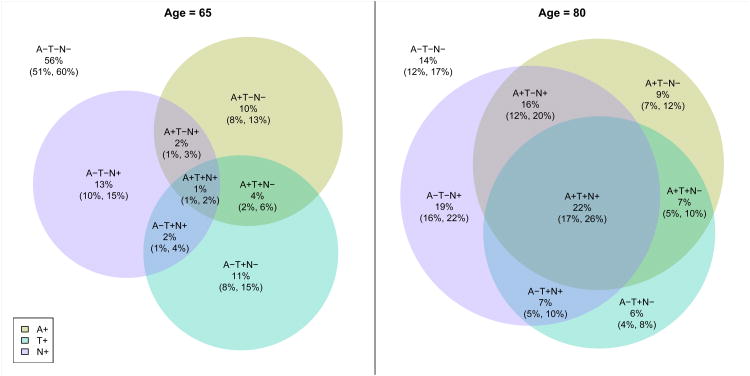
Fig 3 illustrates the proportions of individuals at ages 65 and 80 who have abnormal A, T, and N. This figure illustrates that abnormalities in these three biomarkers mostly do not overlap with each other at young ages. At older ages, the presence of more than one abnormal biomarker is common and there is considerable discordance among the three biomarkers.
Figure 3. Venn diagram of the estimated prevalence of each ATN group at age 65 and age 80.
These estimates are averaged over men and women. Since estimates are for a given age among clinically normal individuals, weighting to the population is not necessary. 95% confidence intervals for the estimates are also shown.
ATN prevalence by age was also computed using HVa instead of cortical thickness as the N measure (Fig S4). While, agreement between the HVa and thickness measures was moderate (kappa = 0·45), overall the ATN prevalence trends by age were similar when either HVa or cortical thickness was used. One notable difference was a higher prevalence of N+ in men than women when using HVa, which is evident when comparing the A−T−N+ curves between men and women (Fig 2A vs S4).
Discussion
Our main findings were the following. A−T−N− prevalence declines from age 50 onward while A−T+N+ and A+T+N+ increase continuously with age for both men and women. A−T−N− is the most prevalent group from age 50 to the late 70s. From the late 70s onward, A+T+N+ is the most prevalent group. The N− groups (A−T+N−, A+T−N−, and A+T+N−) all reached a peak prevalence by age 75 or earlier while the N+ groups (A−T−N+ and A+T−N+) reached a peak prevalence at or above age 84. .
Cross-sectional prevalence curves are a first step in understanding the complex and interdependent evolution of amyloid, tau, and neurodegeneration in aging individuals. Because our sample comes from a geographically stable population secular changes are likely to be minimized as much as possible, and thus we interpret differences in ATN prevalence curves across the 50 to 90 age range as being largely due to transitions between biomarker groups as people age. The declining prevalence of A−T−N− with age is logical since individuals can only transition out of A−T−N−, while the increasing prevalence of A+T+N+ with age makes sense because this is an absorbing biomarker state – i.e. people who remain CN can transition out of all states except A+T+N+ (Fig 2A). Interestingly, the increasing prevalence of A– T+N+ may reflect an absorbing state for those on a non- AD pathway.
For the other five ATN groups, the prevalence increases to a peak with age and then declines. The age at which the prevalence curves peak differs considerably among ATN groups (table 2, table S2, Fig 2B), but peak ages can be grouped into two clusters. The N– groups with evidence of either abnormal amyloid or tau deposition (A– T+N–, A+T– N–, and A+T+N–) all peak by age 75 or earlier while the N+ groups (A– T– N+ and A+T– N+) peak at or above age 84. From age 75 – 85 the prevalence of these three N– groups falls while the prevalence of these two N+ groups rises. This is consistent with the idea that neurodegeneration/neuronal injury is a downstream consequence of antecedent proteinopathies. The fact that A−T−N+ is more frequent than some N −groups in middle age (Fig S3) is consistent with the idea that this group is on a separate, non-AD trajectory where neurodegeneration is not driven by AD proteinopathy.
Overall the effect of sex on the prevalence of all ATN groups is minimal when using cortical thickness (Fig 2A, Fig S2), but more pronounced when using HVa (Fig S4).
APOE4 was more frequent among the A+ than the A− groups (Table 1, Fig 1). Among those who were A-, we found no clear evidence of elevation in APOE e4 frequency among A−T+N−, A−T−N+, or A−T+N+ relative to A−T−N− (Fig 1, table 1). Similarly, among those who are A+, we found no evidence of elevation in APOE ε4 frequency among A+T+N−, A+T−N+, or A+T+N+ relative to A+T−N−. One interpretation of this is that the primary effect of APOE ε4 is to increase amyloidosis, not to enhance tau deposition, neurodegeneration, or both through non-amyloid related mechanisms.
Abnormal biomarker profiles are associated with worse cognition across different domains after adjusting for age (Fig 1, Fig S1, table 1). WMH volume was higher in N+ in comparison to N− groups (p=0·05) (Fig 1, Fig S1, table 1). This supports the position that ischemic brain injury is, among other conditions, 29a likely contributor to N+.
SNAP was first described as A−N+ where N+ was based on FDG PET and MRI findings.7 In the 2011 NIA-AA criteria, the definition of N+ also included abnormal CSF phosphorylated and total tau.10, 11 In our present data, 15% of individuals were classified as SNAP defined by MR and amyloid PET at age 65, and 26% at age 80. Of these, 13% at age 65 and 27% at age 80 also had abnormal tau PET (i.e. A−T+N+) (Fig 3). Thus, tau and neurodegeneration are concordant only in a minority of A−N+ (SNAP) individuals where N+ is defined cortical thickness.30, 31 Mormino et al 32 and Wisse et al 32 reported that tau was not elevated in SNAP relative to A−N− individuals who were classified by amyloid PET and hippocampal volume and/or FDG PET using the NIA-AA plus SNAP 4-group construct. Similarly, we found that the proportions of T+ participants were similar among the A−N−and A−N+ groups, (16% vs 13% at age 65 and 30% vs. 27% at age 80) (Fig 3). However, by classifying A, T and N separately, we demonstrated that tau PET is frequently abnormal in SNAP where N+ is defined by cortical thickness. Tau PET had not yet been studied in humans when SNAP was first described. If the A−T+N−profile is included in the SNAP category where T+ is defined by tau PET, which we believe should be the case, then the proportion of SNAP with evidence of tauopathy is 50% at age 65 and 41% at age 80 (Fig 3).
We postulate that the A−T−N+ profile corresponds to neurodegeneration due to a heterogeneous group of non- AD pathologies that increase in prevalence with age including cerebrovascular disease, Lewy body disease,TDP 43, argyrophilic grains, and hippocampal sclerosis.34 A logical assumption is that the A−T+N−profile corresponds to primary age related tauopathy (PART).35 The A−T+N+ profile may correspond to a combination of PART and the other non-AD pathologies mentioned above. However, imaging - autopsy correlation studies will be needed confirm these hypotheses.
The four A+ profiles represent preclinical AD by the 2011 NIA-AA guidelines. A+T−N− corresponds to NIA- AA preclinical AD stage 1. A+T+N−, A+T−N+, and A+T+N+ all correspond to NIA-AA preclinical AD stage 2/3. Thus, tau and neurodegeneration are discordant in the majority of NIA-AA preclinical AD stage 2/3 individuals at age 65 (86%) and in half at age 80 (51%) (Fig 3).30,31 A model of AD pathogenesis proposes that amyloidosis promotes increased local tau deposition and its spread, which in turn is responsible for neurodegeneration. The ATN biomarker counterpart would be a sequence of A+T−N− to A+T+N− to A+T+N+. The facts that the median ages of these three groups (Table 1, Fig 1) and that the ages at which the prevalence curves peak (table 2, table S2, Fig 2B) increase incrementally lends support to the idea that A+T −N−to A+T+N− to A+T+N+ is the biomarker sequence of preclinical AD. However, longitudinal data will be necessary to confirm this chronological sequence. he A+T−N+ profile, which does not fit into the sequence of preclinical AD proposed above, perhaps indicates individuals in whom two different types of pathologies are evident by biomarkers: a non-AD degenerative process(es) resulting in N+, plus early AD resulting in the A+T−profile.
For our primary analyses, we used cortical thickness rather than commonly used hippocampal volume as our measure of neurodegeneration to avoid necessitating an adjustment for head size. Brain volumes scale with head size and correcting for this is not straightforward since head size is related to sex, yet sex-specific effects on atrophy likely exist. A solution is to use cortical thickness which does not scale closely with head size and consequently does not require an adjustment.37 Overall, the ATN prevalence curves by age were similar when either HVa (Fig S4) or cortical thicknesses (Fig 2A) were used as the N measure. These findings suggest that the ATN prevalences we report should be robust to different definitions of N. However, with only moderate agreement between abnormal HVa and thickness, there may be differences in which individuals are labeled N+ by the two biomarkers. We are uncertain if the more pronounced sex differences when using HVa as the N measure represent an artifact of head size adjustment or a true biological effect.
Our operationalization of the ATN scheme reflected a number of methodological factors and decisions. Both clinical- imaging correlation14-18 and autoradiographic 38,39 evidence, points to AV1451 as a useful measure of the 3R/4R paired helical filament tau deposits that are characteristic of AD and primary age related tauopathy 35.Binding in primary tauopathies (except those that produce 3R/4R fibrillar tau deposits) is less certain. In this study, we used a single reporter tau PET meta ROI that included medial, basal, and lateral temporal lobe areas 23.Our rationale was that tau PET uptake in these areas is consistently associated with characteristics of AD such as the presence of amyloid on PET, worse cognitive performance across the clinical spectrum, and abnormal CSF phosphorylated tau 14-18. This set of ROIs captures a broad dynamic range across the normal to pathologic aging to AD dementia spectrum; it therefore seems to represent a reasonable tau PET summary reporter 23.23
The ATN framework requires defining abnormality in each biomarker. We previously conducted a thorough examination of different methods for selecting cut-points to define abnormality on amyloid PET, tau PET and cortical thickness.23 We regard plaques, tangles and synapse loss to be pathological. While all of these processes increase in frequency and severity with age 34, our cut-points were not age-adjusted. Our position is that while not age-norming the cut-points results in a greater proportion of older individuals being labeled abnormal, the fact that an entity is frequent does not disqualify it from being pathological. While age-norming cognitive tests is a common practice, biomarkers in other fields are typically not age-normed. For example the cut-points used to define diabetes or hypertension are not changed with age. Loss of synapses and dendritic spines and associated cognitive/functional loss seems to be a nearly universal feature of aging in humans and a range of animal species. 40, 41 Whether this should be considered pathological or not is an unresolved question.
The methods of selecting reporter meta ROIs and cut-points used in ATN classification were AD-centric. However, while temporal lobe atrophy is characteristic of AD, it is not diagnostic for AD. A variety of non-AD conditions (argyrophillic grains, hippocampal sclerosis, etc.) may produce atrophy in these brain areas. However, until specific biomarkers of the common non-AD entities are developed, the only available biomarker evidence of their presence is nonspecific indicators of neurodegeneration/neuronal injury.
Our study has limitations. Because eight possible ATN combinations exist, participant numbers in some groups are small. Dichotomizing each biomarker simplifies what is an underlying continuous process. Measurement imprecision will inevitably result in some classification errors particularly for values close to cut-points. With three different biomarker classes per individual, the likelihood of classification error is compounded compared to a situation where only a single biomarker is used. We have not examined individuals in the population who have become clinically impaired; this awaits greater enrollment of impaired individuals in the MCSA. While the most rational explanation for the observed changing ATN prevalances with age is within-subject ATN group transitions, our data are cross-sectional. Our study raises interesting questions for which no answers exist at this time. For example, what are the longitudinal clinical/cognitive outcomes and the pathological underpinnings of these ATN groups? Answers to these questions require longitudinal clinical follow-up in large numbers of well characterized individuals with eventual autopsy correlation. To our knowledge, these data do not exist anywhere at this time for individuals characterized by ATN profile. Data addressing these issues will await maturation of our and other research cohorts.
Supplementary Material
Figure S1. Age adjusted plots of WMH volume and cognition by ATN grouBox plots of partial residuals of the log of WMH volume and cognitive z-scores after regressing out the effect of age. These plots are weighted to the clinically normal Olmsted County population by age and sex.
Figure S2. Estimated prevalence with 95% confidence limits of the ATN biomarker groups by age and sex. Since estimates are for a given age and sex among clinically normal individuals, weighting to the population is not necessary. Differences between men and women within biomarker group are also shown where values above zero indicate ages where the biomarker prevalence is higher in men than women and values below zero where the biomarker prevalence is higher in women than men. Differences are considered statistically significant if the confidence limits do not include zero.
Figure S3. Pairwise differences in prevalences among ATN biomarker groups. Values above zero indicate ages where the biomarker prevalence for the first group listed in the title is higher than in the second group and values below zero where the biomarker prevalence is higher for the second group listed in the title than the first. Differences are considered statistically significant if the confidence limits do not include zero. These curves were averaged over men and women.
Figure 4S. Estimated prevalence of the ATN biomarker groups by age and sex where N is defined using hippocampal volume adjusted for head size. Since estimates are for a given age and sex among clinically normal individuals, weighting to the population is not necessary.
Research in Context.
Evidence before this study
We searched PubMed with the terms “preclinical AD”, “tau PET”, and “amyloid PET” from January 2006 - September 2016, English language only. Clinically normal cohorts have been studied using the NIA-AA staging plus SNAP construct resulting in four different biomarker categories: A−N−, A+N−, A−N+ (SNAP) or A+N+. Proportions of these four groups were roughly similar in many cohorts. APOE ε4 was much more common in A+N− and A+N+ than in A−N− or SNAClinical and psychometric outcomes were uniformly worst in A+N+. These findings were largely the same whether biomarker categorization was done using imaging or CSF. The NIA-AA staging plus SNAP construct has been useful because it provided a common framework for different research groups to communicate their own findings.
Added value of this study
In retrospect, a weakness of the NIA-AA staging plus SNAP construct is the grouping of CSF phosphorylated tau into the same neurodegeneration/neuronal injury category with total tau, MRI and FDG PET. The ATN construct remedies this weakness and enables researchers to investigate multi-domain biomarker associations where the effects of tauopathy (defined by tau PET or CSF phosphorylated tau) and neurodegeneration/neuronal damage (defined by CSF total tau, MRI and FDG PET) at the individual level are segregated. We describe clinical characteristics and age- and sex- specific prevalences of individuals age 50 and older using the ATN construct. To our knowledge, this is the first study to do so. We found that tau and neurodegeneration were often discordant. Among the individuals in our sample who would be labeled NIA-AA stage 2/3 preclinical AD (i.e. A+T+N−, A+T−N+, and A+T+N+), 86% are discordant between T and N at age 65 and 51% at age 80. Among the individuals in our sample who would be labeled SNAP (i.e. A−T+N,− A−T−N+, A−T+N+), 92% are discordant between T and N at age 65 and 78% at age 80.
Implications of all the available evidence
The ATN classification scheme is a useful approach to biomarker characterization with the goal of more fully understanding the underlying heterogeneous pathology in the population. Marked age variation in prevalences requires careful interpretation of biomarker results from studies across cohorts of different ages. Future research will elucidate the within-subject biomarker changes to evaluate amyloid dependent (i.e. AD) and amyloid independent (i.e. SNAP) pathological pathways and sequences of biomarker abnormality.
Acknowledgments
Funding sources: National Institutes of Health (AG11378, AG041851, AG06786), The Alexander Family Professorship of Alzheimers disease research, Mayo Clinic. The GHR Foundation.
The Rochester Epidemiology Project, and AVID radiopharmaceuticals, Inc., for supplying AV-1451 precursor, chemistry production advice, and FDA regulatory cross-filing permission and documentation needed for this work.
Funding: National Institute on Aging; Alexander Family Professorship of Alzheimer's Disease Research. The GHR Foundation.
Footnotes
Contributors: Dr. Jack - conceptualization of the study, analysis and interpretation of data, drafting and revising manuscript. Dr. Jack had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ms. Wiste - conceptualization of the study, analysis and interpretation of data, drafting and revising manuscript, statistical analysis
Mr. Weigand - conceptualization of the study, analysis and interpretation of data, drafting and revising manuscript, statistical analysis
Dr. Therneau - analysis and interpretation of data, drafting and revising manuscript, statistical analysis
Dr. Knopman - drafting and revising manuscript
Dr. Lowe - data collection, drafting and revising the manuscript
Dr. Vemuri - drafting and revising manuscript
Dr. Mielke - drafting and revising manuscript
Dr. Roberts - drafting and revising manuscript
Dr. Machulda - drafting and revising the manuscript
Mr. Senjem - analysis and interpretation of data, drafting and revising manuscript
Dr. Gunter - analysis and interpretation of data, drafting and revising manuscript
Dr. Rocca - drafting and revising manuscript
Dr. Petersen - analysis and interpretation of data, drafting and revising manuscript
Declaration of interests: Dr. Jack reports other from Eli Lilly Co, grants from NIH, outside the submitted work.
Dr. Knopman reports personal fees from Data safety monitoring board DIAN study, personal fees from Data safety monitoring board Lundbeck AD trial, outside the submitted work.
Dr. Lowe reports personal fees from Bayer Pharmaceuticals, grants from GE Health Care, grants from Siemens Molecular Imaging, grants from AVID Radiopharmaceuticals, personal fees from Piramal Imaging, personal fees from Merck Research, outside the submitted work.
Mr. Senjem reports stock/options ownership from Celgene Corporation, Inovio Pharmaceuticals, Medtronic, Parexel International Corporation, and Gilead Sciences, outside the submitted work.
Dr. Petersen reports grants from NIH, during the conduct of the study; personal fees from Hoffman-La Roche; and Consultant Merck, Genentech, Biogen, Eli Lilly, outside the submitted work.
The other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carillo MC, Thies W, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Assocation workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morric JC, Rosser MN, Scheltens P, Thies W, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Assocation Workgrou. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, De Kosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging and Alzheimer's Association Workgroup. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carillo M, Thies W, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet neurology. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O'Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR., Jr Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimer's & dementia the journal of the Alzheimer's Association. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM, Roberts RO, Boeve BF, Petersen RC. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol. 2013;73(4):472–480. doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, Johnson KA, Sperling RA. Synergistic Effect of beta-Amyloid and Neurodegeneration on Cognitive Decline in Clinically Normal Individuals. JAMA neurology. 2014;71(11):1379–85. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet neurology. 2013;12(10):957–65. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Harten AC, Smits LL, Teunissen CE, Visser PJ, Koene T, Blankenstein MA, Scheltens P, van der Flier WM. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81(16):1409–16. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 12.Caroli A, Prestia A, Galluzzi S, Ferrari C, van der Flier WM, Ossenkoppele R, Van Berckel B, Barkhof F, Teunissen C, Wall AE, Carter SF, Scholl M, Choo IH, Grimmer T, Redolfi A, Nordberg A, Scheltens P, Drzezga A, Frisoni GB. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): Prediction of progression. Neurology. 2015;84(5):508–15. doi: 10.1212/WNL.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, Maruff P, Salvado O, Ames D, Martins RN, Masters CL, Rowe CC, Villemagne VL. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. The Lancet Neurology. 2016;15(10):1044–53. doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- 14.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. The Lancet Neurology. 2015;14(1):114–124. doi: 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KA, Shultz A, Betensky RA, Becker JA, Sepulcre J, Rentz DM, Mormino EC, Chhatwal J, Amariglio RE, Papp K, Marshall GA, Albers M, Mauro S, Pepin LC, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson BC, Gomez-Isla T, Hyman BT, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer's disease. Annals of neurology. 2016;79(1):110–9. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabionovici GD, Jagust WJ. PET Imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, Joshi AD, Devous MD, Sr, Mintun MS. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathologicalstages. Brain : a journal of neurology. 2016;139(Pt 5):1539–50. doi: 10.1093/brain/aww023. [DOI] [PubMed] [Google Scholar]
- 18.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer's disease. Science Translational Medicine. 2016;8(338):338ra66–338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. The Lancet Neurology. 2003;2(10):605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, and Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–47. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic Proceedings. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci K, Machulda MM, Mielke MM, Roberts RO, Vemuri P, Reyes D, Petersen RC. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016 doi: 10.1016/j.jalz.2016.08.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klunk WE, Koeppe RA, Price JC, Benzinger T, Devous M, Jagust W, Johnson KA, Mathis CA, Minhas D, Pontecorvo M, Rowe C, Skovronsky D, Minturn M. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimer's & dementia. 2015;11(1):1–15. doi: 10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitwell JL, Tosakulwong N, Weigand SD, Senjem ML, Lowe VJ, Gunter JL, Boeve BF, Knopman DS, Dickerson BC, Petersen RC, Jack CR., Jr Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? Neuroimage Clin. 2013;2:249–57. doi: 10.1016/j.nicl.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, Senjem ML, Vemuri P, Murray ME, Dickson DW, Parisi JE, Kantarci K, Weiner MW, Petersen RC, Jack CR. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage: Clinical. 2016;11:802–12. doi: 10.1016/j.nicl.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Mielke MM, Vemuri P, Lowe V, Senjem ML, Gunter JL, Reyes D, Machulda MM, Roberts R, Petersen RC. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain : a journal of neurology. 2015;138(Pt 12):3747–59. doi: 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, Senjem ML, Gunter JL, Vemuri P, Jack CR, Jr, Miller VM, Kantarci K. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80(10):911–8. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJ. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain : a journal of neurology. 2008;131(Pt 12):3286–98. doi: 10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- 30.Vos SJ, Gordon BA, Su Y, Visser PJ, Holtzman DM, Morris JC, Fagan AM, Benzinger TL. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. doi: 10.1016/j.neurobiolaging.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, Benzinger TL. Longitudinal beta-Amyloid Deposition and Hippocampal Volume in Preclinical Alzheimer Disease and Suspected Non-Alzheimer Disease Pathophysiology. JAMA neurology. 2016 doi: 10.1001/jamaneurol.2016.2642. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mormino EC, Papp KV, Rentz DM, Schultz AP, LaPoint M, Amariglio R, Hanseeuw B, Marshall GA, Hedden T, Johnson KA, Sperling RA. Heterogeneity in Suspected Non-Alzheimer Disease Pathophysiology Among Clinically Normal Older Individuals. JAMA neurology. 2016 doi: 10.1001/jamaneurol.2016.2237. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisse LE, Butala N, Das SR, Davatzikos C, Dickerson BC, Vaishnavi SN, Yushkevich PA, Wolk DA. Suspected non-AD pathology in mild cognitive impairment. Neurobiology of aging. 2015;36(12):3152–62. doi: 10.1016/j.neurobiolaging.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta neuropathologica. 2011;121(5):571–87. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jicha GA, Jellinger KA, Kovacs GG, Knopman D, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee AC, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein TD, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathologica. 2014;128(6):755–66. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53(4):1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML, Vasdev N, Dickerson BC, Gomperts SN, Growdon JH, Johnson KA, Frosch MP, Hyman BT, Gomez-Isla T. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Annals of neurology. 2015;78(5):787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, Kantarci K, Boeve BF, Pandey MK, Bruinsma T, Knopman DS, Jones DT, Petrucelli L, Cook CN, Graff-Radford NR, Dickson DW, Petersen RC, Jack CR, Jr, Murray ME. An autoradiographic evaluation of AV- 1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77(2):219–34. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeoman M, Scutt G, Faragher R. Insights into CNS ageing from animal models of senescence. Nature reviews Neuroscience. 2012;13(6):435–45. doi: 10.1038/nrn3230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Age adjusted plots of WMH volume and cognition by ATN grouBox plots of partial residuals of the log of WMH volume and cognitive z-scores after regressing out the effect of age. These plots are weighted to the clinically normal Olmsted County population by age and sex.
Figure S2. Estimated prevalence with 95% confidence limits of the ATN biomarker groups by age and sex. Since estimates are for a given age and sex among clinically normal individuals, weighting to the population is not necessary. Differences between men and women within biomarker group are also shown where values above zero indicate ages where the biomarker prevalence is higher in men than women and values below zero where the biomarker prevalence is higher in women than men. Differences are considered statistically significant if the confidence limits do not include zero.
Figure S3. Pairwise differences in prevalences among ATN biomarker groups. Values above zero indicate ages where the biomarker prevalence for the first group listed in the title is higher than in the second group and values below zero where the biomarker prevalence is higher for the second group listed in the title than the first. Differences are considered statistically significant if the confidence limits do not include zero. These curves were averaged over men and women.
Figure 4S. Estimated prevalence of the ATN biomarker groups by age and sex where N is defined using hippocampal volume adjusted for head size. Since estimates are for a given age and sex among clinically normal individuals, weighting to the population is not necessary.