Abstract
Non-motor symptoms (NMS) are a key component of Parkinson’s disease (PD). A range of NMS, most notably impaired sense of smell, sleep dysfunction, and dysautonomia are present from the ‘pre-motor’ phase to the final palliative stage. Theories as to the pathogenesis of PD such as those proposed by Braak and others also support the occurrence of NMS in PD years before motor symptoms start. However, research addressing the range and nature of NMS in PD has been confounded by the fact that many NMS arise as part of drug-related side effects. Thus, drug-naive PD (DNPD) patients provide an ideal population to study the differences in the presentation of NMS. The aim of this paper is therefore to systematically review all the available studies of NMS in DNPD patients. We believe this is the first review of its kind. The current review confirms the increasing research being conducted into NMS in DNPD patients as well as the necessity for further investigation into less-studied NMS, such as pain. Moreover, the data confirms non-motor heterogeneity among PD patients, and, therefore, further research into the concept of non-motor subtyping is encouraged. The review suggests that the clinical assessment of NMS should be integral to any assessment of PD in clinical and research settings.
Introduction
Non-motor symptoms (NMS) are a key component of Parkinson’s disease (PD)1 and a major determinant of quality of life and phenotypic expression.1,2 Indeed, there is growing evidence that the overall burden of NMS may have a greater impact on quality of life than motor symptoms, not just in advanced motor disease as commonly perceived but also in early motor PD.2,3
A number of NMS are present from the ‘pre-motor’ stage4 to the final palliative stages of PD.5 The involvement of multiple neurotransmitters from the onset of PD, extra-striatal dopaminergic pathways, as well as early involvement of brainstem and olfactory areas in PD further augment the importance of NMS in PD.6
However, the fact that a range of NMS may arise as part of drug-related side effects confounds this issue further.3 Thus, drug-naive PD (DNPD) patients are an ideal population to study to study the differences in presentation of NMS at the early, or even pre-motor stages of PD and some controlled studies have also reported on the burden of NMS in DNPD.7,8 Yet, it is difficult to obtain large case series of DNPD patients as often patients referred to PD specialist centers or clinical research-orientated neurology departments are already on anti-parkinsonian medication. Thus the aim of this review is to systematically review all the available studies of NMS in DNPD patients and we believe this is the first review of its kind.
Materials and methods
Literature search strategy
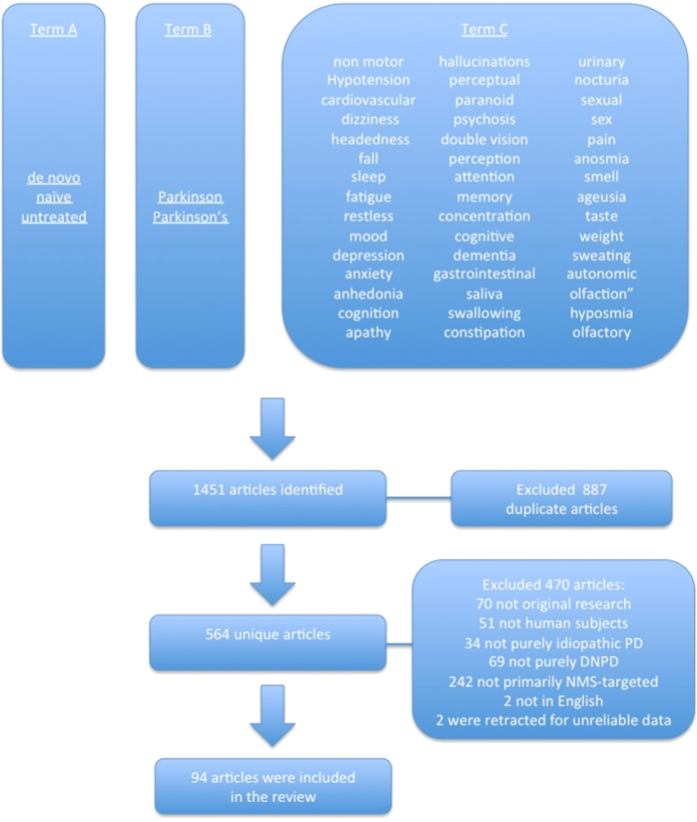
A systematic literature search was carried out on the 4th of February 2015, using the PubMed database, covering all articles published up until that time. For each individual search, we used three Medical Subject Headings terms in either title or abstract (Figure 1). Term A was ‘de novo’, ‘naive’, or ‘untreated’; Term B was ‘Parkinson’ or ‘Parkinson’s’, and Term C was ‘non-motor’, ‘hypotension’, ‘cardiovascular’, ‘dizziness’, ‘headedness’, ‘fall’, ‘sleep’, ‘fatigue’, restless’, ‘mood’, ‘depression’, ‘anxiety’, ‘anhedonia’, ‘cognition’, ‘apathy’, ‘hallucinations’, ‘perceptual’, ‘paranoid’, psychosis’, ‘double vision’, ‘perception’, ‘attention’, ‘memory’, ‘concentration’, ‘cognitive’, ‘dementia’, ‘gastrointestinal’, ‘saliva’, ‘swallowing’, ‘constipation’, ‘urinary’, ‘nocturia’, ‘sexual’, ‘sex’, ‘pain’, ‘anosmia’, ‘smell’, ‘ageusia’, ‘taste’, ‘weight’, ‘sweating’, ‘autonomic’, ‘olfaction’, ‘hyposmia’, and ‘olfactory’.
Figure 1.
Prisma chart summarizing the literature search strategy and the application of inclusion and exclusion criteria.
The alternatives Medical Subject Headings for Term C were based on the Non-Motor Symptoms Scale (NMSS),9,10 and the Non-Motor Symptoms Questionnaire (NMSQuest).11 The NMSS is a 30-item clinician-rated scale designed to assess NMS in PD. The 30 items are grouped into nine domains: cardiovascular (2 items), sleep/fatigue (4 items), mood/cognition (6 items), perceptual problems (3 items), attention/memory (3 items), gastrointestinal (3 items), urinary (3 items), sexual function (2 items), and miscellaneous (4 items: pain, change in ability to taste or smell, change in weight, excessive sweating).9,10 The NMSQuest consists of nine NMS domains (gastrointestinal, urinary, attention/memory, perceptual problems/hallucination, sexual dysfunction, cardiovascular, sleep, mood/apathy, miscellaneous), each of which includes two to seven specific questions with dichotomous (yes/no) answers for a total of 30 items.11
We also perused the reference lists of the papers since the drafting of this paper so as to try and include further papers reporting aspects of NMS in drug-naive PD populations.
Inclusion and exclusion criteria
To be included in the review, the articles had to meet the following inclusion criteria:
to be original papers,
to study human subjects,
to refer to studies which clearly address idiopathic PD only,
to refer to a strictly drug-naive population. As such, we have excluded studies where patients might have had a trial dose of any anti-parkinsonian agent and,
no retrospective studies as the study’s aim was to clinically explore the range and nature of NMS in prospective study-based papers.
Results
Search results
The search strategy described above resulted in the identification of 1,451 articles. Out of these, 887 articles were duplicates as different combinations of Terms A, B, and C resulted in the same article in many cases. After the eligibility assessment, 468 articles were further excluded, as they did not meet the inclusion criteria. Two papers were further excluded as their publication have been retracted for unreliable data. In total, 94 papers were included in the review. Table 1 summarizes the characteristics of these papers. Figure 1 illustrates the study selection process.
Table 1. Characteristics of papers included in the review.
|
Year of publication
| |
| Range | 1987–2015 |
|
Number of publications per decade
| |
| Until 1990 | 6 |
| 1991–2000 | 14 |
| 2001–2010 | 19 |
| 2011–2015 | 55 |
|
Number of DNPD patients per publication
| |
| Range | 8–428 |
| Mean (s.d.) | 83.8 (96.0) |
| Median | 45 |
|
Number of publications studying…
| |
| Holistic profile | 13 |
| Autonomic domain | 19 |
| Neuropsychiatric domain | 30 |
| Sensory domain | 7 |
| Fatigue domain | 3 |
| Gastrointestinal domain | 6 |
| Sleep domain | 16 |
Abbreviation: DNPD, drug-naive Parkinson’s disease.
Holistic NMS profile of drug-naive PD patients
The majority of studies that were designed to describe the holistic NMS profile of drug-naive PD patients used the NMSS and the NMSQuest.
In 2009, Kim et al.12 were the first to use the NMSS to describe the holistic NMS profile of DNPD patients compared with controls. NMS were significantly more frequently present in the DNPD population, with nocturia, forgetfulness, and restless legs being the three most common symptoms. Interestingly, neither age nor duration of disease was correlated with the number of NMS or the total NMSS score. However, a positive correlation between the number of NMS or the total NMSS score and motor disease severity as assessed by Hoehn and Yahr stage was observed.12
A major study of 1,072 patients in 2009 by Barone et al.,13 the PRIAMO study described the NMS profile of 107 DNPD patients via a semi-structured interview (the PRIAMO-Quest), which consisted of 12 NMS domains (gastrointestinal symptoms, pain, urinary symptoms, cardiovascular symptoms, sleep disorders, fatigue, apathy, attention, skin disorders, psychiatric symptoms, respiratory symptoms, other symptoms). The highest prevalence of NMS in DNPD patients was found to be psychiatric NMS, such as anxiety and depression (present in 66% of the DNPD population), followed by fatigue (present in 52% of the DNPD population). The least common NMS was found to be respiratory problems, such as stridor, cough, and dyspnea, (present in 8% of the DNPD population).
In 2011, Müller et al.14 studied the autonomic and sensory symptoms in 207 untreated PD patients using a preliminary version of the Movement Disorder Society-sponsored revised version of the UPDRS (pMDS-UPDRS). They found that both categories of symptoms were more frequent in patients compared with controls. In the patient group, reduced olfaction (59%), urinary problems (47%), increased saliva or drooling (42%), constipation (39%), and sensory complaints such as pain or abnormal sensation (34%) were the most frequent symptoms but mild in severity, although severity was not assessed by validated cut-off scores of specific non-motor instruments.14 Similar to the observation by Kim et al.,12 higher Hoehn and Yahr stage was associated with a larger number of autonomic and sensory symptoms and with the occurrence of gastrointestinal symptoms. However, a recent study by Zis et al.3 suggests that the burden of NMS in DNPD subjects can be severe (26.3%) and very severe (19.3%).
In 2013, an analysis of the baseline data of the DeNoPa cohort was performed and used both NMSQuest as well as NMSS in addition to SCOPA-AUT scale.7 The findings showed that DNPD patients, compared with controls, present with an increase in the total NMSQuest score, the total NMSS scores, and all SCOPA-AUT subscores (gastrointestinal, urinary, cardiovascular, thermoregulatory, pupilomotor, and sexual dysfunction in men) sparing sexual dysfunction in women. In a large DNPD population consisting of 200 patients, Picillo et al.15 found that using the NMSQuest, only 11.5% of patients were free of NMS, whereas 21.5% of healthy controls were free of NMS. All NMS domains were affected, with the exception of perceptual problems/hallucinations. The most prevalent symptoms were anxiety and sadness. Although overall NMSQuest scores did not differ between males and females, the former complained significantly more frequently of sexual problems and taste/smelling difficulties.
In 2013, Kim and colleagues16 compared NMSS symptoms across DNPD and drug-induced parkinsonism groups using the NMSS. Their results suggest that NMS were prevalent in the DNPD group, and symptoms of urinary and sleep disturbance, restless legs syndrome (RLS), attention deficits, and hyposmia were able to differentiate these groups, once controlling for age and gender.
Contrary to the gender findings above, in a similar study conducted by Song et al. in 2014, it was shown that DNPD female patients were more depressed and had more impaired cognition, whereas gender differences were not apparent on motor and other NMS.17 A holistic analysis of the NMS spectrum in the latter study population showed that 71% of the patients report fatigue, 59% of the patients were having sleep problems, 36% of the patients suffered from constipation, and 32% of the patients suffered from depression.18 However, in this study, the authors did not use a single holistic NMS tool, but other specific questionnaires such as the 20-item version of the Center for Epidemiological Studies Depression Scales (CES-D) for depression and the MMSE and Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog) for cognition.
Also in 2014, Zis et al.3 analyzed cross-sectional UK data from a multicenter collaboration, using the NMSS and showed that NMS are common in DNPD patients and over 45% may have severe to very severe burden of NMS, a key determinant of quality of life.
In a recent paper, Yang et al.19 compared the holistic NMS profile between DNPD patients and SWEDD (scans without evidence of dopaminergic deficits) patients. Using validated scales including the NMSS, they showed that DNPD patients have more NMS than newly diagnosed untreated PD patients, suggesting that specific NMS, especially rapid eye movement (REM) sleep behavior disorder (RBD) or olfactory impairment, might aid the differential diagnosis, regardless of what the actual condition underlying SWEDD is.19
The ONSET-PD study aimed to describe the presence and perceived onset of NMS in PD, using the NMSQuest and the SCOPA-aut.20 Anhedonia, apathy, memory complaints, and inattention were found to anticipate the motor onset and occurred more frequently in the DNPD patients compared with controls during a 2-year pre-motor period. Hyposmia, mood disturbances, ageusia, excessive sweating, fatigue, and pain were noted in the 2- to 10-year pre-motor period. Constipation, dream-enacting behavior, excessive daytime sleepiness, and postprandial fullness were frequently perceived more than 10 years before motor symptoms. These findings confirm that NMS are prevalent in early DNPD and frequently reported to occur in the pre-motor period.20
All these studies confirm that NMS are present in early untreated stages of PD and that differences between genders and among populations exist. Such differences in NMS profiling suggest that there is non-motor heterogeneity in PD right from the onset of the motor disorder. Thus, an interesting and elegant study, conducted by Erro et al.21 took this hypothesis further by using a data-driven approach on DNPD. They identified four distinct groups of patients, which they have labeled: (1) benign pure motor; (2) benign mixed motor-non-motor; (3) non-motor dominant; and (4) motor dominant, promoting the hypothesis that non-motor subtyping is possible. The ONSET-PD study also report specific non-motor clustering.20 Specific NMS-based studies (as reviewed below) support such a suggestion and therefore non-motor subtyping is of great interest and has indeed recently gained momentum.
Specific NMS studies
Autonomic NMS
Features of autonomic disturbance include symptoms caused by sympathetic dysfunction, parasympathetic dysfunction, or both. These symptoms may concern, among others, cardiovascular symptoms, urinary symptoms, sexual dysfunction, and other issues such as excessive sweating.
Cardiovascular dysfunction
Untreated PD patients suffer significant failure in cardiovascular nervous system regulation.22 There is reasonable evidence to suggest that DNPD patients show sympathetic dysfunction,22–29 the most prominent symptom of which is orthostatic hypotension, which may lead to considerable morbidity. The prevalence of orthostatic hypotension varies from 4% (ref. 23) to 60%.30 In addition, Hiorth et al.31 observed that 17% of untreated patients may experience falls that could possibly be linked to orthostatic hypertension.
Many studies have aimed to investigate heart rate (HR) variability and resting HR in DNPD patients. Both the HR response to standing and the HR variability to deep breathing are tests used to assess the parasympathetic function. DNPD patients show significant impairments in HR variability22–24,28,29,32,33 compared with controls. Moreover, the DeNoPa study showed that mean HR is increased in DNPD patients, whereas the QT interval is shorter compared with healthy controls.7 However, data on parasympathetic dysfunction in early, untreated, stages of PD remains largely inconclusive. Although the majority of the studies assessing cardiovascular reflexes such as the Ewing’s battery of autonomic tests, showed that parasympathetic dysfunction may be present early in the disease course,22–24,27–29,32,33 some studies have shown that DNPD manifested only sympathetic dysfunction, either assessing cardiovascular reflexes or on the basis of cardiac radioiodinated metaiodobenzylguanidine uptake.34
PD causes dysfunction of the diurnal autonomic cardiovascular regulation.32 Moreover, during sleep, DNPD patients have been found to show defective cardiac autonomic control,35–37 mainly parasympathetic but also sympathetic in nature, despite having normal results in conventional autonomic tests during wakefulness.35
Postprandial hypotension is another cardiovascular symptom experienced in PD. As early as in 1987, Micieli et al.30 showed that over 50% of patients studied showed a marked postprandial systolic fall resembling that observed in chronic autonomic failure. In 2014, Umehara et al.38 concluded that in DNPD, systemic sympathetic denervation, impaired baroreflex-cardiovagal gain, and insufficiency of compensatory sympathetic nervous activation is associated with postprandial hypotension. Thus, systemic sympathetic denervation and baroreflex failure seem to contribute toward the development of postprandial and orthostatic hypotension.
Urinary dysfunction
Urinary NMS include difficulty holding urine (urgency), increased urinary frequency, and nocturia and may be the result of storage or voiding dysfunction. In 2011, Uchiyama et al.39 calculated that 64% of DNPD patients report urinary symptoms, while 82% have abnormal urodynamic studies. However, the ONSET-PD study20 showed that urinary symptoms are not more prevalent in DNPD patients compared with controls, at least in the early PD stage.
Interestingly, urinary symptoms were not found to correlate with gender, disease severity or motor symptom type.20 However, Erro et al.40 have recently showed that DNPD patients with urinary symptoms have higher motor and non-motor disturbances than those without suggesting the existence of a subgroup of patients who have an overall higher motor and non-motor burden and increased likelihood to require levodopa over the first 4 years from diagnosis. On the basis of these findings, Erro et al.40 argued that urinary symptoms might be a clinical marker of severity as well as possibly highlighting a non-motor subtype of PD.
Gastrointestinal dysfunction
Gastrointestinal symptoms in PD include dysphagia, nausea, constipation, and defecatory dysfunction.41 DNPD patients show delayed gastric emptying42,43 and reduced bowel sounds44 compared with healthy controls. Interestingly, Tanaka et al.45 took this further and showed that delay in gastric emptying did not differ between untreated, early-stage, and treated advanced-stage PD patients. In 2011, Tanaka et al.45 showed that gastric emptying of untreated, early-stage PD is already delayed, thus making delayed gastric emptying a potential marker of the preclinical stage of PD.
In 1991, Edwards et al.41 observed that gastrointestinal symptoms are comparable between treated and untreated PD except for defecatory dysfunction, which was significantly more common in treated patients and suggested that the gastrointestinal symptoms of PD reflect direct involvement in the gastrointestinal tract by the primary disease process. In 2011, almost 95% of the patients recruited in the QL-GAT study reported constipation, which was ameliorated after treatment with levodopa.46
Hypersialorrhea (drooling)
Hypersialorrhea is frequently reported in patients with PD and may be the result of excessive production of saliva, swallowing difficulties, or both. In a study designed to prospectively investigate the salivary secretion, Bagheri et al.47 found that DNPD patients show a lower salivary flow compared with controls, whereas no significant differences were seen between untreated and treated patients, suggesting that hypersialorrhea could better be explained on the basis of swallowing difficulties. On the other hand, decreased salivary flow might be explained by autonomic dysfunction.
Rhinorrhea
In a case–control study conducted through interviewing and using a specific questionnaire, it was estimated that half of the DNPD patients suffered from rhinorrhea.48 The authors hypothesize that the high frequency of rhinorrhea in PD patients may be due to sympathetic dysfunction. Moreover, the authors showed an association between rhinorrhea and olfactory dysfunction. Whether rhinorrhea may actually affect olfaction, as tested with objective measures, is yet to be determined.48
Neuropsychiatric symptoms
A wide range of neuropsychiatric symptoms have been described in PD even in early and untreated patients.49 Only few studies have focused on the whole spectrum of neuropsychiatric symptoms in DNPD patients, the majority having investigated only specific symptoms. In 2012, Poletti et al.50 in a small series of DNPD patients administered a neuropsychiatric battery, finding that the most common neuropsychiatric symptoms included depression (33%), alexithymia (20%), anxiety (20%), and impulsivity (10%). In 2014, Aarsland et al.49 used the Neuropsychiatric Inventory in a large unselected sample of patients with incident, non-demented DNPD, and compared the results using age- and education-matched controls. More than half of the patients exhibited at least one symptom, and more than 25% had at least one symptom of clinically significant severity. Several patients had two or more symptoms, and 13% had two or more clinically significant symptoms. Depression, anxiety, sleep disturbances, and apathy were the most common symptoms, whereas psychotic symptoms were very rare.49
In a large controlled study on the global spectrum of neuropsychiatric symptoms, de la Riva et al.51 showed that depression, fatigue, apathy, and anxiety are significantly more prevalent in DNPD patients compared with healthy controls, despite their tendency to remain relatively stable in the early stage, whereas global cognition slightly deteriorates.
Depression
Depressive symptoms are common in DNPD patients, with a prevalence varying from study to study. Younger age of PD onset is associated with depression in DNPD patients52 and, interestingly, Santamaria et al.52 observed that in 91% of depressed DNPD patients, depressive symptoms began before the motor symptoms.
Ravina et al.53 pooled the baseline data from two phase II clinical trials enrolling early, untreated subjects with PD showing that out of a total of 413 patients, 13.8% suffered from depression. In smaller series, depressive symptoms have been shown to be present in approximately one-third of patients.50–52,54 Choi et al.54 showed that after long-term levodopa therapy, up to 18% of depressed patients may recover, whereas 22% of the initially nondepressed patients became depressed. In 2014, Spalletta et al.55 showed that following treatment, depressive symptoms improvement was paralleled by the very expected improvement of motor symptoms severity. However, change in depression severity over a long term was not significantly related to changes in motor symptoms.55
Anxiety
Studies focusing purely on anxiety in DNPD patients are limited. In a small case series, Spalletta et al.55 estimated that the frequency of generalized anxiety disorder was 8.3%, a percentage not altered following treatment.
Apathy
The prevalence of apathy within DNPD ranges from as low as 8.3% (ref. 55) to 33.3%.56 In a large controlled study, Pedersen et al.57 showed that apathy was significantly associated with male gender, higher depression scores, and more severe motor symptoms, but was not associated with greater cognitive impairment. However, Dujardin et al.58 in their DNPD population showed that apathy was associated with lower cognitive status, as well as with fatigue and anhedonia. A self-report version of the Apathy Evaluation Scale has now been validated, for detecting apathy in PD,56 and this tool may help to clarify these conflicting results in future studies.
Alexithymia
In a small controlled study, Poletti et al.59 estimated that 23.8% of the DNPD patients are alexithymic, 26.2% borderline alexithymic, and 50% non-alexithymic. These percentages were not statistically different compared with controls. On the other hand, they observed that DNPD with the postural instability gait difficulty motor subtype presented more alexithymic features than PD patients with tremor-dominant phenotype, suggesting that the postural instability gait difficulty subtype could represent a risk factor for developing alexithymia.60
Impulsivity—compulsivity
The association between impulse control disorders (ICDs), including compulsive gambling, buying, hypersexuality, binge eating, and punding, and dopaminergic medications is well established.61 Conversely, only few studies have investigated the frequency and correlates of impulse control and related behavior symptoms in DNPD patients. In 2011, Antonini et al.62 found that, despite none fulfilled DSM-IV criteria for any ICDs, 18 of 103 (17.5%) DNPD patients screened positive for at least one ICD using the Minnesota Impulsive Disorder Interview and the South Oaks Gambling Scale, a figure similar to that of healthy controls. Similarly, a large study conducted in 2013 by Weintraub et al.,61 estimated that at least one ICD was present in 18.5% of DNPD patients. Specifically, binge eating was the most frequent ICD (7.1%), followed by hobbyism (5.4%), punding (4.8%), hypersexuality (4.2%), buying (3.0%), gambling (1.2%), and walkabout (0.6%). In a study that included a small number of DNPD patients, Nicoletti et al.63 estimated that 50% of them presented with obsessive-compulsive personality disorder. These findings highlight the necessity of a detailed behavioral assessment before starting dopaminergic therapy.
Memory—cognition
Although dementia is a feature of advanced PD, mild cognitive impairment can occur in the early stages of PD.64 Several studies have been conducted and investigated the performance of DNPD patients across multiple neuropsychological tests.55,65–74 Memory impairment in early DNPD mainly reflects deficit of learning and encoding rather than retention or retrieval.66 This pattern of cognitive impairment is distinct from the one occurring in the normal ageing process.65 With medical treatment, selective improvement in memory domains (verbal and visuo-spatial episodic memory) has been observed.55
Compared with controls, DNPD patients have a relative risk ratio of 2.1 to develop mild cognitive impairment.75 The prevalence of mild cognitive impairment varies from study to study ranging from 14.8% (ref. 70) to 43.4% (ref. 76), and this may reflect ongoing uncertainty of the most appropriate diagnostic criteria.77 On the other hand, the presence of dementia seems also to depend on the time of PD onset and, therefore, often on the age of the patient. Reid et al.65,78 estimated that 39% of DNPD patients whose symptoms of PD began after the age of 70 years had dementia, while only 8% of DNPD patients whose symptoms of PD began before the age of 70 years did have dementia.
Another interesting association is the relationship between sleep and cognitive dysfunction.79 Specifically, both RBD and insomnia have been associated with lower scores on several cognitive tests. Given the correlation between sleep disturbances and cognitive impairment, it is possible that sleep symptoms in PD patients might be considered as an early marker of cognitive decline.79
In an interesting study published in 2014, Kwon et al.80 studied the whole NMS spectrum in 80 DNPD patients and suggested that depression, vivid dreaming, RBD, hyposmia, and abnormal stereopsis are closely associated with cognitive decline, and that presence of these NMS predicts the subsequent development of dementia. In a small series by Bae et al.,81 orthostatic hypertension was shown to be associated with cognitive dysfunction in DNPD patients.
Fatigue
Fatigue is a common symptom in PD, since the earliest stage, and it has a negative impact on patients’ activities of daily living.82 Herlofson et al.82 estimated that 55% of the DNPD patients suffer from clinically significant fatigue, an amount almost three times greater than controls. Kang et al.83 estimated that 45% of the DNPD patients suffer from fatigue independently from their motor burden, further showing that depression and difficulties with activities of daily living were independent risk factors for fatigue. Conversely, the analysis of the baseline data of the ELLDOPA study showed that 37% of untreated PD patients suffer from fatigue.84 Fatigue was associated with the severity of PD, and progressed less in patients who subsequently were treated with levodopa.85
Sleep
Apart from nocturia and autonomic dysregulation during sleep, which have been described above, other sleep disturbances may emerge from the early, untreated stages of PD.85–88
Periodic limb movements
In a polysomnographic study, Wetter et al.89 showed that sleep disruption and increased motor activity during REM and non-REM sleep are a frequent finding in DNPD patients. They also found an increased periodic limb movement index, which may be due to a dopaminergic deficit and is probably not associated with dopaminergic treatment.89 However, the same group did not confirm these results in a secondary study.90
Restless leg syndrome
The prevalence of restless leg syndrome (RLS) varies from 5.5% (ref. 91) to 16.5%.92 Angelini et al.91 showed that the frequency of overall life-time RLS did not differ significantly between DNPD patients and controls. In another study, Gjerstad et al. estimated that about 15.5% of DNPD patients meet RLS criteria, a percentage that was not significantly higher compared with controls.93 Nevertheless, they found that leg motor restlessness occurred with a near three-fold higher risk in PD patients as compared with controls.93 These conflicting findings underline the need for more accurate assessments of RLS in PD and support the notion that RLS and PD are different entities.93
REM sleep disorders
Video-polysomnographic studies have demonstrated that 28% of the DNPD patients show REM sleep without atonia, a percentage that is significantly higher compared with controls.85 In 2013, Plomhause et al.94 estimated that 30% of the DNPD patients met the criteria for RBD. In a larger video-polysomnographic study, Sixel-Döring et al.95 detected REM sleep behavioral events in 51% of the DNPD patients, a percentage significantly higher compared with controls. Similar to the study by Plomhause et al.,94 RBD was identified in 25% of the total DNPD population.95
Excessive daytime sleepiness
In 2002, in a case–control study, Fabbrini et al.96 showed that excessive daytime sleepiness does not seem to be a trait of untreated PD but appears only in treated PD patients. This was also shown by Kaynak et al. in 2005.97 However, more recently Giganti et al.98 showed a higher level of sleepiness in the DNPD patients compared with controls in the hours following awakening and in the early afternoon. Their results suggest that sleepiness during specific daytime may be an early manifestation of sleepiness, which will spread later to the whole daytime.98
Sensory dysfunction
Olfactory dysfunction
Olfactory symptoms include reduced ability (hyposmia) or inability (anosmia) to smell. Decreased olfactory function is among the first signs of idiopathic PD and is probably present in the pre-motor phase.99,100 Apart from odor identification, odor discrimination is also affected in DNPD patients.101 The olfactory dysfunction is bilateral and not related to motor symptoms.99
Ophthalmologic dysfunction
A wide spectrum of ophthalmologic features may be present in DNPD patients. In a controlled study, Biousse et al.102 showed that ocular complaints are more common in DNPD compared with controls, with ocular surface irritation being the most common (63.3%), followed by difficulty in reading (26.7%), visual hallucinations (26.7%), and diplopia (10%). Two studies showed that DNPD patients show alterations in chromatic contour perception, which is correlated to the severity of the disease.103,104 Interestingly, up to 87.5% of DNPD patients were found to suffer from dysfunction of stereopsis and visual perception.105
Conclusion
This systematic review aimed to focus on the NMS of DNPD patients. It is the first review on this topic and is of particular interest as it may not only offer a better insight on early symptoms that may contribute to the diagnosis, or be used as potential clinical markers, but also may provide a better understanding of which NMS are affected by dopaminergic treatment. Our review indicates the following key points:
Research in DNPD is increasing. This is exemplified in our data (Table 1), which shows that the number of papers reporting NMS in DNPD patients after 2011 has exceeded the total numbers of papers reporting NMS in DNPD patients up until 2010, a period spanning over 20 years.
While initial studies in DNPD were uncontrolled and usually relied, at one point in time, on cross-sectional design, many are now controlled. DNPD patients are often described as ‘gold dust’ as they are difficult to recruit into studies as often anti-parkinsonian medication would have been been started before their presentation to tertiary, specialist, PD centers. This highlights the necessity of a multicenter approach to studies related to DNPD. This review confirms that there is considerable burden of non-motor symptoms and heterogeneity in DNPD patients in addition to motor presentations.21 These observations underpin the concept for non-motor subtyping within PD as certain NMS are overrepresented in DNPD and are likely to be dominated by these NMS, such as sleep dysfunction, cognitive problems, or dysautonomia, during the course of their condition.
Large-scale studies addressing poorly studied NMS, such as pain and anxiety, are required as these are treatable issues in PD.
Early identification of NMS may have therapeutic implications, especially as some NMS may respond to dopaminergic drugs. In a 2-year follow-up study of a DNPD population, Erro et al.106 were the first to show that indeed specific NMS such as depression and concentration improved following dopaminergic treatment. Therefore, documentation of NMS in DNPD state could allow us to document how NMS respond to dopaminergic and non-dopaminergic treatment. Also, such a systematic documentation allows PD specialists to map the natural history of NMS in a comprehensive manner.
Regarding NMS, DNPD represents a unique and important patient group and allows one also to examine the clinical expression of the Braak staging of PD,107 in particular, expression of specific NMS. It also suggests that specific NMS may dominate the clinical picture of PD, suggesting specific subtypes within the NMS-dominant cluster of DNPD, as suggested by Erro et al.21 The natural history of such NMS-dominant subtypes are unclear and, from a pathophysiological point of view, these NMS-dominant subtypes confirm the differential spread of the pathophysiological process via a limbic or a brainstem route as has been documented by Jellinger.108 Clinically, identification of such subtypes may also have implications on treatment as ‘subtype-specific’ treatment packages may need to be developed. The review data also confirm the growing observation that assessment of NMS using validated clinical ‘holistic’ tools should be an integral part of clinical assessment in PD, supplementing motor assessment.
Acknowledgments
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
CCW is supported by an Australian Postgraduate Award at the University of Sydney. AS is supported by a BRC grant as well as a innovation grant from Parkinson’s UK. KRC, AS, and KRC acknowledge funding by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre and Dementia Unit at South London, and Maudsley NHS Foundation Trust, and King’s College London. The other authors declare no conflict of interest.
References
- Sauerbier A, Ray Chaudhuri K. Non-motor symptoms: the core of multi-morbid Parkinson’s disease. Br J Hosp Med (Lond) 2014; 75: 18–24. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011; 26: 399–406. [DOI] [PubMed] [Google Scholar]
- Zis P, Rizos A, Martinez-Martin P, Pal S, Silverdale M, Sharma JC et al. Non-motor symptoms profile and burden in drug naive versus long-term Parkinson’s disease patients. J Parkinsons Dis 2014; 4: 541–547. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Gaig C, Santamaria J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology 2009; 72: S12–S20. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23: 837–844. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006; 5: 235–245. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Sixel-Doring F, Wicke T, Ebentheuer J, Schaumburg M et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013; 81: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013; 80: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 2007; 22: 1901–1911. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P, Rodriguez-Blazquez C, Abe K, Bhattacharyya KB, Bloem BR, Carod-Artal FJ et al. International study on the psychometric attributes of the non-motor symptoms scale in Parkinson disease. Neurology 2009; 73: 1584–1591. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord 2006; 21: 916–923. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park SY, Cho YJ, Hong KS, Cho JY, Seo SY et al. Nonmotor symptoms in de novo Parkinson disease before and after dopaminergic treatment. J Neurol Sci 2009; 287: 200–204. [DOI] [PubMed] [Google Scholar]
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009; 24: 1641–1649. [DOI] [PubMed] [Google Scholar]
- Muller B, Larsen JP, Wentzel-Larsen T, Skeie GO, Tysnes OB. Parkwest Study G. Autonomic and sensory symptoms and signs in incident, untreated Parkinson’s disease: frequent but mild. Mov Disord 2011; 26: 65–72. [DOI] [PubMed] [Google Scholar]
- Picillo M, Amboni M, Erro R, Longo K, Vitale C, Moccia M et al. Gender differences in non-motor symptoms in early, drug naive Parkinson’s disease. J Neurol 2013; 260: 2849–2855. [DOI] [PubMed] [Google Scholar]
- Kim JS, Youn J, Shin H, Cho JW. Nonmotor symptoms in drug-induced parkinsonism and drug-naive Parkinson disease. Can J Neurol Sci 2013; 40: 36–41. [DOI] [PubMed] [Google Scholar]
- Song Y, Gu Z, An J, Chan P, Chinese Parkinson Study Group. Gender differences on motor and non-motor symptoms of de novo patients with early Parkinson’s disease. Neurol Sci 2014; 35: 1991–1996. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gu Z, An J, Wang C, Chan P. Non-motor symptoms in treated and untreated Chinese patients with early Parkinson’s disease. Tohoku J Exp Med 2014; 232: 129–136. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Kim YE, Yun JY, Kim HJ, Jeon BS. Identifying the clusters within nonmotor manifestations in early Parkinson’s disease by using unsupervised cluster analysis. PLoS One 2014; 9: e91906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R et al. The onset of nonmotor symptoms in Parkinson’s disease (The ONSET PD Study). Mov Disord 2015; 30: 229–237. [DOI] [PubMed] [Google Scholar]
- Erro R, Vitale C, Amboni M, Picillo M, Moccia M, Longo K et al. The heterogeneity of early Parkinson’s disease: a cluster analysis on newly diagnosed untreated patients. PLoS One 2013; 8: e70244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M, Haapaniemi T, Turkka J, Suominen K, Tolonen U, Sotaniemi K et al. Heart rate variability in patients with untreated Parkinson’s disease. Eur J Neurol 2000; 7: 667–672. [DOI] [PubMed] [Google Scholar]
- Aris E, Dotchin CL, Gray WK, Walker RW. Autonomic function in a prevalent Tanzanian population with Parkinson’s disease and its relationship to disease duration and 5-year mortality. BMC Res Notes 2013; 6: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piha SJ, Rinne JO, Rinne UK, Seppanen A. Autonomic dysfunction in recent onset and advanced Parkinson’s disease. Clin Neurol Neurosurg 1988; 90: 221–226. [DOI] [PubMed] [Google Scholar]
- Asahina M, Mathias CJ, Katagiri A, Low DA, Vichayanrat E, Fujinuma Y et al. Sudomotor and cardiovascular dysfunction in patients with early untreated Parkinson’s disease. J Parkinsons Dis 2014; 4: 385–393. [DOI] [PubMed] [Google Scholar]
- Bae HJ, Cheon SM, Kim JW. Orthostatic hypotension in drug-naive patients with Parkinson’s disease. J Mov Disord 2011; 4: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli U, Lucetti C, Del Dotto P, Ceravolo R, Gambaccini G, Bernardini S et al. Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 2003; 60: 1400–1404. [DOI] [PubMed] [Google Scholar]
- Camerlingo M, Ferraro B, Gazzaniga GC, Casto L, Cesana BM, Mamoli A. Cardiovascular reflexes in Parkinson’s disease: long-term effects of levodopa treatment on de novo patients. Acta Neurol Scand 1990; 81: 346–348. [DOI] [PubMed] [Google Scholar]
- Haapaniemi TH, Kallio MA, Korpelainen JT, Suominen K, Tolonen U, Sotaniemi KA et al. Levodopa, bromocriptine and selegiline modify cardiovascular responses in Parkinson’s disease. J Neurol 2000; 247: 868–874. [DOI] [PubMed] [Google Scholar]
- Micieli G, Martignoni E, Cavallini A, Sandrini G, Nappi G. Postprandial and orthostatic hypotension in Parkinson’s disease. Neurology 1987; 37: 386–393. [DOI] [PubMed] [Google Scholar]
- Hiorth YH, Lode K, Larsen JP. Frequencies of falls and associated features at different stages of Parkinson’s disease. Eur J Neurol 2013; 20: 160–166. [DOI] [PubMed] [Google Scholar]
- Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllyla VV. Ambulatory ECG and analysis of heart rate variability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2001; 70: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiainen V, Haapaniemi TH, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllyla VV. Circadian heart rate variability in Parkinson’s disease. J Neurol 2002; 249: 1535–1540. [DOI] [PubMed] [Google Scholar]
- De Luka SR, Svetel M, Pekmezovic T, Milovanovic B, Kostic VS. When do the symptoms of autonomic nervous system malfunction appear in patients with Parkinson’s disease? Vojnosanit Pregl 2014; 71: 346–351. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Franceschi M, Pinto P, Zucconi M, Smirne S. Respiration and heart rate variability during sleep in untreated Parkinson patients. Gerontology 1992; 38: 92–98. [DOI] [PubMed] [Google Scholar]
- Kallio M, Suominen K, Haapaniemi T, Sotaniemi K, Myllyla VV, Astafiev S et al. Nocturnal cardiac autonomic regulation in Parkinson’s disease. Clin Auton Res 2004; 14: 119–124. [DOI] [PubMed] [Google Scholar]
- Camerlingo M, Aillon C, Bottacchi E, Gambaro P, D’Alessandro G, Franceschi M et al. Parasympathetic assessment in Parkinson’s disease. Adv Neurol 1987; 45: 267–269. [PubMed] [Google Scholar]
- Umehara T, Toyoda C, Oka H. Postprandial hypotension in de novo Parkinson’s disease: a comparison with orthostatic hypotension. Parkinsonism Relat Disord 2014; 20: 573–577. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Sakakibara R, Yamamoto T, Ito T, Yamaguchi C, Awa Y et al. Urinary dysfunction in early and untreated Parkinson’s disease. J Neurol Neurosurg Psychiatry 2011; 82: 1382–1386. [DOI] [PubMed] [Google Scholar]
- Erro R, Picillo M, Amboni M, Moccia M, Vitale C, Longo K et al. Nonmotor predictors for levodopa requirement in de novo patients with Parkinson’s disease. Mov Disord 2015; 30: 373–378. [DOI] [PubMed] [Google Scholar]
- Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord 1991; 6: 151–156. [DOI] [PubMed] [Google Scholar]
- Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord 2001; 16: 1041–1047. [DOI] [PubMed] [Google Scholar]
- Unger MM, Moller JC, Mankel K, Schmittinger K, Eggert KM, Stamelou M et al. Patients with idiopathic rapid-eye-movement sleep behavior disorder show normal gastric motility assessed by the 13C-octanoate breath test. Mov Disord 2011; 26: 2559–2563. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Saji E, Yajima R, Onodera O, Nishizawa M. Reduced bowel sounds in Parkinson’s disease and multiple system atrophy patients. Clin Auton Res 2011; 21: 181–184. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kato T, Nishida H, Yamada M, Koumura A, Sakurai T et al. Is there a delayed gastric emptying of patients with early-stage, untreated Parkinson’s disease? An analysis using the 13C-acetate breath test. J Neurol 2011; 258: 421–426. [DOI] [PubMed] [Google Scholar]
- Tateno F, Sakakibara R, Yokoi Y, Kishi M, Ogawa E, Uchiyama T et al. Levodopa ameliorated anorectal constipation in de novo Parkinson’s disease: the QL-GAT study. Parkinsonism Relat Disord 2011; 17: 662–666. [DOI] [PubMed] [Google Scholar]
- Bagheri H, Damase-Michel C, Lapeyre-Mestre M, Cismondo S, O’Connell D, Senard JM et al. A study of salivary secretion in Parkinson’s disease. Clin Neuropharmacol 1999; 22: 213–215. [PubMed] [Google Scholar]
- Friedman JH, Amick MM. Rhinorrhea is increased in Parkinson’s disease. Mov Disord 2008; 23: 452–454. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Alves G, Tysnes OB, Pedersen KF, Ehrt U et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease. J Neurol Neurosurg Psychiatry 2009; 80: 928–930. [DOI] [PubMed] [Google Scholar]
- Poletti M, Lucetti C, Del Dotto P, Berti C, Logi C, Bonuccelli U. Relationship between neuropsychiatric symptoms and cognitive performance in de novo Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2012; 24: E22–E23. [DOI] [PubMed] [Google Scholar]
- de la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 2014; 83: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria J, Tolosa ES, Valles A, Bayes A, Blesa R, Masana J. Mental depression in untreated Parkinson’s disease of recent onset. Adv Neurol 1987; 45: 443–446. [PubMed] [Google Scholar]
- Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D et al. The impact of depressive symptoms in early Parkinson disease. Neurology 2007; 69: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Sohn YH, Lee JH, Kim J. The effect of long-term levodopa therapy on depression level in de novo patients with Parkinson’s disease. J Neurol Sci 2000; 172: 12–16. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Robinson RG, Cravello L, Pontieri FE, Pierantozzi M, Stefani A et al. The early course of affective and cognitive symptoms in de novo patients with Parkinson’s disease. J Neurol 2014; 261: 1126–1132. [DOI] [PubMed] [Google Scholar]
- Santangelo G, Barone P, Cuoco S, Raimo S, Pezzella D, Picillo M et al. Apathy in untreated, de novo patients with Parkinson’s disease: validation study of Apathy Evaluation Scale. J Neurol 2014; 261: 2319–2328. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Alves G, Bronnick K, Aarsland D, Tysnes OB, Larsen JP. Apathy in drug-naive patients with incident Parkinson’s disease: the Norwegian ParkWest study. J Neurol 2010; 257: 217–223. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Langlois C, Plomhause L, Carette AS, Delliaux M, Duhamel A et al. Apathy in untreated early-stage Parkinson disease: relationship with other non-motor symptoms. Mov Disord 2014; 29: 1796–1801. [DOI] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Pagni C, Lucetti C, Del Dotto P, Ceravolo R et al. Alexithymia is associated with depression in de novo Parkinson’s disease. Psychother Psychosom 2011; 80: 251–253. [DOI] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Pagni C, Lucetti C, Del Dotto P, Tognoni G et al. The association between motor subtypes and alexithymia in de novo Parkinson’s disease. J Neurol 2011; 258: 1042–1045. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Papay K, Siderowf A, Parkinson’s Progression Markers Initiative. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 2013; 80: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Siri C, Santangelo G, Cilia R, Poletti M, Canesi M et al. Impulsivity and compulsivity in drug-naive patients with Parkinson’s disease. Mov Disord 2011; 26: 464–468. [DOI] [PubMed] [Google Scholar]
- Nicoletti A, Luca A, Raciti L, Contrafatto D, Bruno E, Dibilio V et al. Obsessive compulsive personality disorder and Parkinson’s disease. PLoS One 2013; 8: e54822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009; 8: 464–474. [DOI] [PubMed] [Google Scholar]
- Reid WG, Broe GA, Hely MA, Morris JG, Williamson PM, O’Sullivan DJ et al. The neuropsychology of de novo patients with idiopathic Parkinson’s disease: the effects of age of onset. Int J Neurosci 1989; 48: 205–217. [DOI] [PubMed] [Google Scholar]
- Bronnick K, Alves G, Aarsland D, Tysnes OB, Larsen JP. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology 2011; 25: 114–124. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain 1991; 114(Pt 5): 2095–2122. [DOI] [PubMed] [Google Scholar]
- Hartikainen P, Helkala EL, Soininen H, Riekkinen P Sr. Cognitive and memory deficits in untreated Parkinson’s disease and amyotrophic lateral sclerosis patients: a comparative study. J Neural Transm Park Dis Dement Sect 1993; 6: 127–137. [DOI] [PubMed] [Google Scholar]
- Miah IP, Olde Dubbelink KT, Stoffers D, Deijen JB, Berendse HW. Early-stage cognitive impairment in Parkinson’s disease and the influence of dopamine replacement therapy. Eur J Neurol 2012; 19: 510–516. [DOI] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 2012; 83: 601–606. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Sprengelmeyer R, Fimm B, Wallesch CW. Cognitive slowing in decision tasks in early and advanced Parkinson’s disease. Brain Cogn 1992; 18: 60–69. [DOI] [PubMed] [Google Scholar]
- Pagni C, Frosini D, Ceravolo R, Giunti G, Unti E, Poletti M et al. Event-based prospective memory in newly diagnosed, drug-naive Parkinson’s disease patients. J Int Neuropsychol Soc 2011; 17: 1158–1162. [DOI] [PubMed] [Google Scholar]
- Domellof ME, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson’s disease. Mov Disord 2011; 26: 2183–2189. [DOI] [PubMed] [Google Scholar]
- Antoniades CA, Demeyere N, Kennard C, Humphreys GW, Hu MT. Antisaccades and executive dysfunction in early drug-naive Parkinson’s disease: the discovery study. Mov Disord 2015; 30: 843–847. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study Group. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009; 72: 1121–1126. [DOI] [PubMed] [Google Scholar]
- Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol 2014; 27: 276–281. [DOI] [PubMed] [Google Scholar]
- Szeto JY, Mowszowski L, Gilat M, Walton CC, Naismith SL, Lewis SJ. Assessing the utility of the Movement Disorder Society Task Force Level 1 diagnostic criteria for mild cognitive impairment in Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 31–35. [DOI] [PubMed] [Google Scholar]
- Reid WG, Hely MA, Morris JG, Broe GA, Adena M, Sullivan DJ et al. A longitudinal of Parkinson’s disease: clinical and neuropsychological correlates of dementia. J Clin Neurosci 1996; 3: 327–333. [DOI] [PubMed] [Google Scholar]
- Erro R, Santangelo G, Picillo M, Vitale C, Amboni M, Longo K et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J Neurol 2012; 259: 1808–1813. [DOI] [PubMed] [Google Scholar]
- Kwon KY, Kang SH, Kim M, Lee HM, Jang JW, Kim JY et al. Nonmotor symptoms and cognitive decline in de novo Parkinson’s disease. Can J Neurol Sci 2014; 41: 597–602. [DOI] [PubMed] [Google Scholar]
- Bae HJ, Lim JH, Cheon SM. Orthostatic hypotension and cognitive impairment in de novo patients with Parkinson’s disease. J Mov Disord 2014; 7: 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlofson K, Ongre SO, Enger LK, Tysnes OB, Larsen JP. Fatigue in early Parkinson’s disease. Minor inconvenience or major distress? Eur J Neurol 2012; 19: 963–968. [DOI] [PubMed] [Google Scholar]
- Kang SY, Ma HI, Lim YM, Hwang SH, Kim YJ. Fatigue in drug-naive Parkinson’s disease. Eur Neurol 2013; 70: 59–64. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Friedman JH, Oakes D, Shulman L, Comella CL, Marek K et al. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology 2008; 71: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskova J, Klempir J, Majerova V, Picmausova J, Sonka K, Jech R et al. Sleep disturbances in untreated Parkinson’s disease. J Neurol 2011; 258: 2254–2259. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Dhoat S, Williams AJ, Dimarco A, Pal S, Forbes A et al. The range and nature of sleep dysfunction in untreated Parkinson’s disease (PD). A comparative controlled clinical study using the Parkinson’s disease sleep scale and selective polysomnography. J Neurol Sci 2006; 248: 158–162. [DOI] [PubMed] [Google Scholar]
- Ferreira T, Prabhakar S, Kharbanda PS. Sleep disturbances in drug naive Parkinson’s disease (PD) patients and effect of levodopa on sleep. Ann Indian Acad Neurol 2014; 17: 416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansdottir S, Gjerstad MD, Tysnes OB, Larsen JP. Subjective sleep problems in patients with early Parkinson’s disease. Eur J Neurol 2012; 19: 1575–1581. [DOI] [PubMed] [Google Scholar]
- Wetter TC, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep 2000; 23: 361–367. [PubMed] [Google Scholar]
- Wetter TC, Brunner H, Hogl B, Yassouridis A, Trenkwalder C, Friess E. Increased alpha activity in REM sleep in de novo patients with Parkinson’s disease. Mov Disord 2001; 16: 928–933. [DOI] [PubMed] [Google Scholar]
- Angelini M, Negrotti A, Marchesi E, Bonavina G, Calzetti S. A study of the prevalence of restless legs syndrome in previously untreated Parkinson’s disease patients: absence of co-morbid association. J Neurol Sci 2011; 310: 286–288. [DOI] [PubMed] [Google Scholar]
- Shin HY, Youn J, Yoon WT, Kim JS, Cho JW. Restless legs syndrome in Korean patients with drug-naive Parkinson’s disease: a nation-wide study. Parkinsonism Relat Disord 2013; 19: 355–358. [DOI] [PubMed] [Google Scholar]
- Gjerstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology 2011; 77: 1941–1946. [DOI] [PubMed] [Google Scholar]
- Plomhause L, Dujardin K, Duhamel A, Delliaux M, Derambure P, Defebvre L et al. Rapid eye movement sleep behavior disorder in treatment-naive Parkinson disease patients. Sleep Med 2013; 14: 1035–1037. [DOI] [PubMed] [Google Scholar]
- Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep 2014; 37: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini G, Barbanti P, Aurilia C, Vanacore N, Pauletti C, Meco G. Excessive daytime sleepiness in de novo and treated Parkinson’s disease. Mov Disord 2002; 17: 1026–1030. [DOI] [PubMed] [Google Scholar]
- Kaynak D, Kiziltan G, Kaynak H, Benbir G, Uysal O. Sleep and sleepiness in patients with Parkinson’s disease before and after dopaminergic treatment. Eur J Neurol 2005; 12: 199–207. [DOI] [PubMed] [Google Scholar]
- Giganti F, Ramat S, Zilli I, Guidi S, Raglione LM, Sorbi S et al. Daytime course of sleepiness in de novo Parkinson’s disease patients. J Sleep Res 2013; 22: 197–200. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1992; 55: 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KK, Waro BJ, Aasly JO. Olfactory dysfunction in sporadic Parkinson’s Disease and LRRK2 carriers. Acta Neurol Scand 2014; 129: 300–306. [DOI] [PubMed] [Google Scholar]
- Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC et al. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord 2001; 16: 41–46. [DOI] [PubMed] [Google Scholar]
- Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ. Ophthalmologic features of Parkinson’s disease. Neurology 2004; 62: 177–180. [DOI] [PubMed] [Google Scholar]
- Buttner T, Kuhn W, Przuntek H. Alterations in chromatic contour perception in de novo parkinsonian patients. Eur Neurol 1995; 35: 226–229. [DOI] [PubMed] [Google Scholar]
- Muller T, Kuhn W, Buttner T, Przuntek H. Distorted colour discrimination in Parkinson’s disease is related to severity of the disease. Acta Neurol Scand 1997; 96: 293–296. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park JH, Kim YH, Koh SB. Stereopsis in drug naive Parkinson’s disease patients. Can J Neurol Sci 2011; 38: 299–302. [DOI] [PubMed] [Google Scholar]
- Erro R, Picillo M, Vitale C, Amboni M, Moccia M, Longo K et al. Non-motor symptoms in early Parkinson’s disease: a 2-year follow-up study on previously untreated patients. J Neurol Neurosurg Psychiatry 2013; 84: 14–17. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 1991; 14: 153–197. [DOI] [PubMed] [Google Scholar]