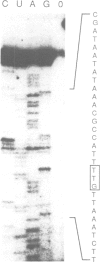
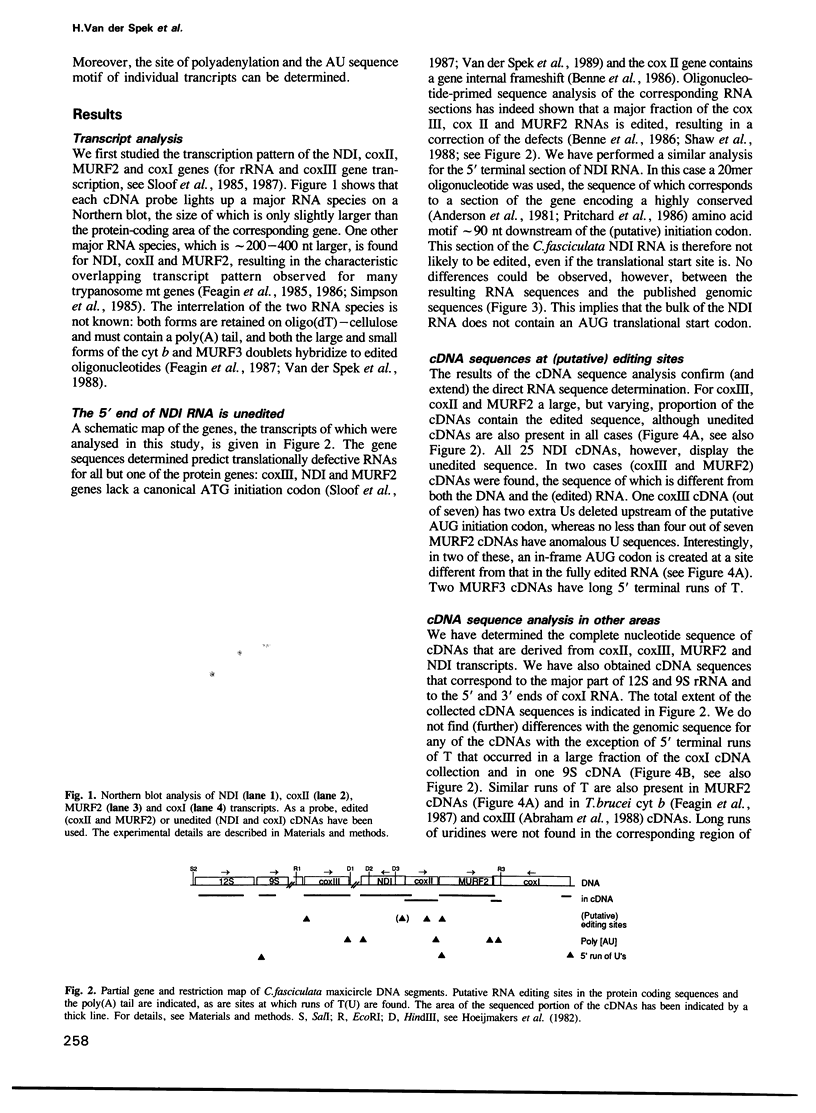
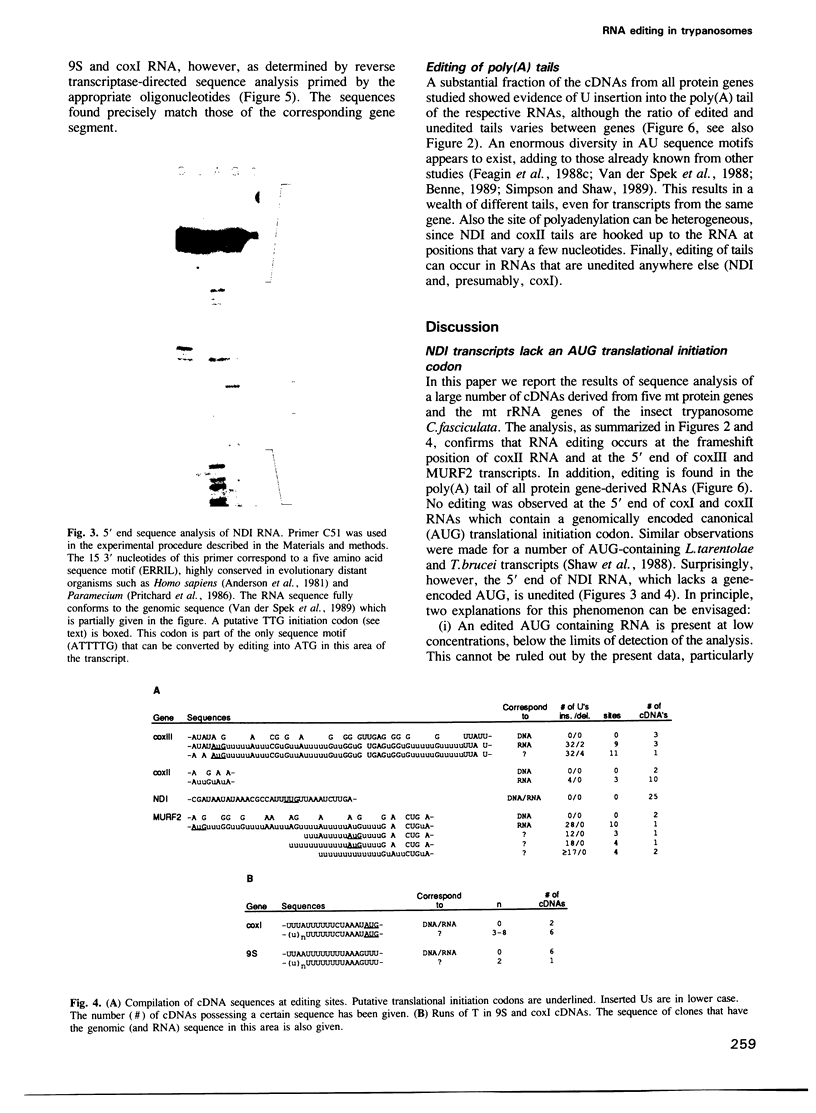
Abstract
With the aid of cDNA and RNA sequence analysis, we have determined to what extent transcripts of mitochondrial maxicircle genes of the insect trypanosome Crithidia fasciculata are altered by RNA editing, a novel mechanism of gene expression which operates via the insertion and deletion of uridine residues. Editing of cytochrome c oxidase (cox) subunit II and III transcripts and of maxicircle unidentified reading frame (MURF) 2 RNA is limited to a small section and results in the creation of a potential AUG translational initiation codon (coxIII, MURF2) or the removal of a frameshift (coxII). No differences with the genomic sequences were observed in the remainder of these RNAs. Surprisingly, NADH dehydrogenase subunit I transcripts were completely unedited in the coding region, implying that an AUG translational initiation codon is absent. The partial ribosomal RNA sequences determined also conform to the gene sequences. Together these results lead to the conclusion that the unusual sequences predicted by the protein and rRNA genes must indeed be present in the gene products. Editing also occurred in the poly(A) tail of RNAs from all protein genes, including those that are unedited in the coding region. The tails display a large variation in AU sequence motifs. Finally, some cDNAs contained sequences absent from both the DNA and the edited RNA. Some of these may represent intermediates in the RNA editing process. We argue, however, that long runs of T may be artefacts of cDNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Feagin J. E., Stuart K. Characterization of cytochrome c oxidase III transcripts that are edited only in the 3' region. Cell. 1988 Oct 21;55(2):267–272. doi: 10.1016/0092-8674(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Benne R., De Vries B. F., Van den Burg J., Klaver B. The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983 Oct 25;11(20):6925–6941. doi: 10.1093/nar/11.20.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R. RNA-editing in trypanosome mitochondria. Biochim Biophys Acta. 1989 Mar 1;1007(2):131–139. doi: 10.1016/0167-4781(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F. The maxi-circle of Trypanosoma brucei kinetoplast DNA. Biochim Biophys Acta. 1979 Nov 22;565(1):1–12. doi: 10.1016/0005-2787(79)90078-9. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Abraham J. M., Stuart K. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell. 1988 May 6;53(3):413–422. doi: 10.1016/0092-8674(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Apocytochrome b and other mitochondrial DNA sequences are differentially expressed during the life cycle of Trypanosoma brucei. Nucleic Acids Res. 1985 Jun 25;13(12):4577–4596. doi: 10.1093/nar/13.12.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Developmentally regulated addition of nucleotides within apocytochrome b transcripts in Trypanosoma brucei. Cell. 1987 May 8;49(3):337–345. doi: 10.1016/0092-8674(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Differential mitochondrial gene expression between slender and stumpy bloodforms of Trypanosoma brucei. Mol Biochem Parasitol. 1986 Sep;20(3):207–214. doi: 10.1016/0166-6851(86)90100-3. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Shaw J. M., Simpson L., Stuart K. Creation of AUG initiation codons by addition of uridines within cytochrome b transcripts of kinetoplastids. Proc Natl Acad Sci U S A. 1988 Jan;85(2):539–543. doi: 10.1073/pnas.85.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Stuart K. Developmental aspects of uridine addition within mitochondrial transcripts of Trypanosoma brucei. Mol Cell Biol. 1988 Mar;8(3):1259–1265. doi: 10.1128/mcb.8.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley I. M., Runswick M. J., Walker J. E. A homologue of the nuclear coded 49 kd subunit of bovine mitochondrial NADH-ubiquinone reductase is coded in chloroplast DNA. EMBO J. 1989 Mar;8(3):665–672. doi: 10.1002/j.1460-2075.1989.tb03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensgens L. A., Brakenhoff J., De Vries B. F., Sloof P., Tromp M. C., Van Boom J. H., Benne R. The sequence of the gene for cytochrome c oxidase subunit I, a frameshift containing gene for cytochrome c oxidase subunit II and seven unassigned reading frames in Trypanosoma brucei mitochondrial maxi-circle DNA. Nucleic Acids Res. 1984 Oct 11;12(19):7327–7344. doi: 10.1093/nar/12.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Schoutsen B., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. I. Sequence evolution and transcription of the maxicircle. Plasmid. 1982 May;7(3):199–209. doi: 10.1016/0147-619x(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Borst P., Weijers P. J. The structure of kinetoplast DNA. I. Properties of the intact multi-circular complex from Crithidia luciliae. Biochim Biophys Acta. 1975 May 1;390(2):155–167. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N., Weiner A. RNA editing. In search of a template. Nature. 1988 Aug 11;334(6182):469–470. doi: 10.1038/334469a0. [DOI] [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at non-AUG triplets in mammalian cells. J Biol Chem. 1989 Mar 25;264(9):5031–5035. [PubMed] [Google Scholar]
- Pritchard A. E., Seilhamer J. J., Cummings D. J. Paramecium mitochondrial DNA sequences and RNA transcripts for cytochrome oxidase subunit I, URF1, and three ORFs adjacent to the replication origin. Gene. 1986;44(2-3):243–253. doi: 10.1016/0378-1119(86)90188-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. M., Feagin J. E., Stuart K., Simpson L. Editing of kinetoplastid mitochondrial mRNAs by uridine addition and deletion generates conserved amino acid sequences and AUG initiation codons. Cell. 1988 May 6;53(3):401–411. doi: 10.1016/0092-8674(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Neckelmann N., de la Cruz V. F., Muhich M. L., Simpson L. Mapping and 5' end determination of kinetoplast maxicircle gene transcripts from Leishmania tarentolae. Nucleic Acids Res. 1985 Aug 26;13(16):5977–5993. doi: 10.1093/nar/13.16.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Neckelmann N., de la Cruz V. F., Simpson A. M., Feagin J. E., Jasmer D. P., Stuart K. Comparison of the maxicircle (mitochondrial) genomes of Leishmania tarentolae and Trypanosoma brucei at the level of nucleotide sequence. J Biol Chem. 1987 May 5;262(13):6182–6196. [PubMed] [Google Scholar]
- Simpson L., Shaw J. RNA editing and the mitochondrial cryptogenes of kinetoplastid protozoa. Cell. 1989 May 5;57(3):355–366. doi: 10.1016/0092-8674(89)90911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Sloof P., Van den Burg J., Voogd A., Benne R., Agostinelli M., Borst P., Gutell R., Noller H. Further characterization of the extremely small mitochondrial ribosomal RNAs from trypanosomes: a detailed comparison of the 9S and 12S RNAs from Crithidia fasciculata and Trypanosoma brucei with rRNAs from other organisms. Nucleic Acids Res. 1985 Jun 11;13(11):4171–4190. doi: 10.1093/nar/13.11.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloof P., van den Burg J., Voogd A., Benne R. The nucleotide sequence of a 3.2 kb segment of mitochondrial maxicircle DNA from Crithidia fasciculata containing the gene for cytochrome oxidase subunit III, the N-terminal part of the apocytochrome b gene and a possible frameshift gene; further evidence for the use of unusual initiator triplets in trypanosome mitochondria. Nucleic Acids Res. 1987 Jan 12;15(1):51–65. doi: 10.1093/nar/15.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Van der Horst G., Osinga K. A., Arnberg A. C. Splicing of large ribosomal precursor RNA and processing of intron RNA in yeast mitochondria. Cell. 1984 Dec;39(3 Pt 2):623–629. doi: 10.1016/0092-8674(84)90469-0. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lake J. A., Simpson A. M., Simpson L. A minimal ribosomal RNA: sequence and secondary structure of the 9S kinetoplast ribosomal RNA from Leishmania tarentolae. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1401–1405. doi: 10.1073/pnas.82.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]
- de la Cruz V. F., Simpson A. M., Lake J. A., Simpson L. Primary sequence and partial secondary structure of the 12S kinetoplast (mitochondrial) ribosomal RNA from Leishmania tarentolae: conservation of peptidyl-transferase structural elements. Nucleic Acids Res. 1985 Apr 11;13(7):2337–2356. doi: 10.1093/nar/13.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spek H., Arts G. J., van den Burg J., Sloof P., Benne R. The nucleotide sequence of mitochondrial maxicircle genes of Crithidia fasciculata. Nucleic Acids Res. 1989 Jun 26;17(12):4876–4876. doi: 10.1093/nar/17.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spek H., van den Burg J., Croiset A., van den Broek M., Sloof P., Benne R. Transcripts from the frameshifted MURF3 gene from Crithidia fasciculata are edited by U insertion at multiple sites. EMBO J. 1988 Aug;7(8):2509–2514. doi: 10.1002/j.1460-2075.1988.tb03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]