Abstract
Introduction
Multiple sclerosis is associated with prolonged cardiac repolarization but the underlying physiology has remained unknown. In this study, we compared cardiac repolarization during the relapsing‐remitting multiple sclerosis (RRMS) disease course in patients with motor and sensory onset symptom.
Methods
Twenty‐five RRMS patients with motor and 33 RRMS patients with sensory onset symptom having 12‐lead electrocardiogram (ECG) recorded at the time of the first demyelinating event (ECG1) as well as at the later disease course (ECG2) were identified from the patient records. The average time interval between ECG1 and ECG2 was 8.6 ± 5.9 y. Heart rate‐corrected QT intervals reflecting cardiac repolarization were calculated by Bazett (QTcBaz), Fridericia (QTcFri), and Karjalainen (QTcKar) formulas.
Results
Heart rate‐corrected QT intervals as well as heart rate were similar in patients with motor and sensory onset symptom in ECG1. However, QTcBaz (p = .002), QTcFri (p = .019), and QTcKar (p = .026) were longer and heart rate was higher (p = .035) in patients with motor than sensory onset symptom in ECG2. Correspondingly, QTcBaz (p = .002), QTcFri (p = .033), and QTcKar (p = .043) prolonged and heart rate tended to increase (p = .060) during the disease course only in the patients with motor onset symptom.
Conclusions
Cardiac repolarization prolonged and heart rate increased during the disease course in RRMS patients with motor but not with sensory onset symptom. This suggests different traits in RRMS according to its initial manifestation and also association of motor onset symptom with more unfavorable cardiovascular prognostic determinants.
Keywords: autonomic nervous system, cardiac repolarization, multiple sclerosis, onset symptom
1. INTRODUCTION
Multiple sclerosis (MS) debilitates a multitude of central nervous system processes, including motor, sensory, and cognitive domains (Wynia, Middel, Van Dijk, De Keyser, & Reijneveld, 2008). In addition, up to two‐thirds of the patients with MS demonstrate cardiac autonomic dysfunction (Acevedo, Nava, Arriada, Violante, & Corona, 2000; Nasseri, Tenvoorde, Adèr, Uitdehaag, & Polman, 1998; Racosta, Kimpinski, Morrow, & Kremenchutzky, 2015).
The central autonomic network (CAN) is a connectome matrix between insular cortex, amygdala, hypothalamus, periaqueductal gray matter, nucleus tractus solitarius, and ventrolateral medulla (Cersosimo & Benarroch, 2013). In addition to other autonomic functions, CAN modulates cardiac repolarization and heart rate (Magnano, Holleran, Ramakrishnan, Reiffel, & Bloomfield, 2002). Cardiac repolarization is mirrored by heart rate‐corrected QT (QTc) interval in an electrocardiogram (ECG) (Postema & Wilde, 2014). Previously, QTc interval has been reported to be longer in patients with MS than in healthy subjects (Drouin, Nataf, Lande, & Louboutin, 1998; de Seze et al., 2000).
Prolonged QTc interval is a predictor for unfavorable cardiovascular events (Oikarinen et al., 2004; Porthan et al., 2009) even in subjects without cardiac disease (Schouten et al., 1991; Siscovick et al., 1996). In patients with MS, cardiovascular mortality has been reported to be higher than in general population (Brønnum‐Hansen, Koch‐Henriksen, & Stenager, 2004; Manouchehrinia, Tanasescu, Tench, & Constantinescu, 2016). On the other hand, the domain of the onset symptom has been demonstrated to predict MS‐related (Sumelahti, Tienari, Wikström, Salminen, & Hakama, 2002) as well as all‐cause (Midgard, Albrektsen, Riise, Kvåle, & Nyland, 1995) mortality in patients with MS. Whether the domain of the onset symptom in relapsing‐remitting MS (RRMS) has an impact on QTc interval during the disease course, however, is not known.
In this study we compared QTc interval and its dynamics during the disease course between RRMS patients demonstrating their onset symptom either in motor or in sensory domain.
2. MATERIAL AND METHODS
We carried out an analysis of data collected retrospectively from the patient records of Mikkeli Central Hospital and Kuopio University Hospital. All the patients with the diagnosis of RRMS and onset symptom either in motor or sensory domain were initially screened from our patient records (n = 183). Only patients in sinus rhythm, with clinically definitive RRMS (according to the McDonald 2010 criteria (Polman et al., 2011)) and without any cardiovascular disease influencing cardiac repolarization at the time of onset symptom were included (n = 168). Finally, 58 of these patients had baseline 12‐lead electrocardiogram (ECG) recorded at the time of the first exacerbation (ECG1) as well as the latest nonacute 12‐lead ECG recorded at the later course of RRMS (ECG2) for comparison, and were included in the analyses.
All the onset symptoms affecting pyramidal tract were defined as motor. On the other hand, the onset symptom was defined as sensory if optic neuritis or somatosensory exacerbation (paresthesia or neuropathic pain) occurred without any motor defect. All the onset symptoms lasted over 24 hr and were considered suggestive of the first demyelinating event by clinicians.
Kuopio University Hospital Research Ethics Committee approved the study protocol and the research was carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. According to the recommendations of local ethics committee, the authorization for using a register data was obtained from the record controller. Informed consent was not required, because of the register‐based nature of the study.
2.1. Patients
The onset symptom was motor in 43% (n = 25) and sensory in 57% (n = 33) of 58 patients. Of the 33 patients with sensory onset symptom of RRMS, 55% (n = 18) had isolated optic neuritis and 45% (n = 15) had somatosensory onset symptom.
The data on concomitant medication and diseases were obtained from the patient records. At the time of ECG1, 20% (5/25) of the patients with motor and 12% (4/33) of the patients with sensory onset symptom had any concomitant disease and the concomitant disease burden was found similar between the groups (p = .479). The prevalence of asthma (12% vs. 6%; p = .643), rheumatoid arthritis (8% vs. 0%; p = .181), osteoporosis (4% vs. 0%; p = .431), depression (0% vs. 6%; p = .501), and schizophrenia (4% vs. 0%; p = .431) showed no statistical difference between patients with motor and sensory onset symptom, respectively.
At the time of ECG2, 48% (12/25) of the patients with motor and 33% (11/33) of the patients with sensory onset symptom had any concomitant disease (p = .258). Namely, diabetes (0% vs. 6%; p = .501), hypertension (8% vs. 12%; p = .690), coronary artery disease (0% vs. 3%; p = 1.000), asthma (12% vs. 6%; p = .643), rheumatoid arthritis (8% vs. 0%; p = .181), osteoporosis (4% vs. 0%; p = .431), depression (12% vs. 6%; p = .643), bipolar disorder (0% vs. 3%; p = 1.000), schizophrenia (4% vs. 0%; p = .431), malignancy (0% vs. 3%; p = 1.000), epilepsy (4% vs. 3%; p = 1.000), and hypothyroidism (4% vs. 0%; p = .431) were recorded, but the prevalence of these in patients with motor and sensory onset symptom did not differ, respectively.
In case of disease‐modifying treatment (DMT), patients were either on interferon beta compounds, glatiramer acetate, or natalizumab. None of the patients were on oral DMTs. Concomitant medication was found similar between the patients with motor and sensory onset symptom both at the time of ECG1 and ECG2 (Table 1).
Table 1.
Medication at the time of onset symptom of relapsing‐remitting multiple sclerosis either in motor or in sensory domain (ECG1) and later disease course (ECG2)
| ECG1 | ECG2 | |||||
|---|---|---|---|---|---|---|
| Motor (n = 25) | Sensory (n = 33) | p‐value | Motor (n = 25) | Sensory (n = 33) | p‐value | |
| Cardiovascular medication | ||||||
| ACE inhibitors | 0 | 0 | 0 | 1 (3) | 1.000 | |
| ATII receptor blockers | 0 | 0 | 1 (4) | 3 (9) | .627 | |
| β‐blocking agents | 0 | 1 (3) | 1.000 | 1 (4) | 4 (12) | .378 |
| Ca‐channel blocking agents | 0 | 0 | 1 (4) | 1 (3) | 1.000 | |
| Diuretics | 0 | 0 | 0 | 2 (6) | .501 | |
| Nitrates | 0 | 0 | 0 | 1 (3) | 1.000 | |
| Anti‐cholinergics | 0 | 5 (15) | .063 | 2 (8) | 7 (21) | .275 |
| Anti‐depressants | 3 (12) | 7 (21) | .490 | 6 (24) | 11 (33) | .439 |
| Anti‐epileptics | 2 (8) | 0 | .181 | 5 (20) | 7 (21) | .910 |
| Anti‐inflammatorics | 11 (44) | 15 (45) | .921 | 9 (36) | 20 (61) | .063 |
| Anti‐psychotics | 1 (4) | 0 | .431 | 1 (4) | 1 (3) | 1.000 |
| Muscle relaxants | 2 (8) | 0 | .181 | 5 (20) | 5 (15) | .731 |
ACE, angiotensin‐converting enzyme; ATII, angiotensin II. Values are number (%) of patients.
2.2. Assessment of cardiac repolarization in electrocardiogram
The 12‐lead ECGs were recorded in supine position as a part of routine practice. QT interval was assessed by automatic analysis and was defined as the interval between the start of QRS complex and the end of T wave. All the analyses were manually confirmed. QT interval is influenced by heart rate and, therefore, needs to be adjusted accordingly. In this study, the calculation of heart‐rate corrected QT intervals was performed using the Bazett (QTcBaz) (Bazett, 1920), the Fridericia (QTcFri) (Fridericia, 1920), and the Karjalainen (QTcKar) (Karjalainen, Viitasalo, Mänttäri, & Manninen, 1994) formulas.
2.3. Statistical analysis
Kolmogorov‐Smirnov test was applied to verify the normal distribution of the variables. Comparisons of continuous variables were performed using the independent samples t test and comparison of categorical variables using the Chi‐square and Fisher's Exact test between the patients with motor and sensory onset symptom as well as between the patients with optic neuritis and somatosensory onset symptom. A paired samples t test was used for within group comparisons. All statistical analyses were performed using IBM SPSS Statistics for Macintosh (Version 22.0; Released 2013; Armonk, NY, USA). Results are expressed as mean ± standard deviation (SD), unless otherwise indicated. A p‐value ≤.05 was considered statistically significant.
3. RESULTS
The RRMS patients with motor and sensory onset symptom were similar with respect to age, gender, systolic and diastolic blood pressure, as well as IgG‐index indicating the ratio between [cerebrospinal fluid immunoglobulin G vs. albumin] and [serum immunoglobulin G vs. albumin], at the time of ECG1 (Table 2). The average time interval between ECG1 and ECG2 was 8.6 ± 5.9 years, with no difference between the patients with motor (8.3 ± 6.9 years) and sensory (8.9 ± 5.0 years) onset symptom (p = .704).
Table 2.
Clinical characteristics of patients with motor and sensory onset symptom of relapsing‐remitting multiple sclerosis
| Motor (n = 25) | Sensory (n = 33) | p‐value | |
|---|---|---|---|
| Age (years) | 35 ± 12 | 35 ± 12 | .964 |
| Female gender (%) | 17 (68) | 15 (45) | .087 |
| Li‐Leuk (×106/L) | 7.4 ± 9.2 | 12.0 ± 19.0 | .313 |
| IgG‐index | 1.12 ± 0.46 | 1.07 ± 0.52 | .720 |
| Na (mmol/L) | 139 ± 2 | 140 ± 3 | .567 |
| K (mmol/L) | 3.9 ± 0.3 | 4.0 ± 0.3 | .132 |
| sBP (mmHg) | 133 ± 16 | 136 ± 23 | .621 |
| dBP (mmHg) | 80 ± 11 | 82 ± 11 | .610 |
Li‐Leuk, leukocyte concentration in cerebrospinal fluid; IgG‐index, the ratio between [cerebrospinal fluid immunoglobulin G vs. albumin] and [serum immunoglobulin G vs. albumin]; Na, plasma sodium concentration; K, plasma potassium concentration; sBP, systolic blood pressure; dBP, diastolic blood pressure. Values are mean ± SD or number (%).
During follow‐up, 81% (47/58) of the patients were on any DMT. DMTs were used in 72% (18/25) of the patients with motor and in 88% (29/33) of the patients with sensory onset symptom, with no difference between the groups (p = .179).
3.1. Cardiac repolarization at the early course of RRMS
Similar heart rate (74 ± 13 bpm vs. 70 ± 14 bpm; p = .238) and QT interval (371 ± 35 ms vs. 379 ± 28 ms; p = .342) were found in ECG1 in patients with motor and sensory onset symptom, respectively.
In ECG1, heart rate corrected QTcBaz (408 ± 17 ms vs. 406 ± 19 ms; p = .549), QTcFri (395 ± 19 ms vs. 396 ± 14 ms; p = .838), and QTcKar (398 ± 17 ms vs. 398 ± 14 ms; p = .957) were similar in patients with motor and sensory onset symptom, respectively.
3.2. Cardiac repolarization during the course of RRMS
The patients with motor onset symptom showed significantly longer QTcBaz (424 ± 25 ms vs. 408 ± 17 ms; p = .002), QTcFri (406 ± 18 ms vs. 395 ± 19 ms; p = .033), as well as QTcKar (407 ± 16 ms vs. 398 ± 17 ms; p = .043) in ECG2 than in ECG1, respectively. Uncorrected QT interval was similar between ECG2 and ECG1 (371 ± 24 vs. 371 ± 35 ms; p = .981), whereas heart rate had a trend to be higher in ECG2 than in ECG1 (79 ± 13 bpm vs. 74 ± 13 bpm; p = .060), respectively.
The patients with sensory onset symptom, on the other hand, showed no change in QTcBaz (406 ± 19 ms vs. 406 ± 19 ms; p = .981), QTcFri (395 ± 15 ms vs. 396 ± 14 ms; p = .675), or in QTcKar (397 ± 15 ms vs. 398 ± 14 ms; p = .735) between ECG2 and ECG1, respectively. Correspondingly, in patients with sensory onset symptom, no change was found in heart rate (70 ± 14 bpm vs. 72 ± 14 bpm; p = .550) or in QT interval (379 ± 28 ms vs. 376 ± 31 ms; p = .503) between ECG1 and ECG2, respectively.
The prolongation in heart rate‐corrected QT interval was more distinct in patients with motor than sensory onset symptom as calculated in absolute units in QTcBaz (16.0 ± 23.4 ms vs. 0.1 ± 21.2 ms; p = .009) (Figure 1), in QTcFri (10.3 ± 22.8 ms vs. −1.2 ± 16.5 ms; p = .030), and in QTcKar (8.7 ± 20.3 ms vs. −0.9 ± 15.8 ms; p = .047) as well as in relative units in QTcBaz (4.0 ± 5.7% vs. 0.2 ± 5.3%; p = .012), in QTcFri (2.8 ± 5.9% vs. −0.2 ± 4.2%; p = .027) and in QTcKar (2.3 ± 5.1% vs. −0.2 ± 4.0%; p = .044), respectively.
Figure 1.
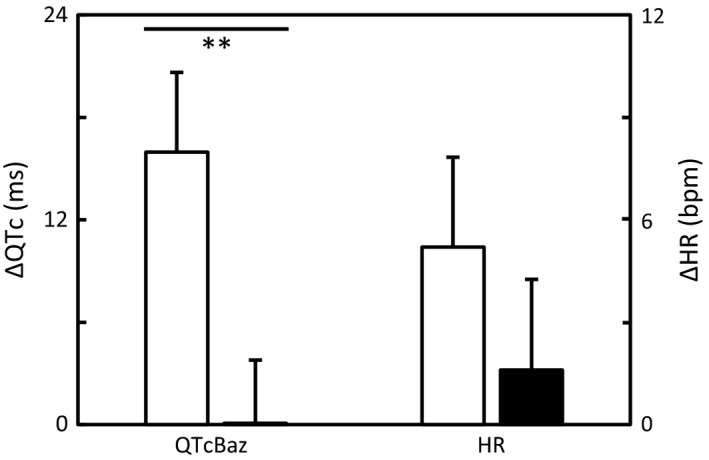
Changes in heart rate‐corrected QT interval (∆QTc) assessed by the Bazett formula (QTcBaz) and in heart rate (∆HR) between the onset symptom (ECG1) and later disease course (ECG2) in patients with motor (white bar) and sensory (black bar) onset symptom of RRMS. Values are mean ± SEM. Significance: **p < .01
3.3. Cardiac repolarization at the later course of RRMS
At the time of ECG2, heart rate was higher in patients with motor (79 ± 13 bpm) than in patients with sensory (72 ± 14 bpm; p = .035) onset symptom. Despite this, uncorrected QT interval was similar between the patients with motor (371 ± 24 ms) and sensory (376 ± 31 ms; p = .564) onset symptom in ECG2.
Significantly longer QTcBaz (424 ± 25 ms vs. 406 ± 19 ms; p = .002), QTcFri (406 ± 18 ms vs. 395 ± 15 ms; p = .019), and QTcKar (407 ± 16 ms vs. 397 ± 15 ms; p = .026) were observed in ECG2 in patients with motor onset symptom as compared to patients with sensory onset symptom, respectively (Figure 2).
Figure 2.

Heart rate‐corrected QT intervals at the later disease course (ECG2) assessed by Bazett (QTcBaz2), Fridericia (QTcFri2), and Karjalainen (QTcKar2) formulas in patients with motor (white bar) and sensory (black bar) onset symptom of RRMS. Values are mean ± SEM. Significances: *p ≤ .05 and **p < .01.
None of the patients had QTc >450 ms in ECG1 as calculated by Bazett, Fridericia, or Karjalainen formula. In ECG2, however, QTcBaz values >450 ms were found in five (20%) patients with motor but only in 1 (3%) patient with sensory onset symptom with borderline statistical significance (p = .075) for difference.
The patients with isolated optic neuritis and those with somatosensory onset symptom demonstrated comparable demographics and heart rate‐corrected QT intervals both at the time of ECG1 and ECG2 (Table 3). We found no change between ECG1 and ECG2 in heart rate (p = .893; p = .473) or in QTcBaz (p = .749; p = .713), QTcFri (p = .542; p = .989), or QTcKar (p = .462; p = .870) in patients with isolated optic neuritis or in patients with somatosensory onset symptom, respectively.
Table 3.
Clinical characteristics of patients with optic neuritis and somatosensory onset symptom of relapsing‐remitting multiple sclerosis
| Optic neuritis (n = 18) | Somatosensory (n = 15) | p‐value | |
|---|---|---|---|
| Age (years) | 33 ± 13 | 37 ± 10 | .307 |
| Female gender (%) | 7 (39) | 8 (53) | .407 |
| Li‐Leuk (×106/L) | 6.9 ± 8.5 | 17.9 ± 25.9 | .150 |
| IgG‐index | 0.96 ± 0.39 | 1.20 ± 0.63 | .210 |
| Na (mmol/L) | 140 ± 3 | 139 ± 2 | .687 |
| K (mmol/L) | 4.0 ± 0.2 | 4.0 ± 0.3 | .960 |
| sBP (mmHg) | 137 ± 28 | 134 ± 15 | .713 |
| dBP (mmHg) | 78 ± 12 | 86 ± 10 | .103 |
| HR1 (bpm) | 69 ± 15 | 71 ± 12 | .791 |
| HR2 (bpm) | 70 ± 14 | 74 ± 15 | .470 |
| QTcBaz1 (ms) | 408 ± 20 | 403 ± 17 | .514 |
| QTcFri1 (ms) | 399 ± 15 | 393 ± 13 | .247 |
| QTcKar1 (ms) | 401 ± 15 | 395 ± 13 | .169 |
| QTcBaz2 (ms) | 406 ± 20 | 405 ± 18 | .928 |
| QTcFri2 (ms) | 397 ± 17 | 393 ± 13 | .483 |
| QTcKar2 (ms) | 399 ± 16 | 395 ± 13 | .481 |
| Follow‐up (y) | 8.0 ± 3.4 | 9.9 ± 6.4 | .305 |
HR, heart rate; QTcBaz, heart rate‐corrected QT interval according to Bazett formula; QTcFri, hear rate‐corrected QT interval according to Fridericia formula; QTcKar, heart rate‐corrected QT interval according to Karjalainen formula; Follow‐up, time interval between ECG1 (1) and ECG2 (2). Other abbreviations are explained in the footnote of the Table 2. Values are mean ± SD or number (%).
4. DISCUSSION
In this study, we demonstrated that cardiac repolarization prolonged during the disease course in patients with motor but not in patients with sensory onset symptom of RRMS. Furthermore, patients with sensory onset symptom either in optic neuritis or somatosensory domain were found to have similar characteristics of cardiac repolarization and heart rate throughout the disease course.
Patients with motor and sensory onset symptom did not differ with respect to cardiac repolarization at the time of the first demyelinating event (ECG1). However, heart rate‐corrected QT interval prolonged during the disease course particularly in patients with motor onset symptom. The motor onset symptom of RRMS has conventionally been associated with worse prognosis and more pronounced disability accumulation (Bsteh et al., 2016; Damasceno, Von Glehn, Brandão, Damasceno, & Cendes, 2013; Eriksson, Andersen, & Runmarker, 2003). Our present finding demonstrates that the RRMS patients with motor onset symptom are more prone also to the prolongation of cardiac repolarization during the disease course. This finding enhances the concept that different traits in RRMS are determined already at the early phase of the disease.
The first demyelinating event in optic neuritis (Confavreux, Vukusic, & Adeleine, 2003; Tintore et al., 2015) or somatosensory (Eriksson et al., 2003) domain are both considered as the signs of more favorable prognosis in RRMS. Correspondingly, patients with sensory onset symptom as a whole or in optic neuritis or somatosensory subgroup demonstrated comparable QTc interval features and heart rate at the time of the first demyelinating event (ECG1) as well as at the later disease course (ECG2). Accordingly, the conventional signs for favorable outcome at the early phase of the disease associates also with lower impact of RRMS on cardiac repolarization.
At the later phase of the disease, heart rate was found higher in patients with motor than in patients with sensory onset symptom of RRMS. Clinical characteristics including age and gender, comorbidities, medication, and the duration of follow‐up were similar between these two groups and thus, may not explain the finding. Higher heart rate in combination with longer heart rate‐corrected QT interval even strengthens the concept of diverse effects of motor and sensory onset symptom on cardiac autonomic regulation during the long‐term RRMS disease course. Lower cardiovascular fitness is associated with higher resting heart rate (Tulppo, Mäkikallio, Seppänen, Laukkanen, & Huikuri, 1998), and this should be borne in mind while interpreting our results. However, on our opinion, differences in heart rate may not significantly confound the interpretation of our findings as heart rate‐corrected QT interval namely allows comparison of cardiac repolarization between different heart rate.
Prolongation in heart rate‐corrected QT interval has previously been shown to be associated with spinal cord atrophy secondary to axonal loss in MS patients (de Seze et al., 2000). Indeed, preganglionic autonomic neurons convey in the lateral horn, whereas motor and sensory neurons locate in their own tracks within spinal cord (Bican, Minagar, & Pruitt, 2013). Possible differences in the devastation in the CAN and spinal autonomic pathways during the motor and sensory RRMS onset disease courses may be one explanation for our findings. Differences in disease activity during the years of RRMS may also have an impact on our findings. However, the differences in disease activity are possibly determined already at initial disease trait as previously demonstrated (Bsteh et al., 2016).
Previously, cardiovascular sympathetic dysfunction has been reported in patients with clinically isolated syndrome (CIS) (Crnošija et al., 2016). However, the entity of CIS is different from RRMS as one‐third of the patients are suggested to remain monophasic without further disease activity after the first demyelinating event (Brownlee & Miller, 2014). In addition, QTc interval is influenced by complex interaction between sympathetic and parasympathetic nervous system at the level of CAN, peripheral nervous system and intrinsic cardiac nervous system (Cersosimo & Benarroch, 2013; Shen & Zipes, 2014) and thus, cannot be considered as a pure marker of cardiovascular sympathetic function.
Evaluation of ECG parameters, an average of 8 years apart, is exceptional in real‐life RRMS research. As Bazett, Fridericia, and Karjalainen formulas yielded comparable results, the methodological concerns related to the QT interval adjustment remain low. On the other hand, the relatively small sample size and the retrospective nature of the study are acknowledged as factors possibly limiting the interpretation of the results.
5. CONCLUSIONS
Disease traits are, in terms of cardiac autonomic regulation, different after motor and sensory onset symptom of RRMS. Cardiac repolarization prolongs and heart rate tends to increase after motor onset symptom but remain stable after sensory onset symptom. This finding enhances the understanding of different traits in RRMS. In addition, the importance of cardiovascular evaluation in RRMS patients is highlighted, as prolongation of cardiac repolarization has clinical implications not only in drug safety issues but also in risk stratification for subsequent cardiovascular events.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the government [grant number 5772804] and the Finnish MS Foundation. The data are available for researchers with appropriate permissions upon request. This observational research was not preregistered in an independent, institutional registry.
Mikkola A, Ojanen A, Hartikainen JEK, Remes AM, Simula S. The impact of multiple sclerosis onset symptom on cardiac repolarization. Brain Behav. 2017;7:e00742 https://doi.org/10.1002/brb3.742
REFERENCES
- Acevedo, A. R. , Nava, C. , Arriada, N. , Violante, A. , & Corona, T. (2000). Cardiovascular dysfunction in multiple sclerosis. Acta Neurologica Scandinavica, 101, 85–88. [DOI] [PubMed] [Google Scholar]
- Bazett, H. C. (1920). An analysis of the time‐relations of electrocardiograms. Heart, 7, 353–370. [Google Scholar]
- Bican, O. , Minagar, A. , & Pruitt, A. A. (2013). The Spinal cord: A review of functional neuroanatomy. Neurologic Clinics, 31, 1–18. [DOI] [PubMed] [Google Scholar]
- Brønnum‐Hansen, H. , Koch‐Henriksen, N. , & Stenager, E. (2004). Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain, 127, 844–850. [DOI] [PubMed] [Google Scholar]
- Brownlee, W. J. , & Miller, D. H. (2014). Clinically isolated syndromes and the relationship to multiple sclerosis. Journal of Clinical Neuroscience, 21, 2065–2071. [DOI] [PubMed] [Google Scholar]
- Bsteh, G. , Ehling, R. , Lutterotti, A. , Hegen, H. , Di Pauli, F. , Auer, M. , … Berger, T. (2016). Long term clinical prognostic factors in relapsing‐remitting multiple sclerosis: Insights from a 10‐year observational study. PLoS ONE, 11, e0158978 https://doi.org/10.1371/journal.pone.0158978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo, M. G. , & Benarroch, E. E. (2013). Central control of autonomic function and involvement in neurodegenerative disorders In Buijs R. M. & Swaab D. F. (Eds.), Handbook of clinical neurology neurol. Vol. 117 (3rd series). (pp. 45–57) New York: Elsevier. [DOI] [PubMed] [Google Scholar]
- Confavreux, C. , Vukusic, S. , & Adeleine, P. (2003). Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain, 126, 770–782. [DOI] [PubMed] [Google Scholar]
- Crnošija, L. , Adamec, I. , Lovrić, M. , Junaković, A. , Krbot Skorić, M. , Lušić, I. , & Habek, M. (2016). Autonomic dysfunction in clinically isolated syndrome suggestive of multiple sclerosis. Clinical Neurophysiology, 127, 864–869. [DOI] [PubMed] [Google Scholar]
- Damasceno, A. , Von Glehn, F. , Brandão, C. O. , Damasceno, B. P. , & Cendes, F. (2013). Prognostic indicators for long‐term disability in multiple sclerosis patients. Journal of the Neurological Sciences, 324, 29–33. [DOI] [PubMed] [Google Scholar]
- Drouin, E. , Nataf, S. , Lande, G. , & Louboutin, J. P. (1998). Abnormalities of cardiac repolarization in multiple sclerosis: Relationship with a model of allergic encephalomyelitis in rat. Muscle and Nerve, 21, 940–942. [DOI] [PubMed] [Google Scholar]
- Eriksson, M. , Andersen, O. , & Runmarker, B. (2003). Long‐term follow up of patients with clinically isolated syndromes, relapsing‐remitting and secondary progressive multiple sclerosis. Multiple Sclerosis, 9, 260–274. [DOI] [PubMed] [Google Scholar]
- Fridericia, L. S. (1920). Die Systolendauer im Elektrokardiogramm bei normalen Menchen und bei Herzkranken. Acta Medica Scandinavica, 53, 469–486. [Google Scholar]
- Karjalainen, J. , Viitasalo, M. , Mänttäri, M. , & Manninen, V. (1994). Relation between QT Intervals and heart rates from 40 to 120 beats/min in rest electrocardiograms of men and a simple method to adjust QT interval values. Journal of the American College of Cardiology, 23, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Magnano, A. R. , Holleran, S. , Ramakrishnan, R. , Reiffel, J. A. , & Bloomfield, D. M. (2002). Autonomic nervous system influences on QT interval in normal subjects. Journal of the American College of Cardiology, 39, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Manouchehrinia, A. , Tanasescu, R. , Tench, C. R. , & Constantinescu, C. S. (2016). Mortality in multiple sclerosis: Meta‐analysis of standardised mortality ratios. Journal of Neurology, Neurosurgery & Psychiatry, 87, 324–331. [DOI] [PubMed] [Google Scholar]
- Midgard, R. , Albrektsen, G. , Riise, T. , Kvåle, G. , & Nyland, H. (1995). Prognostic factors for survival in multiple sclerosis: A longitudinal, population based study in Møre and Romsdal, Norway. Journal of Neurology, Neurosurgery & Psychiatry, 58, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri, K. , Tenvoorde, B. J. , Adèr, H. J. , Uitdehaag, B. M. J. , & Polman, C. H. (1998). Longitudinal follow‐up of cardiovascular reflex tests in multiple sclerosis. Journal of Neurological Sciences, 155, 50–54. [DOI] [PubMed] [Google Scholar]
- Oikarinen, L. , Nieminen, M. S. , Viitasalo, M. , Toivonen, L. , Jern, S. , Dahlöf, B. , … Okin, P. M. (2004). QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: The Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension, 43, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Polman, C. H. , Reingold, S. C. , Banwell, B. , Clanet, M. , Cohen, J. A. , Filippi, M. , … Wolinsky, J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porthan, K. , Viitasalo, M. , Jula, A. , Reunanen, A. , Rapola, J. , Väänänen, H. , … Oikarinen, L. (2009). Predictive value of electrocardiographic QT interval and T‐wave morphology parameters for all‐cause and cardiovascular mortality in a general population sample. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6, 1202–1208. [DOI] [PubMed] [Google Scholar]
- Postema, P. G. , & Wilde, A. A. M. (2014). The Measurement of the QT Interval. Current Cardiology Reviews, 10, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racosta, J. M. , Kimpinski, K. , Morrow, S. A. , & Kremenchutzky, M. (2015). Autonomic dysfunction in multiple sclerosis. Autonomic Neuroscience, 193, 1–6. [DOI] [PubMed] [Google Scholar]
- Schouten, E. G. , Dekker, J. M. , Meppelink, P. , Kok, F. J. , Vandenbroucke, J. P. , & Pool, J. (1991). QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation, 84, 1516–1523. [DOI] [PubMed] [Google Scholar]
- de Seze, J. , Stojkovic, T. , Gauvrit, J. Y. , Saint Michel, T. , Ayachi, M. , Pruvo, J. P. , & Vermersch, P. (2000). Cardiac repolarization abnormalities in multiple sclerosis: Spinal cord MRI correlates. Muscle and Nerve, 23, 1284–1286. [DOI] [PubMed] [Google Scholar]
- Shen, M. J. , & Zipes, D. P. (2014). Role of the autonomic nervous system in modulating cardiac arrhythmias. Circulation Research, 114, 1004–1021. [DOI] [PubMed] [Google Scholar]
- Siscovick, D. S. , Raghunathan, T. E. , Rautaharju, P. , Psaty, B. M. , Cobb, L. A. , & Wagner, E. H. (1996). Clinically silent electrocardiographic abnormalities and risk of primary cardiac arrest among hypertensive patients. Circulation, 94, 1329–1333. [DOI] [PubMed] [Google Scholar]
- Sumelahti, M. L , Tienari, P. , Wikström, J. , Salminen, T. M. , & Hakama, M. (2002). Survival of multiple sclerosis in Finland between 1964 and 1993. Multiple Sclerosis, 8, 350–355. [DOI] [PubMed] [Google Scholar]
- Tintore, M. , Rovira, À. , Río, J. , Otero‐Romero, S. , Arrambide, G. , Tur, C. , … Montalban, X. (2015). Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain, 138, 1863–1874. [DOI] [PubMed] [Google Scholar]
- Tulppo, M. P. , Mäkikallio, T. H. , Seppänen, T. , Laukkanen, R. T. , & Huikuri, H. V. (1998). Vagal modulation of heart rate during exercise: Effects of age and physical fitness. American Journal of Physiology, 274, H424–H429. [DOI] [PubMed] [Google Scholar]
- Wynia, K. , Middel, B. , Van Dijk, J. P. , De Keyser, J. H. A. , & Reijneveld, S. A. (2008). The impact of disabilities on quality of life in people with multiple sclerosis. Multiple Sclerosis, 14, 972–980. [DOI] [PubMed] [Google Scholar]


