Abstract
The onset of steroidogenesis in human fetal adrenal glands (HFA) during the first trimester is poorly investigated. An unresolved question is the capacity of the HFA to produce potent androgen DHT via conventional and/or the backdoor pathway(s) at the end of first trimester, when androgen-responsive organs are developed. Our aim was to explore steroidogenesis and the expression of steroidogenic enzymes and transcription factors in HFA at gestational weeks (GW) 9–12 with focus on their androgenic potential. Steroids in the HFA were analyzed by gas chromatography/mass spectrometry. The expression of steroidogenic enzymes and transcription factors in the HFA at GW9–12 was investigated by qPCR, automated Western blotting and immunohistochemistry. We demonstrated that during GW9–12 HFA produced steroids of the ∆5, ∆4 and the backdoor pathways of the biosynthesis of DHT, though the latter was limited to production of 17α-OH-dihydroprogesterone, androsterone and androstanedione without further conversion to DHT. The only androgens identified in the HFA were testosterone and androsterone, a precursor in the biosynthesis of DHT. We also observed higher levels of CYP17A1 but low expression of 3βHSD2 at GW11–12 in the HFA. Elevated levels of CYP17A1 were associated with an increased expression of SF-1 and GATA-6. Altogether, our data demonstrate that of those steroids analyzed, the only potent androgen directly produced by the HFA at GW9–12 was testosterone. The onset of steroidogenesis in the HFA is a complex process that is regulated by the coordinated action of related transcription factors.
Keywords: human fetal adrenals, steroidogenic enzyme expression, androgens, steroid profiles
Introduction
Steroid hormones produced by the human fetal adrenal glands (HFA) have been proposed to regulate intrauterine homeostasis and the maturation of certain organs required for extrauterine life (1). In this case, appropriate development of and hormonal production by the HFA are critical for normal fetal maturation and survival.
The HFA develop from the intermediate mesoderm and by GW7 have acquired two distinct zones, the inner fetal (FZ) and outer definitive zone (DZ) (2). In parallel, differentiation of the female and male external genitalia begins at GW7 and is completed by GW10, during which period, an overabundance of androgens can lead to abnormal male-directed development referred to as virilization (3). Deficient activity of cytochrome P450 21-hydroxylase (CYP21A2) is one defect in adrenocortical steroidogenesis well known to result in overproduction of androgens by the HFA in response to ACTH, a disorder referred to as congenital adrenal hyperplasia (4).
It has been reported that the HFA has the potential to produce testosterone from androstenedione by action of 17βHSD5 (5), similar to that observed in the adult adrenals, where this enzyme has been found to be expressed in zona reticularis (6). It is also known that testosterone can be further converted to 5α-dihydrotestosterone (DHT) in the target tissue such as the external genital tissue (7), and this metabolic process from cholesterol to DHT by means of testosterone is called the conventional pathway. In addition to this the frontdoor route, it has been demonstrated that 17-hydroxyprogesterone (17-OHP) can be converted to DHT in the testes of pouch young of the tammar wallaby and immature postnatal testes of rodents via an alternative ‘backdoor’ pathway that bypasses the conventional intermediates androstenedione and testosterone (8, 9, 10). In this pathway, progesterone and 17OH-progesterone are subsequently converted to dihydroprogesterone (DHP), allopregnanolone, 17-OH allopregnanolone, androsterone, androstanediol and DHT by the action of 5α-reductase 1 (SRD5A1), CYP17A1, the family of 3α-HSD1–4 (AKR1C1–4), 17βHSD3 and 17βHSD6 (HSD17B3, HSD17B6) (9, 11). Recent studies have reported the presence of a backdoor pathway of DHT synthesis in newborn untreated patients with 21-hydroxylase deficiency (21-OHD), suggesting that the HFA could contribute to virilization of the female fetuses with 21-OHD via production of this potent androgen (12). In line with this, the HFA have been reported to express abundantly AKR1C1, AKR1C3 and AKR1C4 (13), suggesting that synthesis of DHT via the backdoor pathway is probable but not proven in physiological conditions by the HFA. Furthermore, recent study has proposed that DHT can be produced from androstenedione either through 5α-androstanedione (i.e. androstenedione, 5α-androstanedione, DHT) or through 5α-androstanedione, androsterone and androstanediol (i.e. androstenedione, 5α-androstanedione, androsterone, androstanediol, DHT) in the human prostate (14). Given that the HFA produce androstenedione and express many steroidogenic enzymes required for this pathway (5, 13, 15), such a novel pathway for the biosynthesis of DHT cannot be excluded.
One unresolved question in this context is the capacity of the HFA to produce potent androgen DHT via conventional and/or the backdoor pathway(s) at the end of the first trimester, when androgen-responsive organs are developed. Furthermore, due to limited access to appropriate material for research, little is presently known about the potential relationship between the expression of steroidogenic enzymes and of associated transcription factors by the HFA at early stages of the gland development.
Accordingly, in the present study, we carried out a comprehensive analysis of the profile of steroids produced by the HFA at the end of the first trimester. To extend our knowledge concerning the onset of steroidogenic activity of the HFA, we also explored expression of the corresponding steroidogenic enzymes and associated transcription factors during GW9–12. We found that at the end of the first trimester, the HFA have potential to produce testosterone and 5α-reduced precursors of DHT biosynthesis but not this androgen itself. Moreover, the ontogenic expression profiles of the various steroidogenic enzymes differ and are regulated by appropriate transcription factors.
Materials and methods
Ethical approval
These experimental procedures were approved by the Regional Ethics Committee of Stockholm (EPN dnr 2014/1022-32).
Human fetal adrenal collection
HFA were obtained from aborted fetuses in connection with elective termination of pregnancy during the first trimester (9–12 weeks of gestation) at Karolinska University Hospital, Stockholm, Sweden. Fetuses were transported to the laboratory within 30 min of delivery. Gestational age was validated by ultrasound (crown-rump length) and measuring fetal limb length to obtain more precise information about the age of the fetuses as described previously (16). Average value of each gestational week (GW) of the fetuses was as follows: GW9.3 ± 0.04 (n = 9), GW10.3 ± 0.06 (n = 10), GW11.2 ± 0.05 (n = 14) and GW12.0 ± 0 (n = 5). The fetuses were dissected under a binocular microscope in ice-cold PBS and the HFA were removed aseptically. The HFA were isolated from individual fetuses within few minutes and immediately snap-frozen in dry ice and stored at −85°C during one–two weeks before performing analysis of steroidogenic genes expression, steroid levels assay and Western blotting or fixed overnight in neutral-buffered formaldehyde, transferred to 70% ethanol and processed for histology. Adrenals from 38 fetuses were divided into two groups (GW9–10 and GW11–12) and the sexes were pooled.
Isolation of RNA and generation of cDNA
Total RNA was extracted with the RNeasy Mini Kit (74104, Qiagen), in accordance with the manufacturer’s instructions. This RNA was then pretreated with DNAse (RNase-free DNase Set, Qiagen), again as specified by the manufacturer and then quantified by spectrophotometry (BioPhotometer, Hamburg, Germany). The RNA was maintained at −80°C until further processing with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories) employing the manufacturer’s protocol.
Analysis of gene expression by qPCR
The samples were prepared for qPCR utilizing iQ SYBR Green Supermix (170-8882, Bio-Rad Laboratories) and after determining the optimal conditions by running a temperature gradient, cycles run at 95°C for 10 s, 60–62°C for 45 s, 95°C for 60 s and 55°C for 60 s, followed by performance of a melting curve from 55°C to 95°C in steps of 0.5°C and then maintenance at 4°C (iCycler iQ, Bio-Rad Laboratories). To compensate for possible variations in RNA concentration, all values were normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, the product of a housekeeping gene. To monitor efficiency, negative control (RT-) was added to each qPCR assay. The 2−∆Ct method was applied to calculate fold changes in gene expression. An overview of the primers and running conditions employed are presented in Table 1.
Table 1.
qPCR primer sequences and running conditions, bp-base pair.
| Oligo | Sequence | Prod. length (bp) | Temp (°C) |
| GAPDH | F: 5′-gaaggtgaaggtcggagtcaac-3′ | 71 | 55–65 |
| R: 5′-cagagttaaaagcagccctggt-3′ | |||
| CYP11A1 | F: 5′-ctcagtcctggtcaaaggct-3′ | 250 | 60 |
| R: 5′-cttctccctgtaaatcgggc-3′ | |||
| CYP17A1 | F: 5′-ggttcgtatgggcaccaaga-3′ | 111 | 60 |
| R: 5′-agagttgccatttgaggccg-3′ | |||
| GATA4 | F: 5′-gcggaaagaggggatccaaa-3′ | 75 | 60 |
| R: 5′-gaaggagctgctggtgtctt-3′ | |||
| SF-1 | F: 5′-tggtgtgagggggtttctgc-3′ | 249 | 60 |
| R: 5′-aagaagcccttgcagctctca-3′ | |||
| GATA6 | F: 5′-agaagcgcgtgccttcatc-3′ | 158 | 60 |
| R: 5′-catagcaagtggtctgggca-3′ | |||
| CEBPB | F: 5′-gcacagcgacgagtacaaga-3′ | 192 | 60 |
| R: 5′-tgcttgaacaagttccgcag-3′ | |||
| MRAP | F: 5′-agatgaggaacagccccaag-3′ | 243 | 60 |
| R: 5′-gggtcagttcccagaggaga-3′ | |||
| SULT2A1 | F: 5′-tcagttccaaggccaaggtg-3′ | 248 | 60 |
| R: 5′-tcctgtgtcctgtttcagctc-3′ | |||
| AKR1C4 | F: 5′-gaggaacagagctgtgtagaggtcacc-3′ | 185 | 60 |
| R: 5′-tgaaagaaagtgcaccaaagct-3′ | |||
| AKR1D1 | F: 5′-cttgggaggttgagtgccat-3′ | 226 | 60 |
| R: 5′-cgctggatgttgaaacgcaa-3′ | |||
| SP1 | F: 5′-cagcgtccgcgtttttcc-3′ | 93 | 60 |
| R: 5′-tgatcttggtcgctcatggt-3′ | |||
| MC2R | F: 5′-gcacagttcatgtgggatga-3′ | 232 | 60 |
| R: 5′-cacacgaggacagtcggaat-3′ | |||
| HSD17B3 | F: 5′-caccggatgaaatccagagcc-3′ | 253 | 60 |
| R: 5′-gacagcatatggggtcagcac-3′ | |||
| STAR | F: 5′-tgggagctcctacagacaca-3′ | 163 | 60 |
| R: 5′-ttccagccgagaaccgagta-3′ | |||
| AKR1C3 | F: 5′-aagaagtaaagctttggaggtcaca-3′ | 183 | 60 |
| R: 5′-cgatgaaaagtggaccaaagct-3′ | |||
| DAX-1 | F: 5′-tgctctttaacccggacggagtg-3′ | 102 | 60 |
| R: 5′-gcgtcatcctggtgtgttca-3′ | |||
| COUP-TFII | F: 5′-tcctgttcacctcagatgcc-3′ | 131 | 60 |
| R: 5′-ctttccgaatctcgtcggct-3′ | |||
| LRH-1 | F: 5′-tcctggttactgggcaacaa-3′ | 71 | 60 |
| R: 5′-gaggttgttgagggtggctc-3′ | |||
| HSD3B2 | F: 5′-tctaagttacgccctcttctgg-3′ | 207 | 60 |
| R: 5′-ttgtccaaggccctgatctc-3′ | |||
| POR | F: 5′-tgccagcgtttcatgatcaac-3′ | 132 | 60 |
| R: 5′-gagacccacgatgagcgaaa-3′ | |||
| CYB5A | F: 5′-ggagctccatccagaaactct-3′ | 157 | 60 |
| R: 5′-ttcctgcgctgacttctgag-3′ | |||
| AKR1C2 | F: 5′-taaaagtaaagctctagaggccgt-3′ | 183 | 60 |
| R: 5′-cgatgggaattgctccaaagctt-3′ | |||
| SRD5A1 | F: 5′-tctgatgcgaggaggaaagc-3′ | 175 | 60 |
| R: 5′-catgcccgttaaccacaagc-3′ |
Automated Western blotting
All reagents for Wes-automated Western blotting were prepared and used in accordance with the manufacturer’s recommendations (ProteinSimple, San Jose, CA, USA; www.proteinsimple.com/simon.html). The HFA were lysed in CelLytic cell reagent (Sigma) and the lysates diluted with sample buffer to a protein concentration of 0.3 µg in 4 µL were mixed together with the 5x Master Mix (DTT, fluorescence labeled marker, SDS) in a ratio 5:1 and then incubated at 95°C for 5 min. The samples, the biotin-labeled protein ladder, blocking reagent, primary antibodies, HRP-conjugated secondary antibodies, chemiluminescent substrate and stacking matrices were loaded into individual wells of the sample plate. Antibodies were diluted with antibody diluent buffer. After plate loading, the separation electrophoresis and immunodetection steps took place in the capillary system and were fully automated in Wes instrument. Briefly, the capillaries first fill with separation matrix for 200 s, and then stacking matrix for 15 s and finally sample for 9 s with vacuum injection. Separation was then performed at 375 V for 25 min in each capillary. After separation, the capillaries were exposed to UV light, activating the proprietary linking chemistry and locking the separated protein to the capillary wall. Subsequently, the matrix was removed and washed with washing buffer for three times. The capillaries were then blocked with antibody diluent to prevent non-specific binding, and target proteins were immunoprobed with primary antibodies, followed by HRP-conjugated secondary antibodies.
The 30-min incubations with primary antibodies against StAR, CYP17A1, GAPDH and CYP11A1 and 60-min incubations with SF-1, 3βHSD2, DAX1 and GATA6 antisera were followed by incubation with HRP-conjugated goat anti-rabbit secondary antibody for 30 min. The detail information about the antibodies used is presented in Table 2.
Table 2.
List of antibodies used in the study.
| Peptide/protein target | Name of antibody | Manufacturer, catalog no. or name of source | Species raised in monoclonal or polyclonal | Dilution used |
|---|---|---|---|---|
| SF-1 | NR5A1 | NovusBiologicals, NBP1-52823 | Rabbit polyclonal | 1:50 |
| DAX1 | DAX1/NR0B1 | NovusBiologicals, NBP1-32832 | Rabbit polyclonal | 1:50 |
| CYP17A1 | Anti-cytochrome P450 17A1 antibody (EPR6293) | Abcam, ab125022 | Rabbit monoclonal | 1:50 |
| CYP11A1 | Anti-CYP11A1 antibody | Abcam, ab175408 | Rabbit polyclonal | 1:300 |
| GATA-6 | GATA-6 (H-92) | Santa Cruz, sc-9055 | Rabbit polyclonal | 1:25 |
| 3β-HSD | 3β-HSD (H-143) | Santa Cruz, sc-28206 | Rabbit polyclonal | 1:25 |
| StAR | StAR (FL-285) | Santa Cruz, sc-25806 | Rabbit polyclonal | 1:100 |
| GAPDH | GAPDH (FL-335) | Santa Cruz, sc-25778 | Rabbit polyclonal | 1:150 |
| 5αR1 | 5αRED1(H-105) | Santa Cruz, sc-20658 | Rabbit polyclonal | 1:100 |
| AKR1C3 | Human Aldo-keto Reductase 1C3/AKR1C3 | R&D Systems, MAB7678 | Mouse monoclonal | 1:100 |
| 5βR1 | AKR1D1 | Thermo Fisher Scientific, PA5-28963 | Rabbit polyclonal | 1:100 |
| AKR1C1/AKR1C2 | Anti-AKR1C1/AKR1C2 antibody –C-terminal | Abcam, ab170613 | Rabbit polyclonal | 1:100 |
Luminol and peroxide (ProteinSimple) were then added to generate chemiluminescence. The digital images obtained were analyzed with the Compass software (ProteinSimple). Band densities were normalized against GAPDH and the ontogenetic expression of steroidogenic enzymes and related transcription factors were expressed as percentages of the corresponding values of GW9 values. The lane views of the expression of relevant steroidogenic enzymes and system software–generated electropherograms are shown in Supplementary Fig. 1 (see section on supplementary data given at the end of this article).
GC–MS/MS analysis of steroids
Steroids from the HFA at GW9–10 (n = 5) and GW11–12 (n = 7) were extracted twice with diethyl ether and prepared for analysis as described earlier (17). Briefly, tissue samples (from 0.5 to 15 mg) were ground in the presence of methanol/water 80:20 (v:v). The resulting mash was transferred in glass tubes, and the methanol layer was evaporated under N2 stream. Then, an enzymatic deconjugation was performed for hydrolysis of glucuronide and sulfate forms by β-glucuronidase and arylsulfatase, respectively. The deconjugation was operated overnight at 37°C. All total (free + deconjugated) steroids were then extracted twice with diethyl ether. A liquid/liquid partitioning with pentane was used to separate androgens/progestogens from estrogens, and the two resulting fractions were finally submitted to a further purification through silica (SiOH) solid-phase extraction cartridges.
Detection and quantification of androgens and estrogens were performed on a Scion 436 gas chromatograph coupled to a Scion TQ triple quadrupole mass spectrometer (Bruker, Fremont, CA, USA) as described previously (17, 18). The steroids assayed, all involved in the biosynthesis of androgens and estrogens via the ∆4, ∆5 and backdoor pathways for DHT synthesis were as follows: pregnenolone, progesterone, 17-hydroxypregnenolone, 17-hydroxyprogesterone, androstenedione, DHEA, 17β-testosterone, 17α-testosterone, DHT, androsterone, etiocholanolone, 17α-hydroxydihydroprogesterone, 5α-androstanedione, 17αOH-allopregnanolone, allopregnanolone, androstanedione, androstanediol, 5-androstene-3α,17β-diol, 5-androstene-3β,17α-diol, 5α-androstane-3β-17α-diol, epiandrosterone, 5-androstene-3β,17β-diol, 5α-androstane-3β-17β-diol and 17β-estradiol. The coefficients of determination (R2) of the calibration curves performed for each batch of analysis were higher than 0.99. The inter-day repeatability was lower than 10% for a majority of substances, and globally lower than 20% (Supplementary Table 1).
Immunohistochemical analysis
Paraffin-embedded fetal adrenal tissue was cut to a thickness of 5 µm and mounted on microscope slides (P/N10143352, Superfrost Plus, Thermo Scientific) and placed at 60°C for 40 min in the oven. Tissue sections were dewaxed with xylene (P/N 02080, HistoLab, Gothenburg, Sweden) for 20 min and then rehydrated in graded ethanol (99.6, 96 and 70%). Antigen retrieval in 0.01 M citrate buffer (pH 6.0) for 20 min in a water bath was used for all slides.
Samples were incubated with 3% H2O2 in 96% methanol for 10 min at RT for non-specific endogenous peroxidase blocking. After washing with PBS with or without 0.01% Tween 20 (P/N P1379, Sigma Aldrich), the sections were treated with a mixture of 3% goat serum and 1% bovine serum albumin (BSA) in PBS for 60 min at RT to avoid non-specific binding. Slides were subsequently incubated with primary mouse monoclonal antibody to 17βHSD5 and rabbit polyclonal antibodies against AKR1C1/AKR1C2, 5βR1 (AKR1D1) and 5αR1 (Table 2) or unspecific IgGs (for negative control) dissolved in 3% goat serum in PBS overnight at 4°C. After washing with PBS and 0.01% Tween 20, the slides were incubated with biotinylated secondary antibody (ab64256, Abcam), and then with avidin–biotin–peroxidase complex prepared using Vectastain ABC kit (PK-6100, Vector Laboratories, Burlingame, CA, USA) for 60 min at RT. Finally, the slides were stained with DAB (SK-4105, Vector Laboratories) for 20–40 s at RT, washed twice with H2O, counter-stained with Hämalaun solution (Merck), rinsed for five min with running tap water, dehydrated with gradually increasing concentrations of ethanol, cleared with xylene and mounted with cover glass. IgG-negative sections exposed to non-immune rabbit and mouse serum in the absence of primary antibody were included in all immunohistochemistry runs and showed no positive immunostaining.
Statistical analyses
Differences between values were analyzed for statistical significance with Student’s t-test for pairwise comparison and by the one-way analysis of variance (ANOVA) for multi comparison followed by Student–Newman–Keuls analysis or Dunn’s analysis if the normality test failed using the SigmaStat (v 11.00) package (SPSS). P < 0.05 was considered to be statistically significant.
Results
The level of steroids produced by HFA at the end of first trimester
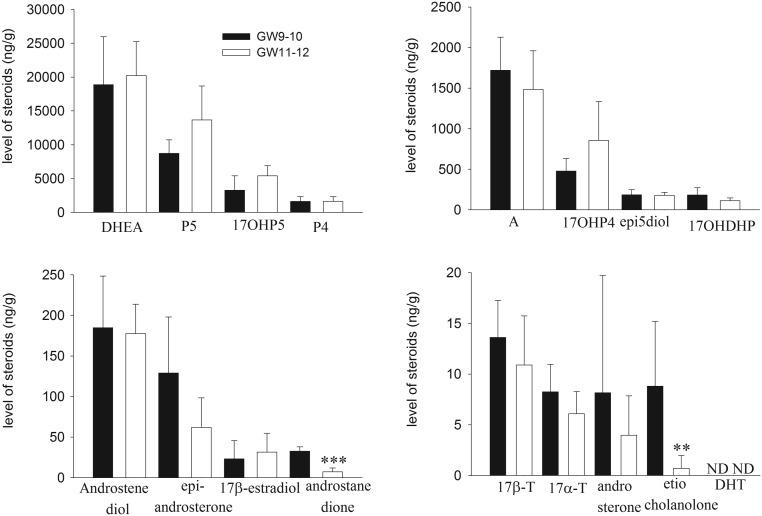
We observed that the HFA at the end of the first trimester produced substantial amount of steroids of ∆5 and ∆4 pathways and several 5α-and 5β-reduced metabolites of DHEA and androstenedione (e.g. androstanedione, epiandrosterone, etiocholanolone) as well as some precursors of DHT biosynthesis via the backdoor pathway (e.g. 17OH-DHP and androsterone), but DHT itself was not detected in the HFA at GW9–12 (Fig. 1). The only androgens identified in the HFA were testosterone, which was found in the form of 17α- and 17β-testosterone and androsterone, a precursor in the biosynthesis of DHT. We also found significant attenuation of the production of reduced steroids, androstanedione and etiocholanolone (by 78 and 92%, P < 0.001, P < 0.01, respectively) by the HFA at GW11–12 compared to GW9–10 (Fig. 1).
Figure 1.
The tissue levels (ng/g) of steroids in the HFA at the end of the first trimester. Concentrations of pregnenolone (5P), 17-OH pregnenolone (17-OHP5), progesterone (P4), 17-OH progesterone (17-OHP4), DHEA, androstenedione (A),17β-testosterone (17β-T), 17α-testosterone (17α-T), androstenediol, androsterone, 17α-OH-dihydroprogesterone (17OHDHP), 5-androstene-3β,17α-diol (epi5-diol), epiandrosterone, androstenedione, etiocholanolone, DHT and 17β-estradiol in the HFA were measured by GC–MS/MS as described in the ‘Materials and methods’ section. The values shown are means ± s.d. for five and seven HFA isolated from individual fetuses at GW9–10 and GW11–12, respectively. **P < 0.01, ***P < 0.001 compared to GW9–10. ND, not detectable.
Ontogeny of steroidogenic enzyme expression in the HFA at the end of the first trimester
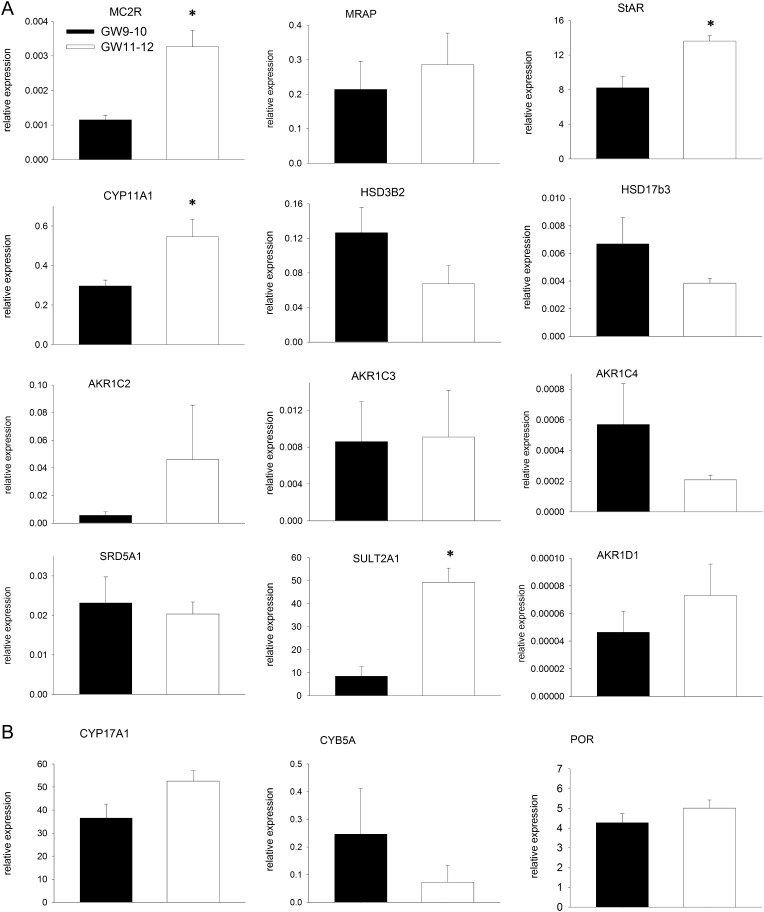
Upon exploring the ontogeny of the expression of steroidogenic enzymes and of the ACTH receptor (MC2R) in the HFA at the end of the first trimester as an indication of steroidogenic potential, we found higher level of MC2R mRNA at GW11–12 then at GW9–10 (by 2.9-fold, P < 0.05), but the expression of its accessory protein MRAP was similar at both ages (Fig. 2A), indicating ongoing differentiation. Similarly, the levels of mRNA encoding steroidogenic genes StAR, CYP11A1 and SULT2A1 were significantly higher in the HFA from GW11–12 then at GW9–10 (Fig. 2A). We also observed that the HFA highly expressed CYP17A1 and genes coding P450 oxidoreductase (POR) and microsomal cytochrome b5 (CYB5A) (Fig. 2B), the accessory proteins that support the catalytic activity of CYP17A1.
Figure 2.
Levels of mRNAs encoding steroidogenic genes in the HFA during GW9–12. The mRNA levels were normalized to GAPDH as a housekeeping gene. The values presented are means ± s.e. for eight and six HFA at GW9–10 and GW11–12, respectively. *P < 0.05 compared to GW9–10.
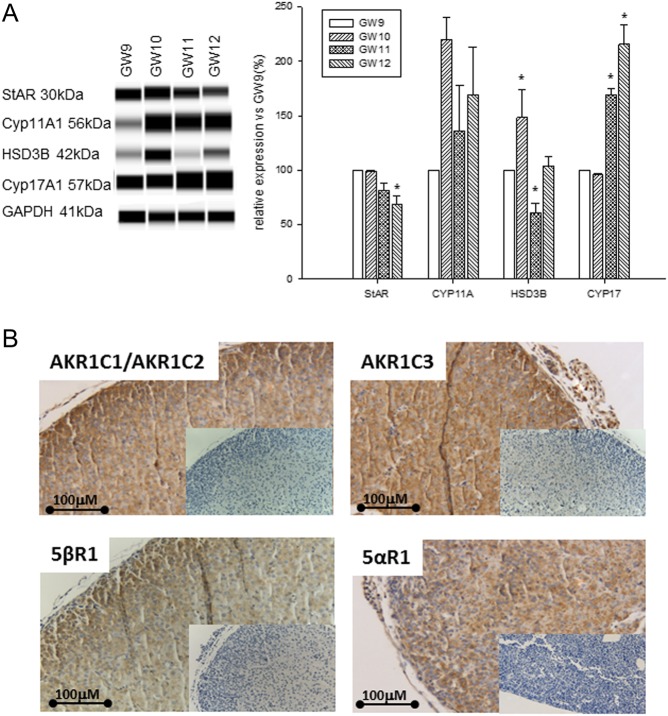
At the protein level, expression of CYP17A1 was upregulated at GW11–12 compared to GW9–10, while the level of 3βHSD2 protein peaked at GW10 and was later reduced at GW11 (Fig. 3A). Immunohistochemical analysis of cross sections of the HFA revealed expression of several androgen-metabolizing enzymes such as 5αRI, 5βRI, AKR1C1/AKR1C2 and AKR1C3 (Fig. 3B). This observation agrees well with our finding of several steroidogenic products (e.g. 17OHDHP, androsterone and etiocholanolone) as a result of the activities of these enzymes.
Figure 3.
Expression of steroidogenic enzyme proteins by the HFA during GW9–12. (A). Protein levels were analyzed by Wes-automated Western blotting and representative blots are shown to the left. To the right, band densities are expressed as a percentage of the corresponding density of GW9. The values shown are means ± s.e. for three (GW9–11) and two (GW12) HFA isolated from individual fetuses. *P < 0.05 compared to GW9. (B) Expression of androgen-metabolizing enzymes in the HFA at GW10 identified by immunohistochemistry. Inserts show the absence of immunostaining in the presence of non-immune serum instead of primary antibody. Scale bar: 100 μm.
The observed differences in the temporal profiles of the expression of steroidogenic enzymes by the HFA suggest a link between the onset of steroidogenesis and the expression of certain transcription factors.
Expression of transcription factors by the HFA during GW9–12
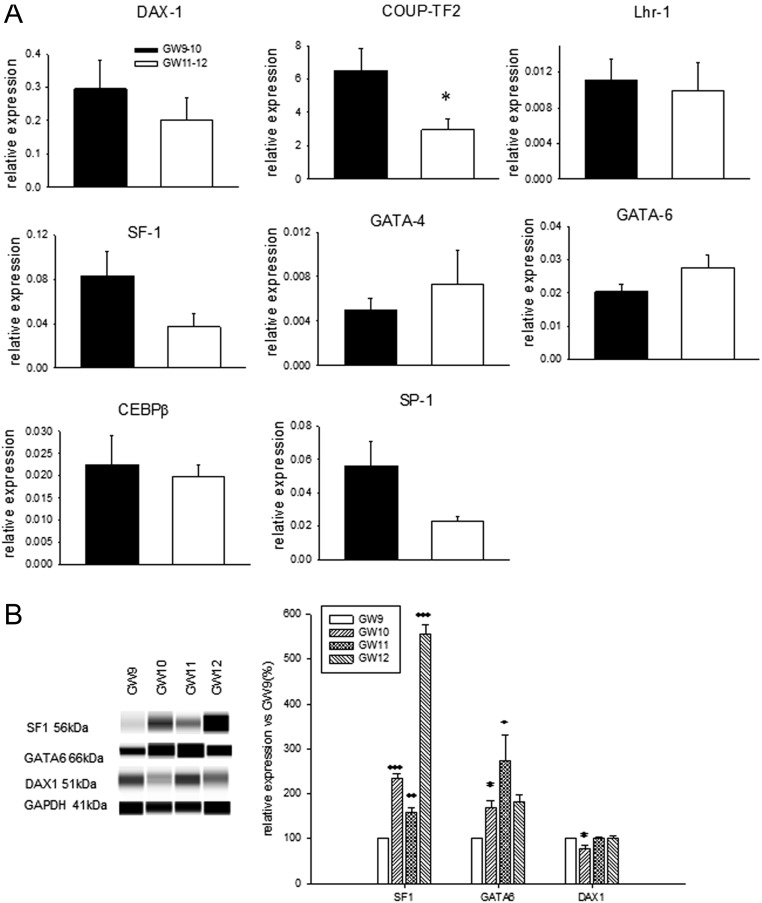
To test this suggestion, we characterized the expression of transcription factors involved in both positive and negative regulation of steroidogenic gene expression. We observed that the level of mRNA encoding the transcription factor COUP-TF2 declined significantly in the HFA at GW11–12 and no significant differences in the levels of mRNA encoding the other transcription factors examined were detected in the HFA at GW9–12 (Fig. 4A). However, at the protein level, expression of SF-1 and GATA-6 was significantly upregulated during GW10–12 and GW10–11, while expression of DAX-1 was slightly, but significantly attenuated by GW10 (Fig. 4B).
Figure 4.
Levels of transcription factors involved in the regulation of steroidogenic enzyme expression in the HFA during GW9–12. (A) The mRNA and (B) protein levels were measured by qPCR and Wes-automated Western blotting, respectively. The values presented are means ± s.e. for the same number of samples as indicated in Figs 2 and 3. (A) *P < 0.05 compared to GW9–10, (B) *P < 0.05, **P < 0.01, ***P < 0.001 compared to GW9.
Discussion
In the present study, we analyzed the steroidogenic machinery of the HFA during a critical phase of the first trimester, when androgen-dependent sexual dimorphism of the external genitalia is established. To our knowledge, this is the first detailed analysis of steroid production by the HFA at the end of the first trimester employing highly sensitive GC–MS/MS technique and linking this production to the levels of steroidogenic enzymes and related transcription factors. We demonstrated that during GW9–12, HFA produced steroids of the ∆5, ∆4 and the backdoor pathways of the biosynthesis of DHT, though the latter was limited to production of 17α-OH-dihydroprogesterone, androsterone and androstanedione without further conversion to DHT. The only androgens identified in the HFA were testosterone and androsterone, a precursor in the biosynthesis of DHT.
Our study has demonstrated that the capacity of the HFA to synthesize testosterone was limited and only less than 1% of androstenedione was converted to the androgen. This finding agrees well with the study reported that organ culture of the HFA at GW8 can produce low levels of testosterone in unstimulated and activator-stimulated conditions (5). It should also be noted that the abundance of DHEA vs androstenedione and androstenediol compared to testosterone in the HFA is likely associated with low expression of 3βHSD2 reported previously (5, 19) and confirmed in the present study.
We also observed for the first time that HFA express 5α-reductase 1 (SRD5A1), whose activity is critical for the operation of the backdoor pathway (8, 20, 21). Similarly, detection of two forms of testosterone, 17α- and 17β-testosterone may indicate that the activities of 17αHSD, 17βHSD3 and 17βHSD5 were present in the HFA to convert androstenedione to both forms of testosterone as reported previously (5, 13, 22). Recently, several studies have reported that 11-oxygenated metabolite of testosterone, 11-ketotestosterone (11KT) can be produced by the human adrenals (23), and it has capacity similar to testosterone to bind to the human androgenic receptor (hAR) and regulates AR-dependent gene expression (23, 24, 25, 26), suggesting that androgenic potential of HFA at the end of first trimester cannot be limited to testosterone action only.
In the present study, we observed that the HFA at GW9–12 had capacity to produce androstanedione and androsterone, the precursors in the biosynthesis of DHT (8, 9). However, further conversion of these steroids into DHT was blocked in the HFA apparently due to inability of 17βHSD3 to convert androstanedione to DHT and androsterone to androstanediol, which were not detectable in all samples (data not shown). The observed restriction of the HFA to produce potent androgen DHT at the end of the first trimester may play an important role in protection of the developing female external genitalia from deleterious effects of androgens and to avoid their masculinization. However, evidence for the presence of the backdoor pathway in newborn untreated patients with 21-hydroxylase deficiency (21-OHD) suggests that the HFA could contribute to DHT biosynthesis in patients with 21-OHD (12).
It has been reported that androsterone can be synthesized from two steroid precursors, androstanedione and 17α-OH-allopregnanolone by reductive activity of 3αHSD and the 17–20 lyase activity of CYP17A1, respectively (8, 20). We did not detect 17α-OH-allopregnanolone in the HFA but taking into account that this steroid is the most efficient substrate for the 17–20 lyase activity (27), one can suggest that this intermediate steroid was rapidly and effectively converted to androsterone by the action of CYP17A1 in the HFA.
Our observation that the expression of MC2R (the receptor for ACTH) in the HFA was elevated during final two weeks of the first trimester may indicate of active differentiation and maturation of the human fetal adrenocortical cells. These findings agree well with an earlier report that the human fetal adrenal cortex is responsive to ACTH and produces corticosteroids and DHEA as early as GW8 (5, 28), strongly indicating functional activity of MC2R at early stages of the HFA development.
In the present study, we found that the major steroid produced by the HFA was DHEA, which was associated with high levels of expression of CYP17A1, its accessory proteins, P450 oxidoreductase (POR) and CYB5A. In addition, low level of expression of 3βHSD2 observed in the HFA (5, 19) may also contribute to excessive production of DHEA. To our knowledge, this is the first time expression of POR and CYB5A by the HFA at the end of the first trimester has been reported. The catalytic activity of CYP17A1 requires a supply of electrons from NADPH via POR (29, 30) and its 17,20-lyase activity is enhanced significantly by microsomal CYB5A (29, 31), which supports interactions with POR allosterically (32). Accordingly, our present findings indicate that the CYP17A1-POR-CYB5 complex is fully functional already at early stages of the HFA development. CYP17A1 is expressed exclusively in the fetal zone (FZ), which produces large amounts of DHEA starting from GW7–8 (33), but the biological role of this steroid in the development and maturation of fetal organs during the first trimester is not yet known. In mice, DHEA has been reported to modulate the growth of embryonic neocortical neurons and may thereby play a crucial role in organizing the neocortex (34). Whether this steroid performs a similar function in the human fetus remains to be determined. DHEA has also been shown to be a ligand for many hepatic nuclear receptors and G-protein-coupled receptors (35).
Further, we also demonstrated the elevated expression of SULT2A1 in the HFA at GW11–12 compared to GW9–10, strongly suggesting that this enzyme plays an important role in the regulation of the availability of pregnenolone, 17OH-pregnenolone and DHEA for androgen production by the HFA (36, 37). In the human adrenals, SULT2A1 converts pregnenolone, 17α-hydroxypregnenolone, DHEA, androsterone and androstenediol to their respective sulfated products (36, 37), which are no longer available as substrates for corresponding enzymes (38).
Our current characterization of the expression of steroidogenic enzymes and related transcription factors by the HFA at the end of the first trimester revealed a partial ontogenetical relationship between the levels of transcription factors that promotes steroidogenic gene expression (e.g. SF-1 and GATA6) and of CYP11A1 and CYP17A1. It has been reported that GATA-6 can work in concert with SF-1 to maximize expression of the enzymes involved in the synthesis of adrenal androgens (39) and can control transcription of CYP17A1 both in a human adrenocortical cell line (40) and virilizing carcinomas (41) as well as is involved in regulating CYP5A transcription (42). Thus, GATA-6 appears to play several roles in connection with development, differentiation and regulation of steroidogenesis in the adrenal cortex (41). Similarly, we observed elevated expression of 3βHSD2 in association with high levels of SF-1 and GATA-6 proteins in the HFA at GW10, while the significant reduction in the level of 3βHSD2 at GW11 was not correlated with the decline in the expression of these steroidogenic factors. It was previously demonstrated that nerve growth factor IB (NGFI-B), an orphan nuclear receptor, can regulate the expression of 3βHSD2 in the HFA during the end of the first trimester (5), indicating that SF-1 and GATA-6 may not be primary transcriptional regulators of the expression of this steroidogenic enzyme.
Altogether, our current study demonstrates that the early onset of steroidogenesis in the HFA is characterized by production of steroids of the ∆5, ∆4 and the backdoor pathways of the biosynthesis of DHT, though the latter was limited to production of 17α-OH-dihydroprogesterone, androsterone and androstanedione without further conversion to DHT. Of those steroids analyzed in the present study, the only potent androgen directly produced by the HFA was testosterone. The similar profiles of the expression of steroidogenic cytochromes and their regulatory transcription factors (e.g. SF-1 and GATA-6) may indicate that these regulatory proteins play a key role in the onset of steroidogenesis in the HFA at a very early stage in their development.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grant from the European Union’s Horizon 2020 research and innovation programme (grant agreement No 634880), the Swedish Research Council, the Children’s Cancer Fund, the Frimurare Barnhuset Foundation, Kronprinsessan Lovisas Foundation, the ‘Sällskapet Barnavård’; the ‘Stiftelsen Sigurd och Elsa Goljes Minne’, the ‘Stiftelsen Olle Engkvist Byggmästare’, the ‘Stiftelsen Gunvor och Josef Anérs’, the ‘Stiftelsen Jane och Dan Olssons’ and the ‘Stiftelsen Tornspiran’.
References
- 1.Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocrine Reviews 2011. 32 317–355. ( 10.1210/er.2010-0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Molecular Endocrinology 2001. 15 57–68. ( 10.1210/mend.15.1.0585) [DOI] [PubMed] [Google Scholar]
- 3.Welsh M, Suzuki H, Yamada G. The masculinization programming window. Endocrine Development 2014. 27 17–27. ( 10.1159/000363609) [DOI] [PubMed] [Google Scholar]
- 4.Speiser PW, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. Clinical Endocrinology 1998. 49 411–417. ( 10.1046/j.1365-2265.1998.00559.x) [DOI] [PubMed] [Google Scholar]
- 5.Goto M, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. Journal of Clinical Investigation 2006. 116 953–960. ( 10.1172/JCI25091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. Journal of Clinical Endocrinology and Metabolism 2009. 94 2192–2198. ( 10.1210/jc.2008-2374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm D, Koopman P. The makings of maleness: towards an integrated view of male sexual development. Nature Reviews Genetics 2006. 7 620–631. ( 10.1038/nrg1903) [DOI] [PubMed] [Google Scholar]
- 8.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends in Endocrinology and Metabolism 2004. 15 432–438. ( 10.1016/S1043-2760(04)00214-0) [DOI] [PubMed] [Google Scholar]
- 9.Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Developmental Dynamics 2013. 242 320–329. ( 10.1002/dvdy.23892) [DOI] [PubMed] [Google Scholar]
- 10.Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 5α-androstane-3α,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology 2003. 144 575–580. ( 10.1210/en.2002-220721) [DOI] [PubMed] [Google Scholar]
- 11.Mahendroo M, Wilson JD, Richardson JA, Auchus RJ. Steroid 5alpha-reductase 1 promotes 5α-androstane-3α,17β-diol synthesis in immature mouse testes by two pathways. Molecular and Cellular Endocrinology 2004. 222 113–120. ( 10.1016/j.mce.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 12.Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative ‘backdoor’ pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. Journal of Clinical Endocrinology and Metabolism 2012. 97 E367–E375. ( 10.1210/jc.2011-1997) [DOI] [PubMed] [Google Scholar]
- 13.Fluck CE, Meyer-Boni M, Pandey AV, Kempna P, Miller WL, Schoenle EJ, Biason-Lauber A. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. American Journal of Human Genetics 2011. 89 201–218. ( 10.1016/j.ajhg.2011.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luu-The V, Belanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Practice and Research Clinical Endocrinology and Metabolism 2008. 22 207–221. ( 10.1016/j.beem.2008.01.008) [DOI] [PubMed] [Google Scholar]
- 15.Savchuk I, Morvan ML, Soeborg T, Antignac JP, Gemzell-Danielsson K, Le Bizec B, Soder O, Svechnikov K. Resveratrol inhibits steroidogenesis in human fetal adrenocortical cells at the end of first trimester. Molecular Nutrition and Food Research 2017. 61 . ( 10.1002/mnfr.201600522) [DOI] [PubMed] [Google Scholar]
- 16.Evtouchenko L, Studer L, Spenger C, Dreher E, Seiler RW. A mathematical model for the estimation of human embryonic and fetal age. Cell Transplant 1996. 5 453–464. ( 10.1016/0963-6897(96)00079-6) [DOI] [PubMed] [Google Scholar]
- 17.Courant F, Antignac JP, Maume D, Monteau F, Andersson AM, Skakkebaek N, Andre F, Le Bizec B. Exposure assessment of prepubertal children to steroid endocrine disrupters 1. Analytical strategy for estrogens measurement in plasma at ultra-trace level. Analytica Chimica Acta 2007. 586 105–114. ( 10.1016/j.aca.2006.11.002) [DOI] [PubMed] [Google Scholar]
- 18.Courant F, Aksglaede L, Antignac JP, Monteau F, Sorensen K, Andersson AM, Skakkebaek NE, Juul A, Le Bizec B. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. Journal of Clinical Endocrinology and Metabolism 2010. 95 82–92. ( 10.1210/jc.2009-1140) [DOI] [PubMed] [Google Scholar]
- 19.Voutilainen R, Ilvesmaki V, Miettinen PJ. Low expression of 3 beta-hydroxy-5-ene steroid dehydrogenase gene in human fetal adrenals in vivo; adrenocorticotropin and protein kinase C-dependent regulation in adrenocortical cultures. Journal of Clinical Endocrinology and Metabolism 1991. 72 761–767. ( 10.1210/jcem-72-4-761) [DOI] [PubMed] [Google Scholar]
- 20.Ghayee HK, Auchus RJ. Clinical implications of androgen synthesis via 5α-reduced precursors. Endocrine Development 2008. 13 55–66. ( 10.1159/000134780) [DOI] [PubMed] [Google Scholar]
- 21.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews 2011. 32 81–151. ( 10.1210/er.2010-0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellemare V, Faucher F, Breton R, Luu-The V. Characterization of 17α-hydroxysteroid dehydrogenase activity (17α-HSD) and its involvement in the biosynthesis of epitestosterone. BMC Biochemistry 2005. 6 12 ( 10.1186/1471-2091-6-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. Journal of Clinical Endocrinology and Metabolism 2013. 98 1182–1188. ( 10.1210/jc.2012-2912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS ONE 2016. 11 e0159867 ( 10.1371/journal.pone.0159867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pretorius E, Arlt W, Storbeck KH. A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Molecular and Cellular Endocrinology 2016. 441 76–85. ( 10.1016/j.mce.2016.08.014) [DOI] [PubMed] [Google Scholar]
- 26.Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. European Journal of Endocrinology 2016. 174 601–609. ( 10.1530/EJE-15-1181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Archives of Biochemistry and Biophysics 2003. 418 151–160. ( 10.1016/j.abb.2003.07.003) [DOI] [PubMed] [Google Scholar]
- 28.Seron-Ferre M, Lawrence CC, Siiteri PK, Jaffe RB. Steroid production by definitive and fetal zones of the human fetal adrenal gland. Journal of Clinical Endocrinology and Metabolism 1978. 47 603–609. ( 10.1210/jcem-47-3-603) [DOI] [PubMed] [Google Scholar]
- 29.Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids 1997. 62 133–142. ( 10.1016/S0039-128X(96)00172-9) [DOI] [PubMed] [Google Scholar]
- 30.Miller WL, Geller DH, Auchus RJ. The molecular basis of isolated 17,20 lyase deficiency. Endocrine Research 1998. 24 817–825. ( 10.3109/07435809809032692) [DOI] [PubMed] [Google Scholar]
- 31.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Archives of Biochemistry and Biophysics 1995. 317 343–347. ( 10.1006/abbi.1995.1173) [DOI] [PubMed] [Google Scholar]
- 32.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. Journal of Biological Chemistry 1998. 273 3158–3165. ( 10.1074/jbc.273.6.3158) [DOI] [PubMed] [Google Scholar]
- 33.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews 1997. 18 378–403. ( 10.1210/er.18.3.378) [DOI] [PubMed] [Google Scholar]
- 34.Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. PNAS 1998. 95 4678–4683. ( 10.1073/pnas.95.8.4678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prough RA, Clark BJ, Klinge CM. Novel mechanisms for DHEA action. Journal of Molecular Endocrinology 2016. 56 R139–R155. ( 10.1530/JME-16-0013) [DOI] [PubMed] [Google Scholar]
- 36.Strott CA. Steroid sulfotransferases. Endocrine Reviews 1996. 17 670–697. ( 10.1210/edrv-17-6-670) [DOI] [PubMed] [Google Scholar]
- 37.Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB. Sulfation and sulfotransferases 1: sulfotransferase molecular biology: cDNAs and genes. FASEB Journal 1997. 11 3–14. [PubMed] [Google Scholar]
- 38.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends in Endocrinology and Metabolism 2002. 13 234–239. ( 10.1016/S1043-2760(02)00609-4) [DOI] [PubMed] [Google Scholar]
- 39.Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology 2003. 144 4285–4288. ( 10.1210/en.2003-0472) [DOI] [PubMed] [Google Scholar]
- 40.Fluck CE, Miller WL. GATA-4. and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Molecular Endocrinology 2004. 18 1144–1157. ( 10.1210/me.2003-0342) [DOI] [PubMed] [Google Scholar]
- 41.Kiiveri S, Liu J, Arola J, Heikkila P, Kuulasmaa T, Lehtonen E, Voutilainen R, Heikinheimo M. Transcription factors GATA-6, SF-1, and cell proliferation in human adrenocortical tumors. Molecular and Cellular Endocrinology 2005. 233 47–56. ( 10.1016/j.mce.2005.01.012) [DOI] [PubMed] [Google Scholar]
- 42.Huang N, Dardis A, Miller WL. Regulation of cytochrome b5 gene transcription by Sp3, GATA-6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Molecular Endocrinology 2005. 19 2020–2034. ( 10.1210/me.2004-0411) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a