Abstract
Subarachnoid hemorrhage (SAH) is a common and frequently life-threatening cerebrovascular disease, which is mostly related with a ruptured intracranial aneurysm. Its complications include rebleeding, early brain injury, cerebral vasospasm, delayed cerebral ischemia, chronic hydrocephalus, and also non neurological problems. Non-coding RNAs (ncRNAs), comprising of microRNAs (miRNAs), small interfering RNAs (siRNAs) and long non-coding RNAs (lncRNAs), play an important role in intracranial aneurysms and SAH. Here, we review the non-coding RNAs expression profile and their related mechanisms in intracranial aneurysms and SAH. Moreover, we suggest that these non-coding RNAs function as novel molecular biomarkers to predict intracranial aneurysms and SAH, and may yield new therapies after SAH in the future.
Keywords: ncRNAs (non-coding RNAs), intracranial aneurysms (IAs), subarachnoid hemorrhage (SAH), expression profiles, mechanisms
1. Introduction
1.1. intracranial aneurysms and subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) is a common and frequently life-threatening cerebrovascular disease that usually occurs at a relatively young age and has an unpredictable onset; in fact, the average age is about 50 years old [1, 2]. It is a devastating condition in which arterial bleeds into the subarachnoid space damaging brain perfusion and function [3]. Although it accounts for only 5% of strokes, its high rates of mortality and disability greatly increase the burden of society and families [4]. Indeed, the mortality rate of SAH ranges from 8.3% to 66.7% [5]. Additionally, the surviving patients suffer neurological injuries that seriously affect the quality of life to the point that productive patients are unable to return to work and likely needing constant care by others [6]. To date, AHA/ASA experts have consensus on the definition that stroke caused by subarachnoid hemorrhage only includes spontaneous SAH, which is mostly related with a ruptured intracranial aneurysm [1, 2, 7, 8].
The conventional secondary complications of SAH include rebleeding, early brain injury, cerebral vasospasm, delayed cerebral ischemia and chronic hydrocephalus [6, 9-11]. Following SAH, non neurological complications are also present; in fact, SAH patients are vulnerable to multiple extracerebral organ dysfunctions, such as neurocardiogenic injury, neurogenic pulmonary injury, neurogenic renal injury, hyperglycemia, electrolyte imbalance and hematological failure [12]. Despite early surgical and endovascular treatment of ruptured aneurysms has been improved greatly, the clinical outcome remains disappointing and leave SAH still a serious health problem. It leaves patients with psychological and physical damage, and also financial loss [6, 13]. Thus, the pathogenesis and treatment of intracranial aneurysms and SAH need further investigation, in order to better understand this disease and reduce related incidence, morbidity and mortality. This review summarises the non-coding RNAs studies in intracranial aneurysm and SAH.
1.2. non-coding RNAs
Non-coding RNAs (ncRNAs) mainly include microRNAs (miRNAs), small interfering RNAs (siRNAs) and long non-coding RNAs (lncRNAs).
1.2.1. miRNAs
miRNAs are a class of endogenous non-coding RNAs with 21-23 bp [14-16]. Most miRNAs are transcribed by RNA polymerase II to pri-miRNAs with characteristic hairpin structures. In the nucleus, the endonuclease Drosha and its partner DGCR8 process the pri-miRNA and release pre-miRNA hairpins. In the cytoplasm, the RNase III enzymes Dicer and its partner TRBP cleave the pre-miRNA to produce miRNA/miRNA* duplexes [17]. These duplexes are transferred to an Argonaute protein that selects a single strand. It is just the specific mature strand that guides RNA silencing complexes (RISC) to targeted mRNAs. Through complementarily base-pairing with the 3′untranslated region (3′UTR) of the targeted mRNA by its seed region, miRNAs can degrade the mRNA transcript and/or suppress the mRNA post-transcriptional translation [18].
Recent studies demonstrate that miRNAs are highly conserved in human and may modulate approximately 30% of all genes in the human genome. miRNAs can regulate diverse biological processes, including cell proliferation, differentiation and apoptosis [19]. miRNAs also exerts critical functions in gene regulatory networks during cellular development and physiology.In particular, miRNA play an important role in neurodevelopment, neuroplasticity and other fundamental neurobiological processes and diseases [16, 20].
1.2.2. siRNAs
RNA interference (RNAi) technology to silence gene was first described in Caenorhabditis Elegans and expanded to plants, Drosophila and fungi. It is processed into short RNA fragments with approximately 21 bp by an RNase type-III enzyme-Dicer. Subsequently, the specific RNA fragments activate an RNA-induced silencing complex (RISC) and degrade complementary mRNA with one strand retrained, after which it is recycled for several rounds [21]. Recently, RNAi-mediated gene depletion in a sequence-specific manner has gained increasingly popularity in mammalian cells by synthetic dsRNA, known as siRNA [22]. This strategy has been used successfully in various cancers and many other diseases, such as autoimmune, dominant genetic and nervous system disorders [23]. Thus, siRNAs offer a new therapeutical strategy for the future control and treatment of diseases.
1.2.3. lncRNAs
Another great achievement of the past decade has been the discovery of lncRNAs. lncRNAs are defined as transcripts greater than 200 bp in length. They are similar to protein coding transcripts in many ways; as in processing steps, histone-modification profiles, splicing signals and exon/intron lengths, however they lack of significant coding sequences [24]. lncRNAs are quite heterogeneous in several aspects, such as genomic context, size, life cycles and mechanisms of regulation. They may be standalone transcription units or transcribed from antisense to other genes with different degrees of overlap from enhancers, promoters, introns of other genes or pseudogenes [25]. Indeed, lncRNAs can be divided into five biotypes according to their proximity to protein-coding genes:sense, antisense, bidirectional, intronic and intergenic [26, 27]. Increasing evidence indicated that lncRNAs play a crucial role in various biological and pathological processes, such as neural differentiation, cell fate decisions, synaptic plasticity, behavior and neuroprotection [25, 28]. Indeed, a number of lncRNAs contribute to central nervous system disorders, including stroke, neuro-immunological, neuro-developmental, neuro-degenerative, neuro-oncological and psychiatric diseases [29, 30].
2. ncRNAs in intracranial aneurysms and subarachnoid hemorrhage
In recent years, increasing studies have focused on non-coding RNAs as regulators of post-transcriptional gene expression and in response to SAH.
2.1. miRNAs
It is well known that miRNAs have served as novel biomarkers and crucial regulators of pathological mechanisms for vascular diseases [31].
In experimentally Sprague-Dawley rats, microarray and qRT-PCR techniques demonstrated that 14 miRNAs were upregulated and 6 downregulated in late age (3 months) of the intracranial aneurysm tissues [32]. Among these dysregulated miRNAs, 3 upregulated miRNAs (miR-21, miR-22, and miR-24) and 1 downregulated miRNA (miR-181d) suppress apoptosis and promote cell proliferation in the vascular smooth muscle cells of intracranial aneurysms [32-34]. Of note, rabbit aneurysm models were recently investigated using RNA-sequencing and RT-qPCR analysis, demonstrating that 3 miRNAs (miR-1, miR-9-5p, and miR-204-5p) were downregulated and 5 upregulated (miR-10a-5p, miR-21-5p, miR-34a-5p, miR-146a-5p, and miR-223-3p) [35].
In peripheral blood of SAH patients, a microarray study indicated that 86 miRNAs were significantly dysregulated. The authors further examined the different phases of the intracranial aneurysms, showing that in daughter aneurysms group (group A), 68 miRNAs were overexpressed and none silenced. Moreover,
in unruptured aneurysms group (group B), 4 miRNAs were overexpressed and 9 downregulated, while in ruptured aneurysms group (group C), 2 miRNAs were upregulated and 13 downregulated [36]. Interestingly, some miRNAs showed differential expression within the groups:, 4 miRNAs (miRNA-21, miRNA-22 miRNA-720 and miRNA-3665) were upregulated in both group A and B, miRNA-3679-5p was upregulated in both group A and C, while 5 miRNAs (miR-1471, miR-3945, miR-4253, miR-4314, and miR-574-5p) were downregulated in both group B and C [36]. Microarray analysis, using the peripheral plasma of SAH patients, revealed that 119 miRNAs were greatly altered in unruptured aneurysms group and 23 in ruptured aneurysms group, with 20 miRNAs unanimously changed in both groups. 99 miRNAs (69 upregulated and 30 downregulated) were specifically validated by separate microarrays. Furthermore, 6 miRNAs (miR-16, miR-25, miR-let-7g, miR-1183, miR-1825, and miR-188-5p) were confirmed by RT-qPCR, with miR-16 and miR-25 significantly upregulated. The latest 2 miRNAs were then identified by logistic regression analysis as potential biomarkers in intracranial aneurysms [37, 38]. Comparison of the two above papers of peripheral blood, demonstrated that approximately one third of the screened miRNAs were repeated and we summarised the data in Table 1.
Table 1.
Repeated miRNAs screened from peripheral blood
| MiRNA | R | P A | FC A | P B | FC B |
|---|---|---|---|---|---|
| has-miR-939 | Up | 0.004 | 14.7 | 0.011 | 14.1 |
| has-miR-1207-5p | Up | 0.005 | 12.8 | 0.006 | 10.0 |
| has-hsa-miR-22 | Up | 0.003 | 11.8 | 0.003 | 8.3 |
| hsa-miR-1275 | Up | 0.021 | 9.5 | 0.005 | 10.2 |
| hsa-miR-762 | Up | 0.027 | 7.4 | 0.006 | 11.8 |
| hsa-miR-144 | Up | 0.025 | 8.0 | 0.018 | 10.3 |
| hsa-miR-638 | Up | 0.007 | 10.2 | 0.010 | 9.8 |
| hsa-miR-30d | Up | 0.007 | 14.4 | 0.006 | 8.5 |
| hsa-miR-1202 | Up | 0.007 | 12.1 | 0.008 | 8.2 |
| hsa-miR-1915 | Up | 0.004 | 11.1 | 0.015 | 6.3 |
| hsa-miR-423-5p | Up | 0.010 | 11.9 | 0.005 | 14.5 |
| hsa-let-7i | Up | 0.019 | 11.2 | 0.006 | 24.8 |
| hsa-miR-483-5p | Up | 0.018 | 10.1 | 0.013 | 4.4 |
| hsa-miR-197 | Up | 0.011 | 7.52 | 0.015 | 3.4 |
| hsa-let-7b | Up | 0.022 | 10.0 | 0.015 | 40.8 |
| hsa-let-7d* | Up | 0.016 | 8.4 | 0.004 | 10.0 |
| hsa-miR-19b | Up | 0.017 | 5.1 | 0.004 | 14.2 |
| hsa-miR-301a | Up | 0.001 | 2.2 | 0.003 | 10.2 |
| hsa-miR-134 | Up | 0.007 | 11.2 | 0.005 | 14.8 |
| hsa-miR-106b | Up | 0.008 | 10.6 | 0.005 | 13.3 |
| hsa-miR-320c | Up | 0.023 | 8.3 | 0.005 | 11.2 |
| hsa-miR-21 | Up | 0.009 | 7.4 | 0.007 | 9.2 |
| hsa-miR-93 | Up | 0.014 | 8.1 | 0.005 | 18.2 |
| hsa-miR-575 | Up | 0.003 | 18.9 | 0.005 | 19.9 |
| hsa-miR-630 | Up | 0.005 | 14.8 | 0.005 | 15.0 |
| hsa-miR-601 | Up | 0.045 | 7.2 | 0.011 | 9.4 |
| hsa-miR-92a | Up | 0.008 | 12.4 | 0.004 | 19.1 |
| hsa-miR-25 | Up | 0.013 | 6.8 | 0.005 | 27.5 |
| hsa-miR-1225-5p | Up | 0.010 | 10.2 | 0.006 | 7.0 |
| hsa-miR-1268 | Up | 0.011 | 9.9 | 0.011 | 7.2 |
| hsa-miR-151-3p | Up | 0.043 | 7.0 | 0.049 | 5.2 |
| hsa-miR-486-5p | Up | 0.005 | 16.8 | 0.004 | 25.0 |
| hsa-miR-320d | Up | 0.005 | 14.4 | 0.005 | 13.8 |
R: Up or down regulation.
P A: P value in reference 67.
FC A: Fold change in reference 67.
P B: P value in reference 68.
FC B:Fold change in reference 68.
In the ruptured intracranial aneurysms of SAH patients, 18 miRNAs were identified to be dysregulated using microarray and qRT-PCR analysis [39]. By post-transcriptional mechanism, miR-29 manipulated aneurysm formation, progression and rupture rate through suppressing protein expression, such as fibrins, elastins, collagens and metalloproteinase-2 [40]. Additionally, in another experiment of ruptured intracranial aneurysms in SAH patients, microarray assays showed that 157 miRNAs were differentially expressed (72 upregulated and 85 downregulated). Of these, few miRNAs were randomly selected for RT-qPCR validation (miR-99b*, miR-340*, miR-493, miR-1208 and miR-648) and their alteration exhibited consistency with the microarray assay analysis [41]. In line with the above report [39], miR-29b and miR-133 were also showed to be dysregulated in intracranial aneurysms in this study. Furthermore, ruptured saccular intracranial aneurysms in SAH patients were also studied by Chen et al. This study revealed that miR-661, miR-1207-5p and miR-1915-3p were predicted to be activated, while miR-33a-5p, miR-659-3p and miR-524-5p were predicted to be inactivated using Sylamer method [42]. Among these results, miR-524-5p, a brain-specific miRNA, suppressed cell proliferation and invasion by directly targeting Jagged-1 and Hes-1 [43].
Some above screened miRNAs were used for bioinformatics and functional analysis. Jin H et al., employed TargetScan Human to predict target genes and Gene Ontology (GO) analysis to identify functional classification of target genes. 9 target genes were found to be associated with the pathogenesis of aneurysms, such as TRIB1, SSX3, ARFIP2, BCL6B, EP300, EDN1, CHAF1A, PDCD6 and FMN2 [36]. In this latest report integrated database (including miRecords, Tarbase and TargetScan Human),Ingenuity Pathway Analysis (IPA), constructed functional analysis and miRNA-mRNA networks were used to predict target genes . They found that the selected 18 miRNAs were observed to potentially target 681 genes screened from mRNA microarrays, in which 11 miRNAs and 54 genes were involved in the top 12 predicted functions, including migration of phagocytes, proliferation of mononuclear leukocytes, cell movement of mononuclear leukocytes, cell movement of smooth muscle cells, differentiation of macrophages, stimulation of T lymphocytes, cell death of vascular endothelial cells, migration of endothelial cells, cell movement of endothelial cells, apoptosis of vascular endothelial cells, proliferation of smooth muscle cells and proliferation of endothelial cells [39]. Additionally, liu D at al., retrieved miRNA target genes from miRecords, TarBase and Ingenuity Knowledge Base, applied DAVID Bioinformatics Resources or the IPA software to create gene functional annotations and used the Cytoscape platform to establish miRNA-mRNA interaction networks. The results indicated that several biological processes, including programmed cell death, extracellular matrix organization, response to oxidative stress, TGF-beta signalling pathway, smooth muscle cell proliferation, and aortic dissection were related to these miRNAs and their target genes [41]. All these pathways may be potentially associated with the mechanisms of intracranial aneurysms and SAH.
Because the microarray results show greater variation compared to RT-qPCR data, [44], here, we summarised RT-qPCR confirmed miRNA information to date (Table 2). Moreover, the screened dysregulated miRNAs together with their target genes and functional analysis are listed in Table 3.
Table 2.
RT-qPCR confirmed miRNAs and related information
| miRNA | Resource | R | Functional Analysis | Literature |
|---|---|---|---|---|
| hsa-mir-1 | IA domes | Down | Target CCL2, CXCL6, CXCR4, involved in the SMC proliferation and differentiation, and et al | [35], [39] |
| hsa-mir-7-1-3p | IA domes | Down | / | [39] |
| hsa-mir-23b-3p | IA domes | Down | / | [39] |
| hsa-mir-23b-5p | IA domes | Down | / | [39] |
| hsa-mir-24-1-5p | IA domes | Down | / | [39] |
| hsa-mir-28-3p | IA domes | Down | Involved in proliferation of mononuclear leukocytes, cell movement of mononuclear | |
| leukocytes, stimulation of T lymphocytes | [39] | |||
| hsa-mir-28-5p | IA domes | Down | Involved in migration of phagocytes, proliferation of mononuclear leukocytes, cell | |
| movement of smooth muscle cells, and et al | [39] | |||
| hsa-mir-29b-2-5p | IA domes | Down | Repressed the expression of extracellular matrix proteins, targeted several extracellular | |
| hsa-mir-29c-3p | IA domes | Down | matrix genes, and et al | [39, 40] |
| hsa-mir-29c-5p | IA domes | Down | ||
| hsa-mir-133b | IA domes | Down | Associated with the development of intracranial aneurysms, inhibited the PDGF-induced | |
| hsa-mir-133a | IA domes | Down | switch towards a synthetic SMC phenotype, and et al | [39] |
| hsa-mir-140-3p | IA domes | Down | / | [39] |
| hsa-mir-143-3p | IA domes | Down | Involved in apoptosis and tumor formation, targeted several genes, and et al | [39] |
| hsa-mir-143-5p | IA domes | Down | ||
| hsa-mir-145-3p | IA domes | Down | Involved in modulation of vascular smooth muscle cell phenotype | [39] |
| hsa-mir-145-5p | IA domes | Down | ||
| hsa-mir-455-5p | IA domes | Down | Involved in migration of phagocytes, cell movement of mononuclear leukocytes, and | |
| proliferation of SMC | [39] | |||
| hsa-mir-99b* | IA walls | Up | / | |
| hsa-mir-340* | IA walls | Down | / | [41] |
| hsa-mir-493 | IA walls | Up | / | |
| hsa-mir-16 | plasma | Up | Involved in regulating the angiogenic functions of the endothelial cell | [36-38] |
| hsa-mir-25 | plasma | Up | Might refect pathological alterations in the vascular tissue | [36-38] |
| hsa-let-7g | plasma | Up | Involved in modulating important endothelial cell functions such as angiogenesis | [37] |
| rno-miR-147 | IA | Up | / | [32] |
| rno-miR-101b | IA | Up | / | [32] |
| rno-miR-21 | IA | Up | Served as an endogenous response to pathological aortic dilatation | [32-34] |
| rno-miR-22-5p | IA | Up | Served as an integrator of Ca++ homeostasis and myobrillar protein content during stress in | [32-34]] |
| heart | ||||
| rno-miR-24-1-5p | IA | Up | Negatively controlled the TGFβ signaling pathway and induced myogenic activity by the | |
| regulation of VSMC phenotype switch | [32-34] | |||
| rno-miR-26b | IA | Up | / | [32] |
| rno-miR-29a | IA | Up | Related to protein metabolism and the miR-29a was related to immune function | |
| rno-miR-29b | IA | Up | [32] | |
| rno-miR-29c | IA | Up | ||
| rno-miR-140 | IA | Up | / | [32] |
| rno-miR-1 | IA | Up | Reduced PAR-1 mediated cardiomyocyte dysfunction and improved cardiac function | [32] |
| rno-miR-181c | IA | Up | ||
| rno-miR-223 | IA | Up | Might play a protective role to vascular homeostasis and inflammation. | [32] |
| rno-miR-451 | IA | Up | / | [32] |
| rno-miR-92b | IA | Down | / | [32] |
| rno-miR-138 | IA | Down | / | [32] |
| rno-miR-181d | IA | Down | Suppressed the apoptosis and promoted the cell proliferation | [32] |
| rno-miR-433 | IA | Down | / | [32] |
| rno-miR-489 | IA | Down | Promoted the transient proliferative expansion of myogenic progenitors | [32] |
| rno-miR-551b | IA | Down | / | [32] |
R: Up or down regulation.
IA: intracranial aneurysm, VSMC: vascular smooth muscle cell, SAH: subarachnoid hemorrhage.
Table 3.
Information of miRNAs dysregulated in at least two literatures
| miRNA | Resource | Target genes and functional analysis | Literature |
|---|---|---|---|
| hsa-mir-1 | rabbits and lAs | Target CCL2, CXCL6, CXCR4, involved in the SMC proliferation and differentiation | [35], |
| [39] | |||
| Has-let-7a | Plasma and IAs | Programmed cell death, Response to oxidative stress, Smooth muscle cell proliferation | [37], |
| [41] | |||
| hsa-mir-133 | lAs | Modulate SMC proliferation, maintain the skeletal muscle homeostasis | [39], |
| [41] | |||
| hsa-mir-29b | IAs | Manipulated aneurysm formation, progression and rupture rate | [39], |
| [41] | |||
| hsa-mir-25 | Serum and plasma | serve as potential biomarkers in intracranial aneurysms | [36], |
| [37] | |||
| hsa-miR-223 | Serum and rabbit | Associated with vascular remodeling, inflammation and homeostasis | [35], |
| [36] |
Interestingly, another study found that miRNAs nucleotide polymorphisms were also involved in the intracranial aneurysms pathogenesis. The miR-34b/c rs4938723CC genotype could potentially decrease the risk of intracranial aneurysms when compared to the TT genotype. In details, the interaction between the miR-34b/c rs4938723CC genotype and TP53 Arg72Pro CG/CC/GG had a significant decreased risk of intracranial aneurysms compared to those carrying the combined genotypes of miR-34b/c rs4938723 CT/TT and TP53 Arg72Pro CG/CC/GG [45]. The findings suggested that miR-34b/c rs4938723CC and TP53 Arg72Pro polymorphisms might play an important role in the formation, development and rupture of intracranial aneurysms.
2.2. siRNAs
Early brain injury (EBI) suffering from SAH was the most considerable reason of disability and mortality [46]. In general, EBI is caused by blood brain barrier (BBB) disruption, inflammation,oxidative stress, brain edema and neural cell apoptosis. Subsequently, neurological deterioration emerges. Thus, decreasing the occurance of these events may provide beneficial effects [10] .
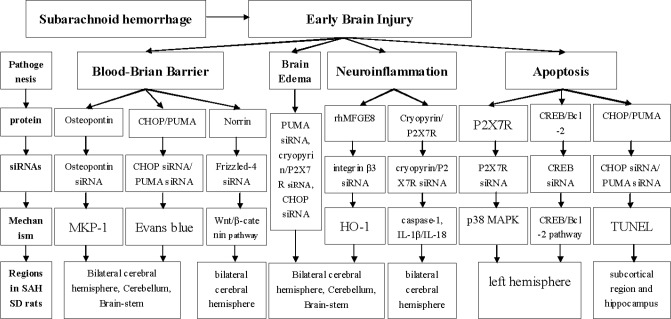
One of the primary pathogenesis of EBI is BBB breakdown (Figure 1). The BBB stability of SAH is mainly investigated by Evans Blue Assay in bilateral cerebral hemisphere, cerebellum and brain-stem of Sprague-Dawley rats. Using siRNA technology specifically blocking the endogenous osteopontin, Suzuki H et al, showed that osteopontin could restore BBB via partly increasing mitogen-activated protein kinase (MAPK) phosphatase-1 (MKP-1) in the brain, suggesting a a potential role for osteopontin as protective factor against BBB disruption [47]. In addition, PUMA and CHOP (C/EBP homologous protein) siRNA significantly reduced the amount of Evans blue extravasations, revealing that PUMA and CHOP siRNA could decrease BBB permeability. Notably, PUMA siRNA could reduce mortality and neurobehavioral deficits after SAH injury [48, 49]. Norrin was proved essential to BBB formation mediated by the Frizzled-4 receptor activation [50]. Extraneous recombinant Norrin showed to protect BBB integrity and improve neurological deficits in bilateral cerebral hemispheres after SAH, while Frizzled-4 siRNA pre treatment could reverse the protective effects. Therefore, it has been suggested that Norrin exerted its action mediated by Frizzled-4 receptor activation [51]. Taken together, it must be noted that siRNA-based therapy might be a promising option for BBB protection after SAH.
Figure 1.
Brain edema is observed post-SAH by the electronic analytical balance (Figure 1). As previously proposed, several siRNAs, such as those targeting PUMA, Cryopyrin/P2X7R and CHOP reduce brain edema after SAH injury [48, 49, 52, 53].
Neuroinflammation also contributes to the pathogenesis of early brain injury after SAH (Figure 1). Cryopyrin and P2X7R siRNA administration decreased cryopyrin and P2X7R protein expression, respectively, significantly abolishing caspase-1 activation and mature IL-1β/IL-18 secretion following SAH. Additionally, cryopyrin/P2X7R siRNA could improve neurobehavioral functions in both hemispheres, such as ameliorated sensorimotor deficits. Thus, the P2X7R/cryopyrin inflammasome axis might contribute to neuroinflammation and their specific siRNAs exerted potentially anti-inflammatory effects following SAH injury [52]. In bilateral cerebral hemispheres, brain stem and cerebellum of SAH rats, administration of recombinant human Milk fat globule-EGF factor-8 (rhMFGE8) increased heme oxygenase-1 (HO-1), while integrin β3 siRNA robustly blocked the upregulation of HO-1 [54, 55]. Taken together, siRNA treatment might be promising for neuroinflammation control post SAH.
Neuronal apoptosis usually occurs in EBI after SAH (Figure 1) [56]. P2X7R stimulation activates p38 mitogen-activated protein kinase (p38 MAPK) and involved in neuronal apoptosis [57]. In the left hemisphere post SAH, P2X7R siRNA robustly reduced p38 MAPK production and cleaved caspase-3, suggesting that P2X7R siRNA could prevent neuronal apoptosis via inhibiting p38 MAPK [58]. In the left hemisphere of another model post SAH by TUNEL assay, JWH133 (CB2R agonist) remarkably increased activated cAMP response element-binding protein (CREB) and Bcl-2 levels while decreased cleaved caspase-3. However, all these effects were reversed by CREB siRNA [59]. Moreover, CHOP siRNA significantly reduced numbers of TUNEL positive cells in the subcortical region and hippocampus, and the effect in hippocampus was also observed with PUMA siRNA treatment [48, 49].
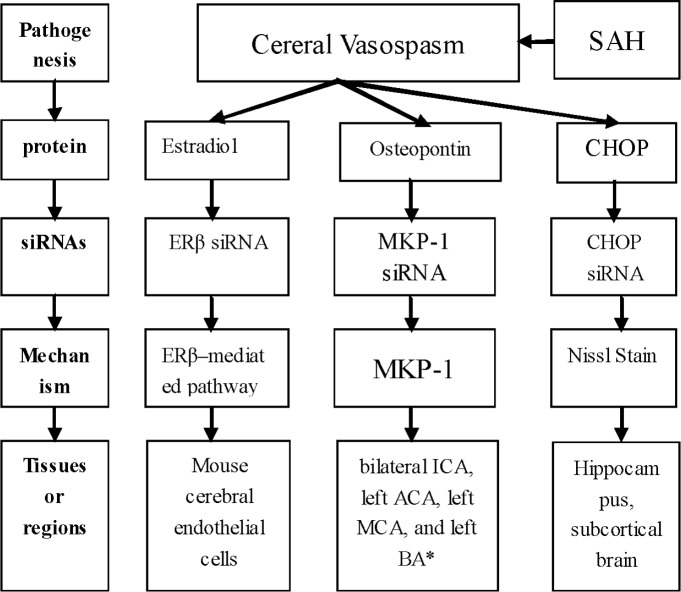
Cerebral vasospasm (CV) is another major complication that leading to poor prognosis of SAH (Figure 2). Cerebral vasospasm means the constriction of smooth muscle in blood vessels, which results in reductions of blood flow in downstream brain. Cerebral vasospasm usually occurs on day 3 after SAH, peaks at days 6 and 8, and lasts for 2-3 weeks [60]. Estradiol treatment prevented cerebral vasospasm after SAH by reducing the nitric oxidesynthase 2 (NOS2) mRNA and protein levels, the NF-kB (Nuclear Factor Kappa B) nuclear translocation and NF-kB binding onto the NOS2 promoter. However, all of these effects could be abolished by Estrogen receptor β (ERβ) siRNA administration [61]. Notably, Suzuki H et al., clarified that osteopontin prevented cerebral vasospasm and remarkably induced MKP-1 (mitogen-activated protein kinase phosphatase-1) in the spastic arteries, including bilateral ICA, left ACA, left MCA and left BA. Nevertheless, the MKP-1 up-regulation could be significantly inhibited by MKP-1 siRNA, which then resulted in worse cerebral vasospasm [62]. By Nissl Stain, CHOP siRNA was considered to ameliorate cerebral vasospasm at 24h after SAH, accompanying with reduced neuronal injury in the hippocampus and subcortical brain [53].
Figure 2.
2.3. lncRNAs
In total, 64 upregulated lncRNAs and 144 downregulated lncRNAs were detected by microarray hybridisation in the brain of adult male wistar rats following SAH. Of note, the lncRNA MRAK038897, with the most obvious alternation, was involved in ankyrin repeat and suppressor of cytokines signaling box 3, which was found to regulate the neuronal inflammatory process of EBI. Thus, it was considered that lncRNA MRAK038897 might play a critical role in the regulation of EBI [63]. The data was confirmed by RT-qPCR assays by randomly selecting 2 upregulated lncRNAs (BC092207, MRuc008hvl) and 3 downregulated lncRNAs (XR_006756, MRAK038897 and MRAK017168), which were consistent with the microarray results [63].
Several SNPs were showed to have significant association with intracranial aneurysms in both European and Japanese individuals, including rs1429412 (Allele G; chr2q), rs700651 (Allele G; chr2q), rs10958409 (AlleleA; chr8q), rs9298506 (Allele A; chr8q) and rs1333040 (Allele T; chr9p). Among these SNPs, the strongest associated SNP, rs1333040, lied 74 kb from the 5′ end of CDKN2B and 88 kb from CDKN2A, with the lncRNA ANRIL also lied within this interval [64]. Coincidentally, the robust association of the SNP rs1333040 with intracranial aneurysms was also found by meta-analysis [65]. Furthermore, genome-wide association studies (GWAS) had surprisingly identified lncRNA ANRIL (NR_003529, in chromosome 9p21; also called CDKN2BAS) as a genetic susceptibility locus related with intracranial aneurysms [66, 67]. Of note, in the specific lncRNA ANRIL, foroud T provided evidence that a SNP (CDKN2BAS; rs6475606) was associated with intracranial aneurysms [68]. Moreover, previous studies reported many other SNPs in this region were also related with intracranial aneurysms. The SNP, rs10757278 (Allele G), was the first common sequence variant that confers rapid progression risk of intracranial aneurysms in European individuals [69]. Another two SNPs, rs10757278 (Allele G) and rs1333045 (Allele A) were demonstrated to have extraordinary associations with intracranial aneurysms. Additionally, the association of rs10757278 (Allele G) remained significant after adjustment for hypertension as well as smoking, while rs1333045 (allele A) did not. Altogether, approximately 24% of the samples were homozygous for the rs10757278 G allele,in line with previous studies [69, 70].
3. Concluding remarks and perspective
As described above, non-coding RNAs (such as miRNAs, siRNAs andlncRNAs) have been recently demonstrated to play an increasingly critical role in the neurodevelopment, neuroplasticity, neural differentiation and neuroprotection [15, 16, 21, 28]. The growing non-coding RNAs studies have drastically increased our understanding on the pathogenesis of intracranial aneurysms and SAH. This review aimed to comprehensively summarise the current knowledge on the role of non-coding RNAs in intracranial aneurysms and SAH, and to effectively offer important insights for further research.
miRNAs are a novel class of endogenous non-coding RNAs with 21-23 bp. Recently, many studies focused on the miRNA expression detection and identified them as novel diagnostic and prognostic biomarkers in intracranial aneurysms and SAH. In general, the researches consisted of rat andrabbit models, peripheral blood of SAH patients and human intracranial aneurysms. All results revealed that miRNAs expression was significantly altered post SAH, and the authors also highlighted the usefulness and potential roles of the dysregulated miRNAs. In peripheral blood of SAH patients, data showed the presence of several repetitive dysregulated miRNAs in two different researches (Table 1). It was indeed suggested that miRNAs were association with SAH pathogenesis and may be used as molecular biomarkers for further investigation. Surprisingly, 20 miRNAs were unanimously changed in both ruptured and unruptured cases in human peripheral blood, regardless the status of intracranial aneurysms. As the samples consisted of unruptured cases, it was suggested that the circulating miRNAs alternation was primordial rather than a secondary complication of aneurysms (such as rupture of aneurysms and neural damages) or the result of clinical treatments (either pharmacological or surgical treatments). Therefore, these circulating miRNAs may play a vital role in the regulation of intracranial aneurysm formation and development, and may be used as novel biological markers to predict the occurrence of intracranial aneurysms rupture [36]. In intracranial aneurysms, miR-29b and miR-133 were also showed dysregulated in both studies, confirming yet again that the changes of miRNA expression were repeatable [39, 41].
Nevertheless, several limitations still existed in miRNAs investigation on intracranial aneurysm and SAH. First, cerebrospinal fluid represents the main alternation of body fluids in SAH patients. However, previous researchers only focused on the peripheral blood without performing synchronous comparison with cerebrospinal fluid. Perhaps, obtaining cerebrospinal fluid immediately after aneurysm rupture is complicated, as lumbar puncture is usually carried out after aneurysm repair [71]. In addition, miRNAs may degrade rapidly, leading to difficult measurement for the low miRNAs levels after surgery. Moreover, studies could focus only at the end-stage of the intracranial aneurysms, either ruptured that has already caused serious injury or large enough that needed surgical intervention. Hence, we could not predict miRNAs expression changes at early stage of intracranial aneurysms. Finally, only several studies conducted the preliminary functional analysis and none, so far, further investigated the intracranial aneurysms or SAH-related pathogenesis, implying thatfurther elucidation is needed.
siRNA were able to suppress deleterious gene expression by targeting specific genes. This strategy has become increasingly popular and successfully employed in killing cancer cells in vitro and in vivo [72]. In intracranial aneurysms and SAH, siRNAs studies have already reached a mature stage and mainly focused on the pathogenesis and pathophysiology. As described above, siRNAs targeting osteopontin, PUMA, CHOP, Frizzled-4, cryopyrin, P2X7R, integrin β3 and CREB were involved in early brain injury (Figure 1), while those against Estrogen receptor β, MKP-1 and CHOP were involved in cerebral vasospasm after SAH (Figure 2). Almost all siRNAs exerted their functions through combining with target genes. Indeed, siRNAs have been proved to be irreplaceable in various regulatory mechanisms in many diseases, and might become novel promising therapeutic targets for future clinical treatment. However, because of their potential off-target effects, siRNAs-based targeting requires further validation of their efficacy, [73], especially in the humans.
As described above, lncRNAs are transcripts greater than 200 bp in length. Evidences indicated that abundant lncRNAs are dysregulated expressed in the brain and played a crucial role in neural differentiation, synaptic plasticity, behaviour and neuroprotection. In adult rats of ischema stroke, 359 lncRNAs were upregulated and 84 lncRNAs were downregulated, with 62 lncRNAs showed > 90% sequence homology with exons of protein-coding genes [74]. Another rats study showed that 177 increased lncRNAs were associated with either coREST or Sin3A after ischemia stroke. Among these lncRNAs, 11 lncRNAs enriched with coREST and 26 lncRNAs enriched with Sin3A were upregulated following ischemia stroke [75]. However, lncRNAs expression profiles were only investigated in SAH rats, and no studies focused on the SAH patients, either in body fluids (peripheral blood and cerebrospinal fluid) or intracranial aneurysm tissues. In addition, although the specific lncRNA ANRIL and its SNPs were reported, there was no study focused on whether lncRNAs modulated SAH pathogenesis and pathophysiology, leaving the topic still unclear. The lncRNAs stability resulted better than other small non coding RNAs and, perhaps, lncRNAs modulation of intracranial aneurysms and SAH would need a better understanding.
However, some other limitations exist in this review. Most studies are observational results carried out by microarray and RT-qPCR analysis, demonstrating the dysregulated expression of non-coding RNAs in intracranial aneurysms and SAH. Experiments that explore SAH mechanism of non-coding RNAs are still patially lacking and only few studies have been validated. As such, it is still unclear whether these alterations are causative, prognostic or merely associated with intracranial aneurysms and SAH.
In conclusion, we reviewed several non-coding RNAs expression profiles and related mechanisms in intracranial aneurysms and SAH, and found that these non-coding RNAs play important roles. We suggested that non-coding RNAs would function as novel molecular biomarkers to predict the intracranial aneurysms and SAH, and may yield new therapies in the future.
Acknowledgments
This study was supported by Outstanding Youth Fund of the First People’s Hospital of Chenzhou (No.N2015-001, to Fengzhen Huang), Research projects of the First People’s Hospital of Chenzhou (No.N2014-020, to Xiaoxi Yao; No.N2015-014, to Tieqiao Zhou), the National Basic Research Program (973 Program) (Nos. 2012CB944601, 2012CB517902 and 2011CB510002 to Hong Jiang), the National Natural Science Foundation of China (Nos. 81471156, 81271260 to Hong Jiang), Hunan Funds for Distinguished Young Scientists (No. 14JJ1008 to Hong Jiang), and High-level medical personnel of Hunan province “225”Project.
References
- [1].Chen S., Feng H., Sherchan P., Klebe D., Zhao G., Sun X.. et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Progress in neurobiology. 2014:11564–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sacco R. L., Kasner S. E., Broderick J. P., Caplan L. R., Connors J. J., Culebras A.. et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association, Stroke. a journal of cerebral circulation. 2013;44(7):2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kooijman E., Nijboer C. H., van Velthoven C. T., Kavelaars A., Kesecioglu J., Heijnen C. J.. The rodent endovascular puncture model of subarachnoid hemorrhage: mechanisms of brain damage and therapeutic strategies. Journal of neuroinflammation. 2014:112. doi: 10.1186/1742-2094-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gangloff A., Nadeau L., Perry J. J., Baril P., Emond M.. Ruptured aneurysmal subarachnoid hemorrhage in the emergency department: Clinical outcome of patients having a lumbar puncture for red blood cell count, visual and spectrophotometric xanthochromia after a negative computed tomography. Clinical biochemistry. 2015;48:634–9. doi: 10.1016/j.clinbiochem.2015.03.011. (10-11) [DOI] [PubMed] [Google Scholar]
- [5].Nieuwkamp D. J., Setz L. E., Algra A., Linn F. H., de Rooij N. K., Rinkel G. J.. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. The Lancet Neurology. 2009;8(7):635–42. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- [6].Connollyyg E. S., Rabinstein A. A., Carhuapoma J. R., Derdeyn C. P., Dion J., Higashida R. T.. et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association, Stroke. a journal of cerebral circulation. 2012;43(6):1711–37. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- [7].Kingwell K.. Stroke: Improving the management of patients at risk of haemorrhagic stroke. Nature reviews Neurology. 2014;10(1):1. doi: 10.1038/nrneurol.2013.257. [DOI] [PubMed] [Google Scholar]
- [8].Li M. H., Chen S. W., Li Y. D., Chen Y. C., Cheng Y. S., Hu D. J.. et al. Prevalence of unruptured cerebral aneurysms in Chinese adults aged 35 to 75 years: a cross-sectional study. Annals of internal medicine. 2013;159(8):514–21. doi: 10.7326/0003-4819-159-8-201310150-00004. [DOI] [PubMed] [Google Scholar]
- [9].Shimamura N., Ohkuma H.. Phenotypic transformation of smooth muscle in vasospasm after aneurysmal subarachnoid hemorrhage. Translational stroke research. 2014;5(3):357–64. doi: 10.1007/s12975-013-0310-1. [DOI] [PubMed] [Google Scholar]
- [10].Fujii M., Yan J., Rolland W. B., Soejima Y., Caner B., Zhang J. H.. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Translational stroke research. 2013;4(4):432–46. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dupont S., Rabinstein A. A.. Extent of acute hydrocephalus after subarachnoid hemorrhage as a risk factor for poor functional outcome. Neurological research. 2013;35(2):107–10. doi: 10.1179/1743132812Y.0000000122. [DOI] [PubMed] [Google Scholar]
- [12].Chen S., Li Q., Wu H., Krafft P. R., Wang Z., Zhang J. H.. The harmful effects of subarachnoid hemorrhage on extracerebral organs. BioMed research international. 2014 doi: 10.1155/2014/858496. 2014858496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sehba F. A., Hou J., Pluta R. M., Zhang J. H.. The importance of early brain injury after subarachnoid hemorrhage. Progress in neurobiology. 2012;97(1):14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi Y., Huang F., Tang B., Li J., Wang J., Shen L.. et al. MicroRNA profiling in the serums of SCA3/MJD patients. The International journal of neuroscience. 2014;124(2):97–101. doi: 10.3109/00207454.2013.827679. [DOI] [PubMed] [Google Scholar]
- [15].Huang F., Long Z., Chen Z., Li J., Hu Z., Qiu R.. et al. Investigation of Gene Regulatory Networks Associated with Autism Spectrum Disorder Based on MiRNA Expression in China. PloS one. 2015;10(6) doi: 10.1371/journal.pone.0129052. e0129052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang F., Zhang L., Long Z., Chen Z., Hou X., Wang C.. et al. miR-25 alleviates polyQ-mediated cytotoxicity by silencing ATXN3. FEBS letters. 2014;588(24):4791–8. doi: 10.1016/j.febslet.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Starega-Roslan J., Koscianska E., Kozlowski P., Krzyzosiak W. J.. The role of the precursor structure in the biogenesis of microRNA. Cellular and molecular life sciences : CMLS. 2011;68(17):2859–71. doi: 10.1007/s00018-011-0726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Humphreys D. T., Hynes C. J., Patel H. R., Wei G. H., Cannon L., Fatkin D.. et al. Complexity of murine cardiomyocyte miRNA biogenesis, sequence variant expression and function. PloS one. 2012;7(2) doi: 10.1371/journal.pone.0030933. e30933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He Z., Jiang J., Kokkinaki M., Tang L., Zeng W., Gallicano I.. et al. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem cells. 2013;31(10):2205–17. doi: 10.1002/stem.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aw S., Cohen S. M.. Time is of the essence: microRNAs and age-associated neurodegeneration. Cell research. 2012;22(8):1218–20. doi: 10.1038/cr.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hong C. A., Nam Y. S.. Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics. 2014;4(12):1211–32. doi: 10.7150/thno.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miele E., Spinelli G. P., Miele E., Di Fabrizio E., Ferretti E., Tomao S.. et al. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. International journal of nanomedicine. 2012:73637–57. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shi B., Keough E., Matter A., Leander K., Young S., Carlini E.. et al. Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2011;59(8):727–40. doi: 10.1369/0022155411410885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H.. et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome research. 2012;22(9):1775–89. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kung J. T., Colognori D., Lee J. T.. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–69. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fritah S., Niclou S. P., Azuaje F.. Databases for lncRNAs: a comparative evaluation of emerging tools. Rna. 2014;20(11):1655–65. doi: 10.1261/rna.044040.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang J., Song Y. X., Ma B., Wang J. J., Sun J. X., Chen X. W.. et al. Regulatory Roles of Non-Coding RNAs in Colorectal Cancer. International journal of molecular sciences. 2015;16(8):19886–919. doi: 10.3390/ijms160819886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Batagov A. O., Yarmishyn A. A., Jenjaroenpun P., Tan J. Z., Nishida Y., Kurochkin I. V.. Role of genomic architecture in the expression dynamics of long noncoding RNAs during differentiation of human neuroblastoma cells. BMC systems biology. 2013;7 doi: 10.1186/1752-0509-7-S3-S11. Suppl 3S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saugstad J. A.. Non-Coding RNAs in Stroke and Neuroprotection. Frontiers in neurology. 2015:650. doi: 10.3389/fneur.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vemuganti R.. All’s well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochemistry international. 2013;63(5):438–49. doi: 10.1016/j.neuint.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Rooij E., Olson E. N.. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nature reviews Drug discovery. 2012;11(11):860–72. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee H. J., Yi J. S., Lee H. J., Lee I. W., Park K. C., Yang J. H.. Dysregulated Expression Profiles of MicroRNAs of Experimentally Induced Cerebral Aneurysms in Rats. Journal of Korean Neurosurgical Society. 2013;53(2):72–6. doi: 10.3340/jkns.2013.53.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gurha P., Abreu-Goodger C., Wang T., Ramirez M. O., Drumond A. L., van Dongen S.. et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation. 2012;125(22):2751–61. doi: 10.1161/CIRCULATIONAHA.111.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maegdefessel L., Azuma J., Toh R., Deng A., Merk D. R., Raiesdana A.. et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Science translational medicine. 2012;4(122) doi: 10.1126/scitranslmed.3003441. 122ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Holcomb M., Ding Y. H., Dai D., McDonald R. J., McDonald J. S., Kallmes D. F.. et al. RNA-Sequencing Analysis of Messenger RNA/MicroRNA in a Rabbit Aneurysm Model Identifies Pathways and Genes of Interest. AJNR American journal of neuroradiology. 2015;36(9):1710–5. doi: 10.3174/ajnr.A4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jin H., Li C., Ge H., Jiang Y., Li Y.. Circulating microRNA: a novel potential biomarker for early diagnosis of intracranial aneurysm rupture a case control study. Journal of translational medicine. 2013:11296. doi: 10.1186/1479-5876-11-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li P., Zhang Q., Wu X., Yang X., Zhang Y., Li Y.. et al. Circulating microRNAs serve as novel biological markers for intracranial aneurysms. Journal of the American Heart Association. 2014;3(5) doi: 10.1161/JAHA.114.000972. e000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Williams Z., Ben-Dov I. Z., Elias R., Mihailovic A., Brown M., Rosenwaks Z.. et al. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(11):4255–60. doi: 10.1073/pnas.1214046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jiang Y., Zhang M., He H., Chen J., Zeng H., Li J.. et al. MicroRNA/mRNA profiling and regulatory network of intracranial aneurysm. BMC medical genomics. 2013:636. doi: 10.1186/1755-8794-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maegdefessel L., Azuma J., Tsao P. S.. MicroRNA-29b regulation of abdominal aortic aneurysm development. Trends in cardiovascular medicine. 2014;24(1):1–6. doi: 10.1016/j.tcm.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu D., Han L., Wu X., Yang X., Zhang Q., Jiang F.. Genome-wide microRNA changes in human intracranial aneurysms. BMC neurology. 2014:14188. doi: 10.1186/s12883-014-0188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen L., Wan J. Q., Zhou J. P., Fan Y. L., Jiang J. Y.. Gene expression analysis of ruptured and un-ruptured saccular intracranial aneurysm. European review for medical and pharmacological sciences. 2013;17(10):1374–81. [PubMed] [Google Scholar]
- [43].Chen L., Zhang W., Yan W., Han L., Zhang K., Shi Z.. et al. The putative tumor suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in glioma. Carcinogenesis. 2012;33(11):2276–82. doi: 10.1093/carcin/bgs261. [DOI] [PubMed] [Google Scholar]
- [44].Mestdagh P., Hartmann N., Baeriswyl L., Andreasen D., Bernard N., Chen C.. et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nature methods. 2014;11(8):809–15. doi: 10.1038/nmeth.3014. [DOI] [PubMed] [Google Scholar]
- [45].Li L., Sima X., Bai P., Zhang L., Sun H., Liang W.. et al. Interactions of miR-34b/c and TP53 polymorphisms on the risk of intracranial aneurysm. Clinical & developmental immunology. 2012 doi: 10.1155/2012/567586. 2012567586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang Z., Ji C., Wu L., Qiu J., Li Q., Shao Z.. et al. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PloS one. 2014;9(5) doi: 10.1371/journal.pone.0097685. e97685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Suzuki H., Hasegawa Y., Kanamaru K., Zhang J. H.. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke; a journal of cerebral circulation. 2010;41(8):1783–90. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yan J., Li L., Khatibi N. H., Yang L., Wang K., Zhang W.. et al. Blood-brain barrier disruption following subarchnoid hemorrhage may be faciliated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Experimental neurology. 2011;230(2):240–7. doi: 10.1016/j.expneurol.2011.04.022. [DOI] [PubMed] [Google Scholar]
- [49].He Z., Ostrowski R. P., Sun X., Ma Q., Huang B., Zhan Y.. et al. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2012;43(2):484–90. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang Y., Rattner A., Zhou Y., Williams J., Smallwood P. M., Nathans J.. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151(6):1332–44. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen Y., Zhang Y., Tang J., Liu F., Hu Q., Luo C.. et al. Norrin protected blood-brain barrier via frizzled-4/beta-catenin pathway after subarachnoid hemorrhage in rats. Stroke; a journal of cerebral circulation. 2015;46(2):529–36. doi: 10.1161/STROKEAHA.114.007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chen S., Ma Q., Krafft P. R., Hu Q., Rolland W.. Sherchan P.. et al. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiology of disease. 2013:58296–307. doi: 10.1016/j.nbd.2013.06.011. 2nd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].He Z., Ostrowski R. P., Sun X., Ma Q., Tang J., Zhang J. H.. Targeting C/EBP homologous protein with siRNA attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Experimental neurology. 2012;238(2):218–24. doi: 10.1016/j.expneurol.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu F., Hu Q., Li B., Manaenko A., Chen Y., Tang J.. et al. Recombinant milk fat globule-EGF factor-8 reduces oxidative stress via integrin beta3/nuclear factor erythroid 2-related factor 2/heme oxygenase pathway in subarachnoid hemorrhage rats. Stroke; a journal of cerebral circulation. 2014;45(12):3691–7. doi: 10.1161/STROKEAHA.114.006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li E., Noda M., Doi Y., Parajuli B., Kawanokuchi J., Sonobe Y.. et al. The neuroprotective effects of milk fat globule-EGF factor 8 against oligomeric amyloid beta toxicity. Journal of neuroinflammation. 2012:9148. doi: 10.1186/1742-2094-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hasegawa Y., Suzuki H., Sozen T., Altay O., Zhang J. H.. Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta neurochirurgica Supplement. 2011;110(Pt 1):43–8. doi: 10.1007/978-3-7091-0353-1_8. [DOI] [PubMed] [Google Scholar]
- [57].Papp L., Vizi E. S., Sperlagh B.. P2X7 receptor mediated phosphorylation of p38MAP kinase in the hippocampus. Biochemical and biophysical research communications. 2007;355(2):568–74. doi: 10.1016/j.bbrc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- [58].Chen S., Ma Q., Krafft P. R., Chen Y., Tang J., Zhang J.. et al. P2X7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Critical care medicine. 2013;41(12):e466–74. doi: 10.1097/CCM.0b013e31829a8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fujii M., Sherchan P., Soejima Y., Hasegawa Y., Flores J., Doycheva D.. et al. Cannabinoid receptor type 2 agonist attenuates apoptosis by activation of phosphorylated CREB-Bcl-2 pathway after subarachnoid hemorrhage in rats. Experimental neurology. 2014:261396–403. doi: 10.1016/j.expneurol.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ciurea A. V., Palade C., Voinescu D., Nica D. A.. Subarachnoid hemorrhage and cerebral vasospasm - literature review. Journal of medicine and life. 2013;6(2):120–5. [PMC free article] [PubMed] [Google Scholar]
- [61].Chen L. C., Lee W. S.. Estradiol reduces ferrous citrate complex-induced NOS2 up-regulation in cerebral endothelial cells by interfering the nuclear factor kappa B transactivation through an estrogen receptor beta-mediated pathway. PloS one. 2013;8(12) doi: 10.1371/journal.pone.0084320. e84320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Suzuki H., Hasegawa Y., Chen W., Kanamaru K., Zhang J. H.. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Annals of neurology. 2010;68(5):650–60. doi: 10.1002/ana.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zheng B., Liu H., Wang R., Xu S., Liu Y., Wang K.. et al. Expression signatures of long non-coding RNAs in early brain injury following experimental subarachnoid hemorrhage. Molecular medicine reports. 2015;12(1):967–73. doi: 10.3892/mmr.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bilguvar K., Yasuno K., Niemela M., Ruigrok Y. M., von Und Zu, Fraunberg M., van Duijn C. M.. et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nature genetics. 2008;40(12):1472–7. doi: 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Alg V. S., Sofat R., Houlden H., Werring D. J.. Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. Neurology. 2013;80(23):2154–65. doi: 10.1212/WNL.0b013e318295d751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yasuno K., Bilguvar K., Bijlenga P., Low S. K., Krischek B., Auburger G.. et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nature genetics. 2010;42(5):420–5. doi: 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pasmant E., Sabbagh A., Vidaud M., Bieche I.. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(2):444–8. doi: 10.1096/fj.10-172452. [DOI] [PubMed] [Google Scholar]
- [68].Foroud T., Koller D. L., Lai D., Sauerbeck L., Anderson C., Ko N.. et al. Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke; a journal of cerebral circulation. 2012;43(11):2846–52. doi: 10.1161/STROKEAHA.112.656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Helgadottir A., Thorleifsson G., Magnusson K. P., Gretarsdottir S., Steinthorsdottir V., Manolescu A.. et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nature genetics. 2008;40(2):217–24. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- [70].Olsson S., Csajbok L. Z., Jood K., Nylen K., Nellgard B., Jern C.. Association between genetic variation on chromosome 9p21 and aneurysmal subarachnoid haemorrhage. Journal of neurology, neurosurgery, and psychiatry. 2011;82(4):384–8. doi: 10.1136/jnnp.2009.187427. [DOI] [PubMed] [Google Scholar]
- [71].Diringer M. N.. Management of aneurysmal subarachnoid hemorrhage. Critical care medicine. 2009;37(2):432–40. doi: 10.1097/CCM.0b013e318195865a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gao Y., Wu H., He D., Hu X., Li Y.. Downregulation of BCL11A by siRNA induces apoptosis in B lymphoma cell lines. Biomedical reports. 2013;1(1):47–52. doi: 10.3892/br.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jung H. S., Rajasekaran N., Ju W., Shin Y. K.. Human Papillomavirus: Current and Future RNAi Therapeutic Strategies for Cervical Cancer. Journal of clinical medicine. 2015;4(5):1126–55. doi: 10.3390/jcm4051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dharap A., Nakka V. P., Vemuganti R.. Effect of focal ischemia on long noncoding RNAs. Stroke; a journal of cerebral circulation. 2012;43(10):2800–2. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dharap A., Pokrzywa C., Vemuganti R.. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN neuro. 2013;5(4):283–9. doi: 10.1042/AN20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]