Abstract
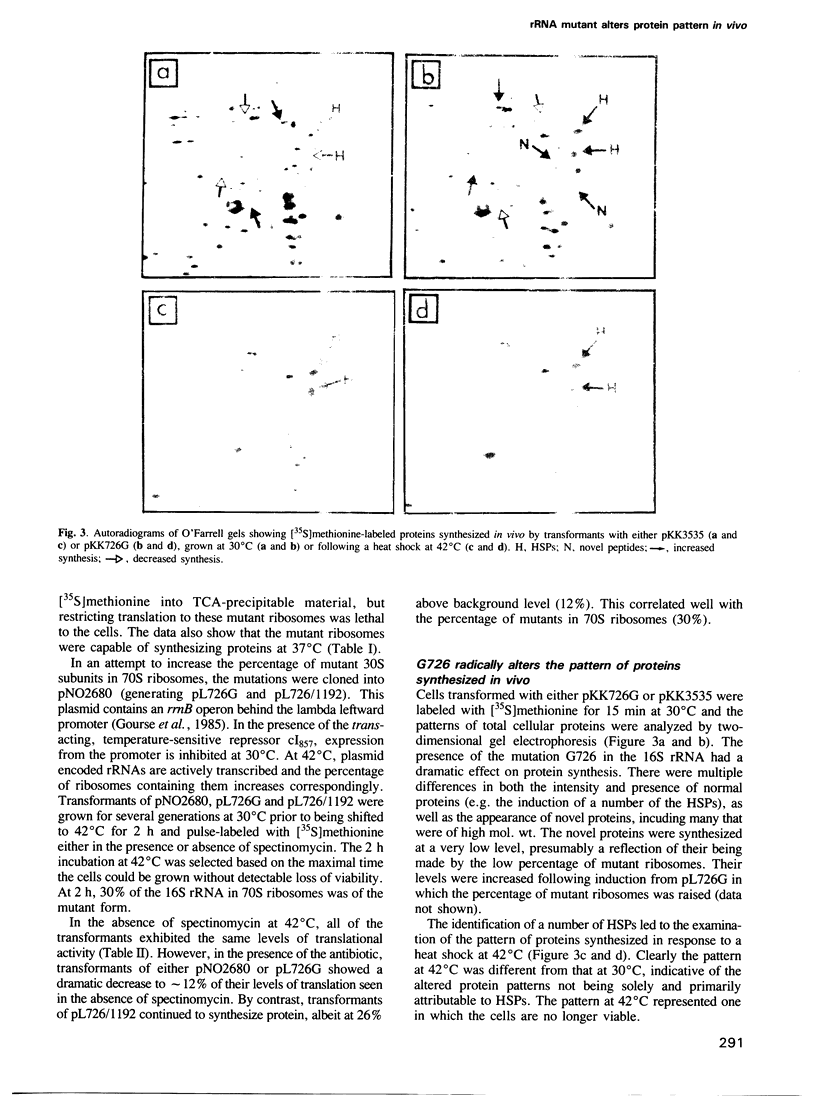
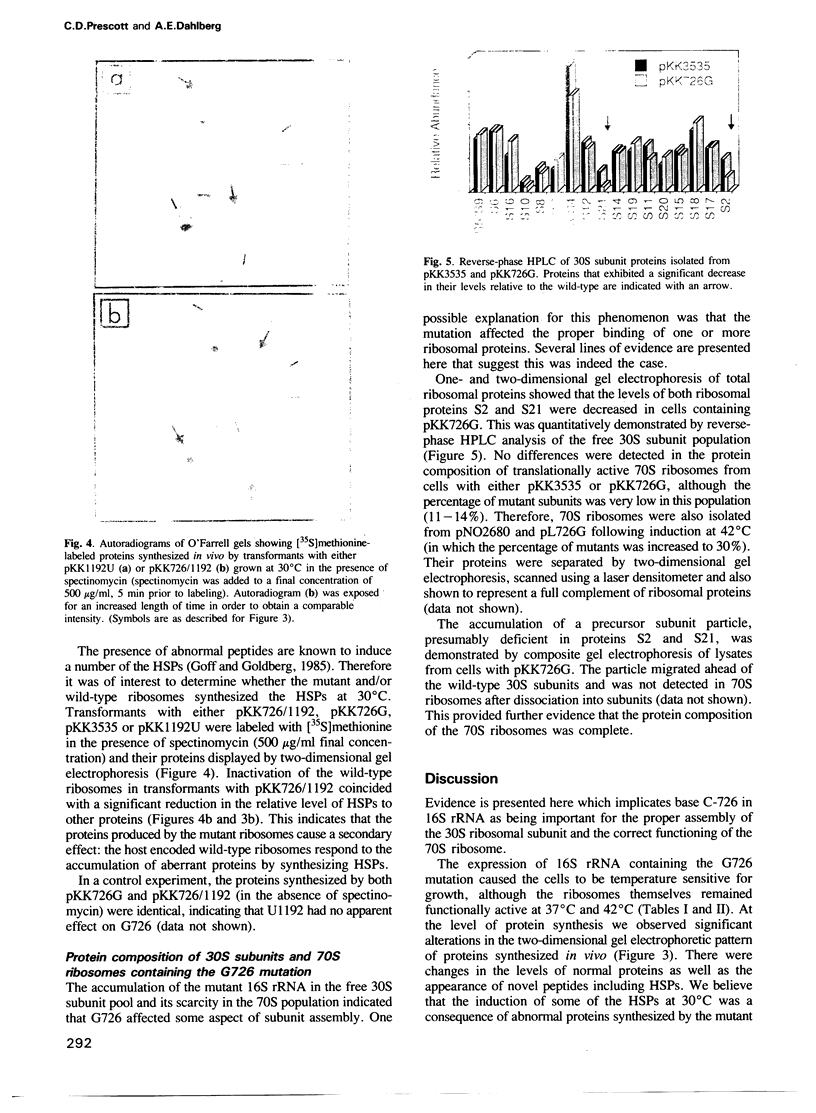
A single base change in 16S rRNA (C-726 to G) was constructed by site-directed mutagenesis and cloned into the multicopy plasmid pKK3535 (generating pKK726G) which contains the complete rrnB operon from Escherichia coli. The mutant 16S rRNA was found predominantly in the 30S subunit fraction but was present in the 70S ribosomes. Protein analyses of the free 30S subunits revealed a decrease in the levels of ribosomal proteins S2 and S21 while the composition of the 70S ribosomes was as the wild-type. Transformants of pKK726G were temperature sensitive for growth, although the mutant ribosomes themselves were translationally active in vivo at 37 and 42 degrees C. Two-dimensional gel electrophoresis of the proteins translated in vivo revealed an altered protein profile which included novel proteins, changes in the levels of normal proteins, and the presence of heat shock proteins (HSPs) at 30 degrees C. Inactivation of the host encoded wild-type ribosomes coincided with a significant decrease in the synthesis of the HSPs. We therefore believe the induction of the HSPs to be a secondary response by the cells to the presence of the abnormal proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backendorf C., Ravensbergen C. J., Van der Plas J., van Boom J. H., Veeneman G., Van Duin J. Basepairing potential of the 3' terminus of 16S RNA: dependence on the functional state of the 30S subunit and the presence of protein S21. Nucleic Acids Res. 1981 Mar 25;9(6):1425–1444. doi: 10.1093/nar/9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Cooperman B. S., Weitzmann C. J., Buck M. A. Reversed-phase high-performance liquid chromatography of ribosomal proteins. Methods Enzymol. 1988;164:523–532. doi: 10.1016/s0076-6879(88)64067-5. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Miskin R., Zamir A. N-ethyl maleimide as a probe for the study of functional sites and conformations of 30 S ribosomal subunits. J Mol Biol. 1973 Sep 25;79(3):481–494. doi: 10.1016/0022-2836(73)90400-2. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Stark M. J., Dahlberg A. E. Regions of DNA involved in the stringent control of plasmid-encoded rRNA in vivo. Cell. 1983 Apr;32(4):1347–1354. doi: 10.1016/0092-8674(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Takebe Y., Sharrock R. A., Nomura M. Feedback regulation of rRNA and tRNA synthesis and accumulation of free ribosomes after conditional expression of rRNA genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1069–1073. doi: 10.1073/pnas.82.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G. A., Traut R. R. A modified two-dimensional gel system for the separation and radioautography of microgram amounts of ribosomal proteins. Methods Enzymol. 1974;30:526–539. doi: 10.1016/0076-6879(74)30052-3. [DOI] [PubMed] [Google Scholar]
- Jacob W. F., Santer M., Dahlberg A. E. A single base change in the Shine-Dalgarno region of 16S rRNA of Escherichia coli affects translation of many proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4757–4761. doi: 10.1073/pnas.84.14.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo D. K., Steen R., Stark M. J., Dahlberg A. E. Analysis of plasmid-coded ribosomal RNA maxicell techniques. Methods Enzymol. 1988;164:691–706. doi: 10.1016/s0076-6879(88)64078-x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Aminoacyl-tRNA binding at the recognition site is the first step of the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 1977 May;74(5):1903–1907. doi: 10.1073/pnas.74.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makosky P. C., Dahlberg A. E. Spectinomycin resistance at site 1192 in 16S ribosomal RNA of E. coli: an analysis of three mutants. Biochimie. 1987 Aug;69(8):885–889. doi: 10.1016/0300-9084(87)90216-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell. 1986 Dec 26;47(6):985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Moazed D., Van Stolk B. J., Douthwaite S., Noller H. F. Interconversion of active and inactive 30 S ribosomal subunits is accompanied by a conformational change in the decoding region of 16 S rRNA. J Mol Biol. 1986 Oct 5;191(3):483–493. doi: 10.1016/0022-2836(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Hijazi K. A., Göringer H. U., Dahlberg A. E. Mutant 16S ribosomal RNA: a codon-specific translational suppressor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4162–4165. doi: 10.1073/pnas.85.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V. R., Yabuki S., Sillers I. Y., Schindler D. G., Engelman D. M., Moore P. B. Positions of proteins S6, S11 and S15 in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1981 Dec 15;153(3):739–760. doi: 10.1016/0022-2836(81)90416-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Gourse R. L., Dahlberg A. E. Site-directed mutagenesis of ribosomal RNA. Analysis of ribosomal RNA deletion mutants using maxicells. J Mol Biol. 1982 Aug 15;159(3):417–439. doi: 10.1016/0022-2836(82)90292-3. [DOI] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989 May 19;244(4906):783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- Tapprich W. E., Goss D. J., Dahlberg A. E. Mutation at position 791 in Escherichia coli 16S ribosomal RNA affects processes involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4927–4931. doi: 10.1073/pnas.86.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin J., Wijnands R. The function of ribosomal protein S21 in protein synthesis. Eur J Biochem. 1981 Sep 1;118(3):615–619. doi: 10.1111/j.1432-1033.1981.tb05563.x. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]