Abstract
Polycyclic aromatic hydrocarbons (PAHs), the by-products of incomplete combustion of organic materials, are commonly found on particulate matter (PM) and have been associated with the development of asthma and asthma exacerbation in urban populations. We examined time spent in the home and outdoors as predictors of exposures to airborne PAHs and measured urinary 1-hydroxypyrene-glucuronide (1-OHPG) as internal dose of PAHs in 118 children aged 5–12 years from Baltimore, MD. During weeklong periods (Saturday–Saturday) in each of four seasons: daily activities were assessed using questionnaires, indoor air nicotine and PM concentrations were monitored, and urine specimens were collected on Tuesday (day 3) and Saturday (day 7) for measurement of 1-OHPG. Time spent in non-smoking homes was associated with significantly decreased 1-OHPG concentration in urine (β = −0.045, 95% CI (−0.076, −0.013)), and secondhand smoke (SHS) exposures modified these associations, with higher urinary 1-OHPG concentrations in children spending time in smoking homes than non-smoking homes (P-value for interaction = 0.012). Time spent outdoors was associated with increased urinary 1-OHPG concentrations (β=0.097, 95% CI (0.037, 0.157)) in boys only. Our results suggest that SHS and ambient (outdoor) air pollution contribute to internal dose of PAHs in inner city children.
Keywords: polycyclic aromatic hydrocarbons, secondhand smoke, 1-hydroxypyrene-glucuronide
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are formed from the incomplete combustion or pyrolysis of organic materials (e.g., coal, wood, fuel, and oil) and are commonly found on PM2.5 (particulates with aerodynamic diameter ≤2.5 µm).1,2 Ambient (outdoor) airborne PAH sources include motor vehicle emissions (combustion products from diesel and conventional gasoline engines), burning fossil fuels (e.g., coal and oil), and industrial activities, whereas smoking tobacco, cooking with gas stoves, home heating, and burning incense are common indoor air PAH sources.3–5 The mutagenic and carcinogenic properties of PAHs are well known, as PAHs and PAH mixtures have been classified as human carcinogens (Group 1) (e.g., benzo[a]pyrene and coal tar pitch), probable carcinogens (Group 2A), and possible carcinogens (Group 2B) by the International Agency for Research on Cancer (IARC).2 PAHs have also been associated with the development of asthma and asthma exacerbation,6,7 low birth weight,8 and neurodevelopmental deficiencies9 in inner city children. PAHs are important organic constituents of PM2.5 because of irritant, oxidative potential, and carcinogenic properties.10 Exposure to PAHs from indoor air are of particular concern, as people spend over 80% of their day indoors,11 and the potency per unit mass and concentrations of indoor particulates may be greater than ambient (outdoor) particulates in some cases.12,13
Several studies have assessed PAH exposures by measuring PAH concentrations from PM samples gathered using personal, in-home stationary, or fixed-site outdoor air monitors.1,14–17 Biomarkers of low-level environmental exposures and internal dose of PAHs, such as urinary 1-hydroxypyrene-glucuronide (1-OHPG), have also been measured in epidemiological studies.18–22 Both 1-hydroxypyrene (1-OHP) and 1-OHPG are commonly used urinary biomarkers of PAH exposure, but 1-OHPG is more easily detected, as the addition of glucuronide confers 3–5 times more fluorescence per molecule than 1-OHP alone.23–25 Previous studies have demonstrated increased urinary 1-OHPG concentrations with recent exposures through inhalation, ingestion, and dermal absorption of PAHs from environmental, occupational, dietary, or medicinal sources.20,21,26–31 Predictors of 1-OHP(G) concentrations include gender,27,32–34 dietary exposures,25,27,35,36 secondhand smoke (SHS) exposure,27,37–40 high traffic volume,41–43 and living in large cities/urban environments.22,44
Exposures to air pollutants in urban environments are especially important, as inner city residents experience high exposures to PAHs, PM, SHS, and other air pollutants and may be more susceptible to developing or exacerbating environmentally related pulmonary diseases.45–50 Combustion particulates (e.g., PM2.5) are of concern because they are readily deposited in the small airways and interact with the lung interstitium.17 PAHs on the surface of combustion particulates have been associated with redox activities and oxidative stress responses10 and asthma exacerbation7 in inner city children. Several studies conducted by the Johns Hopkins Center for Childhood Asthma in the Urban Environment (CCAUE) have reported high levels of PM, SHS, and nitrogen dioxide (NO2) in the homes of asthmatic children in Baltimore City.46–48,50–52 To our knowledge, there has not been a comprehensive longitudinal panel study examining exposures to airborne PAHs and internal dose of PAHs, seasonally, among inner city children. As part of our ongoing longitudinal study of inner city children with asthma, we examined the relationship between predictors of PAH exposure and internal dose (measured by urinary 1-OHPG) and evaluated possible effect modifiers of these associations in a cohort of 118 children in Baltimore City.
MATERIALS AND METHODS
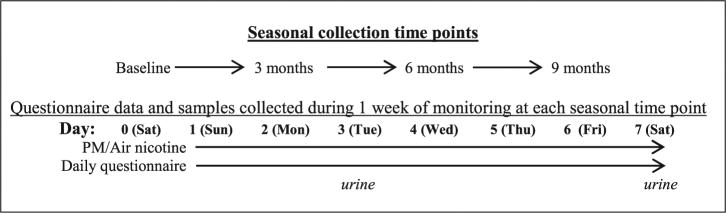
The DISCOVER study, a component of the CCAUE research program, is a panel study examining relationships between environmental pollutants and asthma morbidity in children in Baltimore City. A total of 180 children (100 atopic asthmatic, 50 non-atopic asthmatic, and 30 non-asthmatic children) from 9 contiguous zip codes in Baltimore, MD, were recruited into the study from 2009 to 2013.53 The study was approved by the Johns Hopkins Institute Review Board. Written informed consent was obtained from parents or legal guardians for all measurements. Inclusion criteria for our study included age 5–12 years and residence in Baltimore City. Children with asthma had a physician diagnosis of asthma and symptoms of asthma and/or reliever medication use in past 6 months. Children were excluded from the study if they had a current diagnosis of another major pulmonary disease, if they were planning to relocate residence during the study period, were currently taking antioxidant supplements, or were unable to carry a small backpack for personal monitoring. The children’s homes were monitored for 8 days (Saturday–Saturday; labeled as days 0–7) at baseline, and at 3, 6, and 9 months (Figure 1). Most of the children had four seasonal visits, and the number of visits per participant in the cohort ranged from one to five visits. During each weeklong period, indoor air nicotine and PM concentrations were measured, and daily activity questionnaires were administered. The daily activity diaries assessed smoking in the home and where the child spent his/her time (hours spent inside the home, outdoors, and indoors in other buildings and vehicles). Each participant was followed every 3 months for 9 months. During each environmental monitoring period (each child had up to 5), urine samples were collected in the afternoon to early evening (1500–1900 h) on day 3 (Tuesday), and in the morning (0830–1200 h) on day 7 (Saturday). Our study was comprised of children who had at least one available urine specimen (n = 118).
Figure 1.
DISCOVER study framework.
Urinary 1-OHPG
Spot urine samples were analyzed for urinary 1-OHPG concentrations using immunoaffinity chromatography (IAC) and synchronous fluorescence spectroscopy (SFS), as previously described by Strickland et al.19 The limit of detection was 0.05 pmol/ml, a level of sensitivity sufficient to detect urinary 1-OHPG in 89% of samples. The coefficient of variation of the assay is typically 6–10% (interbatch) in our laboratory.
Urinary Creatinine
Creatinine concentrations in spot urine samples were determined using a modified version of the Jaffe method using a creatinine assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). Absorbance was read at 450– 500 nm using a Biotek ELx800 absorbance microplate reader. Creatinine concentrations were determined using a creatinine standard curve that was estimated in each batch from analysis of standard creatinine.
Particulate Matter
Airborne particulate matter monitoring was conducted in the child’s bedroom using integrated sampling methods for a 5–7-day period. Air samples for both particulate matter ≤ 10 µm (PM10) and ≤ 2.5 µm (PM2.5) were collected on Teflon filters (Pall Gelman, Ann Arbor, MI, USA) using SKC personal environmental monitors (SKC, Eighty Four, PA, USA) and BGI 400S pumps (BGI, Waltham, MA, USA).51 Coarse PM (PM2.5–10) was calculated by subtracting PM2.5 from PM10.
Air Nicotine
Passive sampling badges were placed in the child’s bedroom and the TV/family room at 3–5 feet off the floor. The passive air samplers consist of a sodium-bisulfate-treated filter contained in a 37 mm polystyrene cassette covered with a polycarbonate filter diffusion screen. Nicotine content was analyzed using gas chromatography with a nitrogen-phosphate detector.51 The limit of detection for the passive air nicotine badges was 0.003 µg/m3.
Statistical Analysis
Summary statistics are reported for urinary 1-OHPG, and indoor PM2.5, PM2.5–10, PM10, and air nicotine concentrations. 1-OHPG, PM2.5, PM2.5–10, PM10, and air nicotine concentrations were log-transformed to adjust for positively skewed distributions. Urinary 1-OHPG concentrations on day 3 (Tuesday; n = 255) and day 7 (Saturday; n = 339), and averages for the 2 days were analyzed separately. Urine collected in the afternoon or early evening of day 3 (Tuesday) was compared with pooled estimates of indoor and outdoor PAH exposures on days 0–2 (Saturday–Monday), representing exposures from 24 to 96 h before urine collection (24–48 h for day 2, Monday; 48–72 h for day 1, Sunday; and 72–96 h for day 0, Saturday). Urine collected in the morning to early afternoon on day 7 (Saturday) was compared with pooled estimates of exposures on days 4–6 (Wednesday–Friday), representing exposures from 18 to 90 h before urine collection.
Urinary 1-OHPG concentrations were adjusted for urinary creatinine by including creatinine concentrations in the model as an independent variable.54 Multivariate linear regressions with generalized estimating equations (GEEs) were used to assess associations between potential predictors and individual 1-OHPG concentrations, while adjusting for repeated measurements (i.e., visits) and possible confounders. Of the 118 children in the cohort, 16 were controls (i.e., did not have asthma). There were no significant differences between children with and without asthma in age, race, time spent indoors and outdoors, urinary 1-OHPG, and the presence of adults smoking in the home (data not shown). We therefore used the complete cohort (i.e., both children with and without asthma) in our analyses (n = 118). The explanatory variables age, gender, BMI percentile, atopic status, season, household income, caregivers educational attainment, health insurance type, distance to the street curb, and type of home heating were considered possible confounders if the coefficient changed by >10% after inclusion of the exposure variable in the model or if the variable was significantly associated with the exposure or outcome. Based on these criteria, our final models for multivariate linear regression with GEE were adjusted for age, gender, season, atopic status, and caregiver educational attainment. Race was not included in models because 95% of the participants were African Americans. Sources of indoor air PAH exposures such as self-reported stove use, burning food, burning candles or incense, and time with windows open were also analyzed. Effect modification was assessed using pairwise interaction terms for independent variables. Comparisons of two groups of exposure variables were performed using Wilcoxon sign-rank test. Spearman’s rho was used to examine correlations between internal dose biomarkers and indoor air concentrations. Two-sided tests were considered statistically significant at α < 0.05.
Age, 1-OHPG, creatinine, air nicotine, PM2.5, PM2.5–10, and PM10, self-reported exposure variables (e.g., average time in the home and average time outdoors) were analyzed as continuous variables; gender and health insurance were binary variables; season was a categorical variable; and caregiver education, BMI percentile, and annual household income were ordinal variables. Seasons were defined based on calendar days, and heating season was defined as November 1 through March 1.55 Atopy was defined as having allergic responses to at least one of 13 aeroallergens from a skin prick test or by radioallergosorbent test (RAST). Urinary 1-OHPG concentrations were reported as pmol/ml and µmol/mol creatinine, and concentrations for indoor air nicotine, PM2.5, PM2.5–10, and PM10 were reported as µg/m3. Duplicate measures for indoor air nicotine concentration were averaged. PM2.5 and PM10 measurements were excluded if airflow through the monitor was not sufficient, PM2.5 concentrations were greater than PM10 concentrations, or if there were equipment malfunctions. The highest PM2.5–10 value, 215.3 µg/m3 (over 20-fold higher than the median), was considered an outlier and was therefore not included in analyses. Reasons for missing data included: interviewer was unable to contact the caregiver and obtain urine samples from the child (especially during the weekday (day 3) and on the first day of the monitoring period (day 0)), incorrect or unusable questionnaire data (e.g., daily time apportionment exceeding 24 h), and technical problems with the air nicotine and PM monitors. Missing values were not included in the analyses. All data were analyzed using Stata 11.1 (College Station, TX, USA).
RESULTS
Time Spent in the Home
The children spent an average of 16 h in their homes each day, with no differences by gender (Table 1). More time was spent in the home during the first 3-day period of the monitoring week (days 0–2; Saturday–Monday) (median 17.0 h) as compared to the latter 3-day period (days 4–6; Wednesday–Friday) (median 15.7 h) (Wilcoxon sign-rank test P = 0.005). Overall, time spent in the home on days 4–6 (Wednesday–Friday) showed a significant inverse association with 1-OHPG from day 7 (Saturday) urine (β=−0.045, 95% CI (−0.076, −0.013); P = 0.005), whereas spending time in the home on days 0–2 (Saturday–Monday) was only moderately associated with decreased 1-OHPG concentrations in day 3 (Tuesday) urine (β = - 0.026, 95% CI (−0.055, 0.003)); P = 0.076) (Table 2). Sensitivity analyses showed that associations between time spent in the home and urinary 1-OHPG concentrations remained after individually adjusting for indoor PM2.5, PM2.5–10, and PM10 concentration (data not shown).
Table 1.
Descriptive statistics for urinary 1-OHPG, indoor PM, and indoor air nicotine concentrations.
| 1-OHPG (pmol/ml) |
1-OHPG (µmol/mol Cr) |
Indoor PM2.5 (µg/m3) |
Indoor PM10 (µg/m3) |
Indoor PM2.5–10 (µg/m3) |
Air nicotine (µg/m3) | |
|---|---|---|---|---|---|---|
| Number of samples | 594 | 594 | 309 | 308 | 280 | 346 |
| Arithmetic mean (SD) | 1.76 (1.92) | 0.17 (0.22) | 28.4 (22.8) | 41.1 (29.0) | 12.7 (14.3) | 0.76 (1.26) |
| Geometric mean | 0.89 | 0.09 | 21.8 | 33.3 | 9.0 | 0.17 |
| Median (IQR) | 1.24 (0.42–2.43) | 0.11 (0.05–0.22) | 21.6 (13.0–34.9) | 32.6 (21.6–52.5) | 9.8 (5.4–15.8) | 0.13 (0.03–0.96) |
| 95 Percentile | 5.4 | 0.54 | 72.1 | 97.5 | 30.8 | 3.4 |
| Range | 0.05–14.59 | 0.003–2.13 | 3.7–133.2 | 5.8–268.8 | 0.71–215.3 | 0.005–8.8 |
| % Below LOD | 11% | 11% | – | – | – | – |
| All samples | Boys | Girls | P-valuea | P-valueb | |
|---|---|---|---|---|---|
| Time spent in the home | |||||
| Avg. time spent in the home on days 0–6 (Sat–Fri) | 16.1 (13.6–18.1) | 15.9 (12.7–18.0) | 16.4 (14.7–18.3) | 0.067 | 0.161 |
| Avg. time spent in the home on days 0–2 (Sat–Mon) | 17.0 (13.7–20.3) | 16.7 (11.5–19.5) | 17.8 (14.3–20.3) | 0.059 | 0.089 |
| Avg. time spent in the home on days 4–6 (Wed–Fri) | 15.7 (13.7–17.7) | 15.6 (14.0–17.6) | 15.7 (13.7–18.0) | 0.733 | 0.704 |
| Difference between days 0–2 and days 4–6c | P = 0.005 | ||||
| Time spent outdoors | |||||
| Avg. time spent outdoors on days 0–6 (Sat–Fri) | 1.9 (0.7–3.9) | 2.1 (0.8–4.7) | 1.6 (0.7–3.3) | 0.053 | 0.028 |
| Avg. time spent outdoors on days 0–2 (Sat–Mon) | 2.0 (0.5–4.3) | 2.0 (0.7–5.3) | 1.7 (0.3–3.7) | 0.053 | 0.033 |
| Avg. time spent outdoors on days 4–6 (Wed–Fri) | 1.5 (0.3–4.0) | 1.9 (0.7–4.3) | 1.0 (0.3–3.3) | 0.026 | 0.044 |
| Difference between days 0–2 and days 4–6 c | P = 0.404 |
Average time (hours) spent in the home and time spent outdoors (median (IQR)), and differences by gender.
Wilcoxon rank-sum test for difference by gender.
Multivariable linear regression with GEE: adjusted for age and season.
Wilcoxon signed-rank test. Bold value is statistically significant (P < 0.05).
Table 2.
Associations between average time (h) spent in the home and 1-OHPG concentrations.
| Crudea
|
Adjustedb
|
|||
|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | |
| Avg. time in the home days 0–2 (urine day 3) | −0.024 (−0.054, −0.005) | 0.105 | −0.026 (−0.055, −0.003) | 0.076 |
| Avg. time in the home days 4–6 (urine day 7) | −0.038 (−0.072, −0.005) | 0.024 | −0.045 (−0.076, −0.013) | 0.005 |
| By gender | ||||
| Boys | ||||
| Avg. time in the home days 0–2 (urine day 3) | −0.043 (−0.079, −0006) | 0.022 | −0.045 (−0.079, −0.011) | 0.010 |
| Avg. time in the home days 4–6 (urine day 7) | −0.041 (−0.090, 0.008) | 0.105 | −0.051 (−0.099, −0.003) | 0.038 |
| Girls | ||||
| Avg. time in the home days 0–2 (urine day 3) | 0.014 (−0.033, −0.061) | 0.555 | 0.011 (−0.038, −0.059) | 0.668 |
| Avg. time in the home days 4–6 (urine day 7) | −0.047 (−0.091, −0.004) | 0.033 | −0.046 (−0.082, −0.010) | 0.013 |
Adjusted for urinary creatinine.
Adjusted for urinary creatinine, gender, age, atopic status, season, and caregiver’s education. Bold value is statistically significant (P < 0.05).
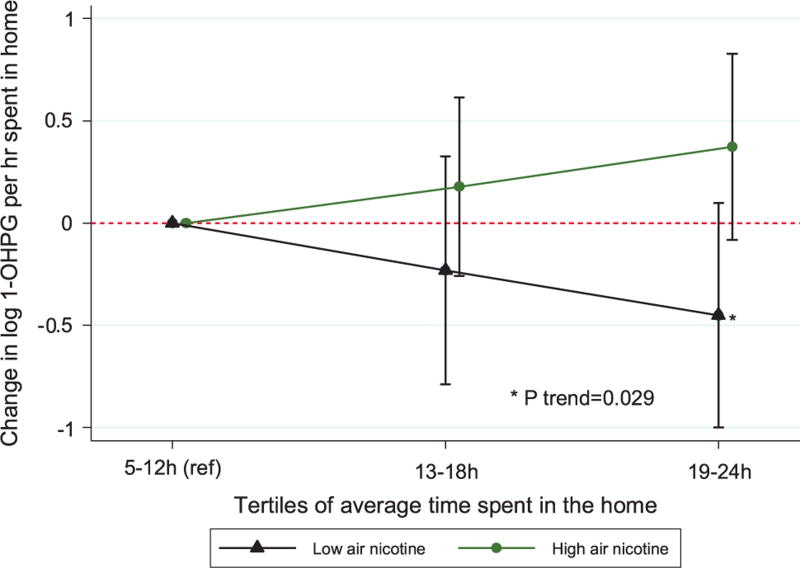
Indoor air nicotine was significantly correlated with urinary 1-OHPG (Spearman’s rho = 0.12, P = 0.031), and significantly associated with elevated 1-OHPG concentrations from day 3 urine only (β = 0.129, 95% CI (0.035, 0.233); P = 0.007) (Table 3a). Overall, time spent in homes with low air nicotine concentration (below the median: 0.13 µg/m3) was significantly inversely associated with urinary 1-OHPG concentration (β= −0.061, 95% CI (−0.106, 0.017); P = 0.007), and there was a significant trend of lower 1-OHPG levels with increasing time spent in low air nicotine (e.g., non-smoking) homes (Cusick’s test for trend P-value = 0.029) (Table 3b). Conversely, time spent in high-nicotine homes (above the median) was positively associated with urinary 1-OHPG (Figure 2). Time spent in homes with low air nicotine concentration on days 4–6 (Wednesday–Friday) was independently associated with significantly decreased urinary 1-OHPG concentrations from urine collected on day 7 (Saturday) (β=− 0.081, 95% CI (−0.121, 0.041); P < 0.001) (Table 3b). SHS exposures (measured by indoor air nicotine concentration and self-reported smoking in the home) also modified the associations between time spent in the home on days 4–6 and urinary 1-OHPG collected on day 7, with moderately stronger inverse associations in homes with low air nicotine concentrations compared with high air nicotine concentrations (P-value for interaction = 0.074) (Table 3b); and significantly stronger inverse associations in self-reported non-smoking homes compared with smoking homes (P-value for interaction = 0.012) (Table 3c).
Table 3.
| a. Associations between urinary 1-OHPG concentrations and indoor air nicotine, PM2.5, PM2.5–10, and PM10 concentrations. | ||||
|---|---|---|---|---|
| Crudea
|
Adjustedb
|
|||
| β (95% CI) | P-value | β (95% CI) | P-value | |
| Air nicotine | ||||
| Urine collected on day 3 | 0.157 (0.061, 0.252) | 0.001 | 0.129 (0.035, 0.223) | 0.007 |
| Urine collected on day 7 | 0.007 (−0.131, 0.144) | 0.923 | −0.006 (−0.147, 0.134) | 0.928 |
| Indoor PM2.5 | ||||
| Urine collected on day 3 | 0.040 (−0.211, 0.290) | 0.757 | 0.020 (−0.257, 0.296) | 0.888 |
| Urine collected on day 7 | 0.014 (−0.215, 0.244) | 0.902 | 0.046 (−0.199, 0.292) | 0.711 |
| Indoor PM2.5–10 | ||||
| Urine collected on day 3 | 0.113 (−0.100, 0.326) | 0.297 | 0.083 (−0.127, 0.293) | 0.437 |
| Urine collected on day 7 | 0.144 (−0.003, 0.291) | 0.055 | 0.177 (0.027, 0.326) | 0.021 |
| Indoor PM10 | ||||
| Urine collected on day 3 | 0.143 (−0.165, 0.450) | 0.364 | 0.094 (−0.240, 0.429) | 0.580 |
| Urine collected on day 7 | 0.142 (−0.085, 0.370) | 0.220 | 0.104 (−0.133, 0.342) | 0.390 |
| b. Associations between time spent in the home and 1-OHPG, stratified by indoor air nicotine concentration (dichotomized at the median: 0.13 µg/m3). | |||||
|---|---|---|---|---|---|
| Low air nicotine
|
High air nicotine
|
P-interaction | |||
| β (95% CI) | P-value | β (95% CI) | P-value | ||
| Avg. time in home, all samples | −0.061 (−0.106, −0.017) | 0.007 | 0.005 (−0.039, 0.048) | 0.833 | 0.049 |
| Avg. time in home days 0–2 (urine day 3) | −0.034 (−0.083, 0.014) | 0.167 | −0.008 (−0.041, 0.026) | 0.658 | 0.357 |
| Avg. time in home days 4–6 (urine day 7) | −0.081 (−0.121, −0.041) | <0.001 | −0.024 (−0.069, 0.020) | 0.285 | 0.074 |
| By gender | |||||
| Boys | |||||
| Avg. time in home days 0–2 (urine day 3) | −0.055 (− −0.116, 0.006) | 0.078 | −0.023 (−0.061, 0.014) | 0.221 | 0.264 |
| Avg. time in home days 4–6 (urine day 7) | −0.088 (−0.149, −0.027) | 0.005 | −0.053 (−0.127, 0.021) | 0.164 | 0.623 |
| Girls | |||||
| Avg. time in home days 0–2 (urine day 3) | 0.011 (−0.056, 0.079) | 0.743 | 0.032 (−0.049, 0.113) | 0.442 | 0.478 |
| Avg. time in home days 4–6 (urine day 7) | −0.067 (−0.119, −0.016) | 0.011 | −0.024 (−0.075, 0.027) | 0.363 | 0.381 |
| c. Associations between average time spent (hours) in the home and urinary 1-OHPG, stratified by adults smoking in the home. | |||||
|---|---|---|---|---|---|
| No smoking
|
Smoking
|
P-interaction
|
|||
| β (95% CI) | P-value | β (95% CI) | P-value | ||
| Avg. time in the home, all samples | −0.013 (−0.065, 0.039) | 0.612 | −0.024 (−0.065, 0.018) | 0.261 | 0.758 |
| Avg. time in the home days 0–2 (urine day 3) | −0.033 (−0.075, 0.010) | 0.135 | −0.013 (−0.056, 0.030) | 0.566 | 0.628 |
| Avg. time in the home days 4–6 (urine day 7) | −0.092 (−0.144, −0.040) | < 0.001 | −0.001 (−0.044, 0.043) | 0.966 | 0.012 |
Adjusted for urinary creatinine.
Adjusted for urinary creatinine, gender, age, atopic status, season, and caregiver’s education. Bold value is statistically significant (P < 0.05).
Multivariable linear regression with GEE: adjusted for urinary creatinine, gender, age, atopic status, season, and caregiver’s education. Bold value is statistically significant (P < 0.05).
Adjusted for urinary creatinine, gender, age, atopic status, season, and caregiver’s education. Bold value is statistically significant (P < 0.05).
Figure 2.
Comparison of associations between urinary 1-OHPG concentration and tertiles of time spent in the home, stratified by indoor air nicotine concentration.
Time Spent Outdoors
The children spent ~2 h each day outdoors, and boys spent more time outdoors daily, compared with girls (Wilcoxon signed-rank test P = 0.053) (Table 1). Among boys, time spent outdoors on days 4–6 was significantly associated with increased 1-OHPG in day 7 urine (β = 0.097, 95% CI [0.037, 0.157); P = 0.002) (Table 4). However, time spent outdoors was not associated with urinary 1-OHPG in girls. Average time spent outdoors also varied significantly by season (Kruskal–Wallis rank test P < 0.001) (Supplementary Table 1). The children spent the most amount of time outdoors in spring, followed by summer and fall, and were outdoors least during the winter. Only time spent outdoors in the summer was associated with significantly increased urinary 1-OHPG concentrations (β=0.147, 95% CI [0.048, 0.246); P = 0.003) (Supplementary Table 2a). In addition, season modulated associations between time spent outdoors and urinary 1-OHPG, with significantly stronger associations during summer compared with the other seasons (P-interaction = 0.001) (Supplementary Table 2b). These associations and seasonal interactions remained after individually adjusting for indoor PM2.5 and air nicotine concentrations.
Table 4.
Associations between average time spent outdoors and urinary 1-OHPG concentration.
| Crudea
|
Adjustedb
|
|||
|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | |
| Avg. time outdoors days 0–2 (urine day 3) | 0.002 (−0.061, 0.066) | 0.939 | 0.019 (−0.058, 0.096) | 0.635 |
| Avg. time outdoors days 4–6 (urine day 7) | 0.030 (−0.014, 0.075) | 0.178 | 0.048 (−0.001, 0.098) | 0.054 |
| By gender | ||||
| Boys | ||||
| Avg. time outdoors days 0–2 (urine day 3) | −0.009 (−0.082, 0.063) | 0.798 | −0.008 (−0.095, 0.080) | 0.861 |
| Avg. time outdoors days 4–6 (urine day 7) | 0.085 (0.026, 0.144) | 0.005 | 0.097 (0.037, 0.157) | 0.002 |
| Girls | ||||
| Avg. time outdoors days 0–2 (urine day 3) | 0.034 (−0.088, 0.156) | 0.585 | 0.060 (−0.085, 0.206) | 0.417 |
| Avg. time outdoors days 4–6 (urine day 7) | −0.019 (−0.087, 0.049) | 0.588 | −0.019 (−0.080, 0.043) | 0.552 |
Adjusted for urinary creatinine.
Adjusted for urinary creatinine, gender, age, atopic status, season, and caregiver’s education. Bold value is statistically significant (P < 0.05).
A total of 118 participants were enrolled in our study and the mean age was 10 years. Participants were slightly more female (52%), predominantly African American (95%), and from low socioeconomic status (SES) households (Supplementary Table 3). Most children had asthma (86%) and resided in households with adults smoking in the home at some time during the study (53%). 1-OHPG concentrations, analyzed from 594 spot urine samples, had an arithmetic mean (SD) of 1.76 (1.92) pmol/ml, and median (interquartile range) of 1.24 (0.42–2.43) pmol/ml (Table 1).
Urinary 1-OHPG concentration from urine collected on days 3 and 7 were correlated (Spearman’s rho = 0.34; P < 0.001), and were significantly associated using linear regression with GEE in crude (β = 0.190, 95% CI (0.065, 0.316); P = 0.003) and adjusted models (β = 0.204, 95% CI (0.085, 0.323); P = 0.001) (Supplementary Table 4). Girls had higher creatinine adjusted urinary 1-OHPG concentrations than boys (β = 0.381, 95% CI (0.093, 0.668); P = 0.010) (Supplementary Table 5). Age, asthma status, atopic status, BMI percentile, and being obese (compared with normal weight) were not significantly associated with 1-OHPG concentrations, and there were no significant differences in 1-OHPG concentration by season or during the heating season.
Indoor airborne PAH sources, such as burning food and burning candles or incense, and type of stove and stove use were also not associated with urinary 1-OHPG. Sources associated with the infiltration of outdoor airborne PAHs into homes, such as time with open windows, proximity of the home to the street, and type of curb (e.g. parking lot, arterial street, or side street) were also not associated with urinary 1-OHPG concentrations (Supplementary Table 5). Median air nicotine concentration was ~ 20 times higher (0.77 vs 0.04 µg/m3), and geometric mean (GM) air nicotine concentration was ~ 9 times higher (0.52 vs 0.06 µg/m3) in smoking homes than non-smoking homes, respectively (Supplementary Table 6). Assessing the contributions of PAHs on indoor particulates to 1-OHPG, only indoor PM2.5–10 (coarse PM) was significantly associated with 1-OHPG (β= 0.177, 95% CI (0.027, 0.326); P = 0.021) from day 7 urine (Table 3a). Surprisingly, indoor PM2.5 (fine PM) concentration was not significantly associated with 1-OHPG concentrations from urine collected on day 3 or day 7.
DISCUSSION
Mean and median urinary 1-OHPG concentration in this study was higher than the US national average, and elevated urinary 1-OHPG levels were associated with exposures to SHS in the home and ambient (outdoor) air pollution. The GM urinary 1-OHPG concentration for children in this study was higher than the levels reported in the second (1999–2000), third (2001–2002), and fourth (2003–2004) National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional study in the United States (Supplementary Table 7). GM and median urinary 1-OHPG levels in our study were also higher than in most international studies in children. It should be noted that the international studies that reported higher 1-OHPG levels were conducted mostly in regions with major industrial activities (e.g., steel mills, coal-fired power plants, and oil refineries)41,42,56,57 or high vehicular traffic volume.58 Although our study comprised mostly asthmatic children, we found no differences in 1-OHPG concentration by asthma status, likely because of similar environmental PAH exposure profiles for children with and without asthma.
Most of the children (53%) lived in households that reported adults smoking in the home during the study period, and this is higher than the US average for children living with a smoker (18%).59 Median indoor air nicotine concentrations in our study (0.13 µ/m3) were also comparable to a similar study of children with asthma in Baltimore, MD.53 Some studies have reported associations between SHS exposure and urinary 1-OHP(G) concentration in children,27,32,38,40,60,61 whereas others have reported no association.22,27,39,58 In our study indoor air nicotine concentrations were independently associated with elevated 1-OHPG levels in Tuesday urine only. Specifically, increased exposures to SHS in the home on the weekend and early weekday (Saturday–Monday) likely contributed to 1-OHPG in urine collected on an early weekday (Tuesday). This may be because of children spending more time in the home during the weekend and early weekday than during the latter part of the week (Wednesday–Friday). We reported significant effect modification of associations between time spent in home and urinary 1-OHPG by SHS exposure (with similar results using indoor air nicotine concentration and self-reported smoking in the home as cigarette smoke exposure metrics). These consistently strong independent associations and interactions with SHS exposures (using multiple exposure metrics) therefore suggest that SHS is a major contributor to urinary 1-OHPG concentrations.
We found a “protective effect” of spending time in homes with little or no smoking on lower 1-OHPG concentration. This inverse association was stronger for 1-OHPG analyzed from Saturday urine (reflecting Wednesday–Friday exposures) than Tuesday urine (reflecting Saturday–Monday exposures), suggesting that children may have been exposed to less cigarette smoke during the latter half of the week. During the school year, children were likely in school during weekday mornings and early afternoons. If the primary exposures to PAHs were from SHS in the home and outdoor air, spending much of the day in schools during the weekdays—which are presumably smoke free—may enhance the protective effect.
We hypothesized that exposures to indoor air pollutants, especially indoor PM, would be primary predictors of increased urinary 1-OHPG. However, we observed the opposite effect: an inverse, protective, relationship between time spent in the home and urinary 1-OHPG (especially in non-smoking homes). In addition, despite high indoor PM2.5 concentrations in our study homes, indoor PM2.5 concentrations were not correlated or associated with 1-OHPG concentrations. Proximity of the home to the street, open windows, and stove use, which are commonly associated with PM and PAH concentrations,1,62 were also not associated with 1-OHPG in our study. In addition, cooking/burning of food and the burning of candles and incense had little effect on PAH internal dose. The absence of an association between indoor PM2.5 and urinary 1-OHPG levels may be because of low PAH content on indoor particulates, significant concentrations of indoor PAHs (i.e., pyrene) in the gas phase, or indoor PM2.5 concentrations measured in the home not reflecting personal exposures to PAHs on PM. Specifically, children may not have spent sufficient time in the PM monitoring areas such that their exposures to PAHs on PM would be reflected in urinary 1-OHPG levels. Our findings of high PM levels and an inverse relationship between time spent in the home and urinary 1-OHPG in this study therefore suggests that exposures to indoor air PAHs from PM2.5 likely had little influence on urinary 1-OHPG concentration.
Exposures to ambient outdoor PAHs may be a significant predictor of 1-OHPG levels, as exposures to outdoor PAHs (measured by time spent outdoors) were independently associated with increased urine 1-OHPG concentrations, especially in boys. Overall, time spent outdoors late in the week was associated with increased urinary 1-OHPG concentrations, with the strongest associations among Saturday (day 7) urine samples, reflecting weekday exposures. Similarly, a study of children in Copenhagen, Denmark, showed positive correlations between spending time outdoors on Monday–Thursday and 1-OHP in urine collected the following day.22 Exposures to outdoor (ambient) air may be more potent than indoor air because of higher concentrations of semivolatile PAHs and particles containing PAHs, and higher PAH content on each particle.10 Vehicular exhaust, especially diesel exhaust particulates (DEPs), are common components of ambient air pollution and are rich in PAH content.63 Larsen and Baker5 reported that diesel exhaust contributes 16–26% of the PAH in ambient air in Baltimore City. In addition, studies in Montreal, Canada, have found that ambient PAH concentrations from high traffic roads around kindergarten schools were 3–12 times higher than low traffic roads, and indoor PAH concentrations were 6 times higher in kindergarten schools around high traffic roads than low traffic roads in the summertime.35,64 Exposures to PAHs from vehicular exhaust in areas with high traffic volumes may therefore be substantial. Tonne et al.17 also reported that time (hours per day) spent outdoors significantly predicted pyrene exposures using personal monitors in predominantly African-American and Latino neighborhoods in northern Manhattan and the Bronx, New York City. Ambient PAHs are key components of air pollution in inner cities and urban areas, and therefore exposures to ambient air pollution while outdoors in Baltimore City are likely a significant source for inhaled PAHs in our study.
Seasonally, spending time outdoors in summer was associated with significantly increased 1-OHPG concentrations in boys only. In our study, boys spent twice as much time outdoors in the summer as girls and were likely exposed to more ambient PAHs. Ambient PAH concentrations may also exhibit seasonal patterns in urban environments. A study by Tonne et al.17 reported that personal monitors measured higher concentrations of airborne pyrene in the summer compared with the winter in New York City.17 An analysis of environmental influences and attributes of PAHs showed that low-molecular-weight PAHs (such as pyrene and phenanthrene) volatilize with increasing temperature and are found predominantly in gaseous phase in warmer weather.4 The gaseous phase PAH, and PM2.5 are readily deposited in the small airways and alveoli. Fine and ultrafine (PM0.1) particulates also have high surface areas per mass, thereby increasing the PAH carrying capacity, making them potent toxicants.10 Collectively, our results suggest that children are exposed to substantial levels of ambient PAHs while outdoors, with increased exposures in summertime (possibly because of volatilization of low-molecular-weight PAHs).
To our knowledge, this is the first panel study examining, seasonally, environmental predictors of 1-OHPG concentrations in inner city children. A major strength of this study is the longitudinal study design that allowed us to address temporality, seasonal influences, and intraindividual differences. Our exposure metric, self-reported time spent in different environments (i.e., in the home and outdoors), was averaged for the 3 days before urine collection to capture various attributes (e.g., weekend vs weekday exposures) and normalize interday variability. In addition, administering daily questionnaires, measuring indoor air pollutants, and collecting urine specimens at multiple time points allowed us to characterize temporally relevant exposures for our outcome, PAH internal dose, measured by urinary 1-OHPG.
Our study has several limitations, including not estimating PAH exposures through ingestion. Dietary intake (e.g., eating roasted, charbroiled, or smoked foods) has been shown to explain some of the internal dose of PAH, as ingested PAHs can account for a significant portion of total PAH exposure.35,64 Outdoor ambient PM and PAHs were also not measured during the monitoring period. Although indoor air pollution was the focus of this study, exposures to outdoor air pollutants probably had a substantial impact on internal dose biomarkers. There were no detailed assessments of exposures to traffic-related pollution, such as geospatial analyses of proximity of the children’s homes and schools to major roads, highways, bus and truck depots, and other areas with high traffic volume. We used a crude exposure metric (i.e., self-reported daily time spent in microenvironments) that may not incorporate exposures while the child is away from home (e.g., at school or in the homes of other smokers) and may bias the PAH exposure–internal dose associations. Internal dose biomarkers of cigarette smoke exposure, such as urinary cotinine concentration, were also not measured in this study. In addition to quantifying internal dose of SHS exposures, urinary cotinine analysis may identify firsthand smokers (which may be a confounder of associations between our current environmental predictors and urinary 1-OHPG).
In summary, our results suggest that two primary sources of airborne PAHs, SHS in the home and outdoor ambient air pollution, positively influenced urinary 1-OHPG concentrations in inner city children. In addition, urinary 1-OHPG concentrations in our study were higher than the US national average and many international studies, reflecting the disproportionate burden of exposures to environmental PAHs for inner city predominantly African-American communities of low SES. Based on our findings, avoiding exposures to SHS and reducing exposures to outdoor air pollutants may lead to reduced morbidity in inner city children. Limiting exposures to airborne PAHs in asthmatic children is especially important, as they experience greater health impacts from exposures to air pollutants.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grants P50ES015903 and P01ES018176; and Environmental Protection Agency Grants RD83451001 and RD83615201. Dr. Peters was supported by a Bloomberg School of Public Health Diversity and Health Disparities Fellowship, and a National Institute of Occupational Safety and Health, Education and Research Center Training Grant, Predoctoral Fellowship (T42-OH008428).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
References
- 1.Dubowsky SD, Wallace LA, Buckley TJ. The contribution of traffic to indoor concentrations of polycyclic aromatic hydrocarbons. J Expo Anal Environ Epidemiol. 1999;9:312–321. doi: 10.1038/sj.jea.7500034. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for the Research of Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to humans. [Accessed March 21 2014];Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. 2010 92 Available at: http://monographs.iarc.fr/ENG/Monographs/vol92/mono92.pdf. [PMC free article] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for polycyclic aromatic hydrocarbons. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1995. [Accessed 14 March 2014]. Available at: http://www.atsdr.cdc.gov/ToxProfiles/tp69.pdf. [PubMed] [Google Scholar]
- 4.Dimashki M, Lim LH, Harrison RM, Harrad S. Temporal trends, temperature dependence, and relative reactivity of atmospheric polycyclic aromatic hydrocarbons. Environ Sci Technol. 2001;35:2264–2267. doi: 10.1021/es000232y. [DOI] [PubMed] [Google Scholar]
- 5.Larsen RK, 3rd, Baker JE. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods. Environ Sci Technol. 2003;37:1873–1881. doi: 10.1021/es0206184. [DOI] [PubMed] [Google Scholar]
- 6.Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KH, Hsu SI, Yan B, Moors K, Chillrud SN, Ross J, et al. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic Biol Med. 2001;31:1132–1138. doi: 10.1016/s0891-5849(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 11.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 12.Wallace LA, Mitchell H, O'Connor GT, Neas L, Lippmann M, Kattan M, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111:1265–1272. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi H, Perera F, Pac A, Wang L, Flak E, Mroz E, et al. Estimating individual-level exposure to airborne polycyclic aromatic hydrocarbons throughout the gestational period based on personal, indoor, and outdoor monitoring. Environ Health Perspect. 2008;116:1509–1518. doi: 10.1289/ehp.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, et al. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20:775–782. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 16.Rosa MJ, Jung KH, Perzanowski MS, Kelvin EA, Darling KW, Camann DE, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med. 2011;105:869–876. doi: 10.1016/j.rmed.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect. 2004;112:754–759. doi: 10.1289/ehp.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchet JP, Gennart JP, Mercado-Calderon F, Delavignette JP, Cupers L, Lauwerys R. Evaluation of exposure to polycyclic aromatic hydrocarbons in a coke production and a graphite electrode manufacturing plant: assessment of urinary excretion of 1-hydroxypyrene as a biological indicator of exposure. Br J Ind Med. 1992;49:761–768. doi: 10.1136/oem.49.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strickland PT, Kang D, Bowman ED, Fitzwilliam A, Downing TE, Rothman N, et al. Identification of 1-hydroxypyrene glucuronide as a major pyrene metabolite in human urine by synchronous fluorescence spectroscopy and gas chromatography-mass spectrometry. Carcinogenesis. 1994;15:483–487. doi: 10.1093/carcin/15.3.483. [DOI] [PubMed] [Google Scholar]
- 20.Strickland P, Kang D. Urinary 1-hydroxypyrene and other PAH metabolites as biomarkers of exposure to environmental PAH in air particulate matter. Toxicol Lett. 1999;108:191–199. doi: 10.1016/s0378-4274(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 21.Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2001;45:3–13. [PubMed] [Google Scholar]
- 22.Hansen AM, Raaschou-Nielsen O, Knudsen LE. Urinary 1-hydroxypyrene in children living in city and rural residences in Denmark. Sci Total Environ. 2005;347:98–105. doi: 10.1016/j.scitotenv.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Tucek M, Maxa K, Tenglerova J, Weyand EH. A rapid and simple method for the analysis of 1-hydroxypyrene glucuronide: a potential biomarker for polycyclic aromatic hydrocarbon exposure. Carcinogenesis. 1995;16:2909–2915. doi: 10.1093/carcin/16.12.2909. [DOI] [PubMed] [Google Scholar]
- 24.Strickland P, Kang D, Sithisarankul P. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environ Health Perspect. 1996;104(Suppl 5):927–932. doi: 10.1289/ehp.96104s5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang D, Lee KH, Lee KM, Kwon HJ, Hong YC, Cho SH, et al. Design issues in cross-sectional biomarkers studies: urinary biomarkers of PAH exposure and oxidative stress. Mutat Res. 2005;592:138–146. doi: 10.1016/j.mrfmmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Kang D, Lee KH, Ichiba M, Zhang J, Tomokuni K, et al. Influence of GSTM1 genotype on association between aromatic DNA adducts and urinary PAH metabolites in incineration workers. Mutat Res. 2002;514:213–221. doi: 10.1016/s1383-5718(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Vermeulen R, Lenters V, Cho SH, Strickland PT, Kang D. Determinants of urinary 1-hydroxypyrene glucuronide in South Korean children. Int Arch Occup Environ Health. 2009;82:961–968. doi: 10.1007/s00420-008-0385-2. [DOI] [PubMed] [Google Scholar]
- 28.Chien YC, Yeh CT. Amounts and proportion of administered pyrene dose excreted as urinary 1-hydroxypyrene after dietary exposure to polycyclic aromatic hydrocarbons. Arch Toxicol. 2010;84:767–776. doi: 10.1007/s00204-010-0570-4. [DOI] [PubMed] [Google Scholar]
- 29.Deziel NC, Wei WQ, Abnet CC, Qiao YL, Sunderland D, Ren JS, et al. A multi-day environmental study of polycyclic aromatic hydrocarbon exposure in a high-risk region for esophageal cancer in China. J Expo Sci Environ Epidemiol. 2013;23:52–59. doi: 10.1038/jes.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai CH, Jaakkola JJ, Chuang CY, Liou SH, Lung SC, Loh CH, et al. Exposure to cooking oil fumes and oxidative damages: a longitudinal study in Chinese military cooks. J Expo Sci Environ Epidemiol. 2013;23:94–100. doi: 10.1038/jes.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prado GF, Zanetta DM, Arbex MA, Braga AL, Pereira LA, de Marchi MR, et al. Burnt sugarcane harvesting: particulate matter exposure and the effects on lung function, oxidative stress, and urinary 1-hydroxypyrene. Sci Total Environ. 2012;437:200–208. doi: 10.1016/j.scitotenv.2012.07.069. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Grainger J, Patterson DG, Jr, Turner WE, Caudill SP, Needham LL, et al. Comparison of 1-hydroxypyrene exposure in the US population with that in occupational exposure studies. Int Arch Occup Environ Health. 2004;77:491–498. doi: 10.1007/s00420-004-0529-y. [DOI] [PubMed] [Google Scholar]
- 33.(CDC) Centers for Disease Control and Prevention, Department of Health and Human Services. Third national report on human exposure to environmental chemicals [revised] 2005 [Google Scholar]
- 34.Sul D, Ahn R, Im H, Oh E, Kim JH, Kim JG, et al. Korea National Survey for Environmental Pollutants in the human body 2008: 1-hydroxypyrene, 2-naphthol, and cotinine in urine of the Korean population. Environ Res. 2012;118:25–30. doi: 10.1016/j.envres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Fiala Z, Vyskocil A, Krajak V, Viau C, Ettlerova E, Bukac J, et al. Environmental exposure of small children to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 2001;74:411–420. doi: 10.1007/s004200100239. [DOI] [PubMed] [Google Scholar]
- 36.Bostrom CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jongeneelen FJ. Biological monitoring of environmental exposure to polycyclic aromatic hydrocarbons; 1-hydroxypyrene in urine of people. Toxicol Lett. 1994;72:205–211. doi: 10.1016/0378-4274(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 38.van Wijnen JH, Slob R, Jongmans-Liedekerken G, van de Weerdt RH, Woudenberg F. Exposure to polycyclic aromatic hydrocarbons among Dutch children. Environ Health Perspect. 1996;104:530–534. doi: 10.1289/ehp.96104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siwinska E, Mielzynska D, Bubak A, Smolik E. The effect of coal stoves and environmental tobacco smoke on the level of urinary 1-hydroxypyrene. Mutat Res. 1999;445:147–153. doi: 10.1016/s1383-5718(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 40.Mucha AP, Hryhorczuk D, Serdyuk A, Nakonechny J, Zvinchuk A, Erdal S, et al. Urinary 1-hydroxypyrene as a biomarker of PAH exposure in 3-year-old Ukrainian children. Environ Health Perspect. 2006;114:603–609. doi: 10.1289/ehp.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuntawiroon J, Mahidol C, Navasumrit P, Autrup H, Ruchirawat M. Increased health risk in Bangkok children exposed to polycyclic aromatic hydrocarbons from traffic-related sources. Carcinogenesis. 2007;28:816–822. doi: 10.1093/carcin/bgl175. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Salinas RI, Elena Leal M, Batres-Esquivel LE, Domínguez-Cortinas G, Calderón J, Díaz-Barriga F, et al. Exposure of children to polycyclic aromatic hydrocarbons in Mexico: assessment of multiple sources. Int Arch Occup Environ Health. 2010;83:617–623. doi: 10.1007/s00420-009-0482-x. [DOI] [PubMed] [Google Scholar]
- 43.Fan R, Wang D, Mao C, Ou S, Lian Z, Huang S, et al. Preliminary study of children’s exposure to PAHs and its association with 8-hydroxy-2’-deoxyguanosine in Guangzhou, China. Environ Int. 2012;42:53–58. doi: 10.1016/j.envint.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect. 2010;118:579–583. doi: 10.1289/ehp.0901077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perera FP, Illman SM, Kinney PL, Whyatt RM, Kelvin EA, Shepard P, et al. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98:167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. 2007;84:577–590. doi: 10.1007/s11524-007-9205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117:294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miranda ML, Edwards SE, Keating MH, Paul CJ. Making the environmental justice grade: the relative burden of air pollution exposure in the United States. Int J Environ Res Public Health. 2011;8:1755–1771. doi: 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116:1428–1432. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butz AM, Halterman JS, Bellin M, Tsoukleris M, Donithan M, Kub J, et al. Factors associated with second-hand smoke exposure in young inner-city children with asthma. J Asthma. 2011;48:449–457. doi: 10.3109/02770903.2011.576742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansel NN, Matsui EC, Rusher R, McCormack MC, Curtin-Brosnan J, Peng RD, et al. Predicting future asthma morbidity in preschool inner-city children. J Asthma. 2011;48:797–803. doi: 10.3109/02770903.2011.604887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsui EC, Hansel NN, Aloe C, Schiltz AM, Peng RD, Rabinovitch N, et al. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. Am J Respir Crit Care Med. 2013;188:1210–1215. doi: 10.1164/rccm.201305-0889OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung KH, Patel MM, Moors K, Kinney PL, Chillrud SN, Whyatt R, et al. Effects of heating season on residential indoor and outdoor polycyclic aromatic hydrocarbons, black carbon, and particulate matter in an urban birth cohort. Atmos Environ. 2010;44:4545–4552. doi: 10.1016/j.atmosenv.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JH, Kim JK, Son BK, Oh JE, Lim DH, Lee KH, et al. Effects of air pollutants on childhood asthma. Yonsei Med J. 2005;46(2):239–244. doi: 10.3349/ymj.2005.46.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu SW, Chan YJ, Hsu HT, Wu KY, Chang Chien GP, Shie RH, et al. Urinary levels of 1-hydroxypyrene in children residing near a coal-fired power plant. Environ Res. 2011;111:1185–1191. doi: 10.1016/j.envres.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Kang JW, Cho SH, Kim H, Lee CH. Correlation of urinary 1-hydroxypyrene and 2-naphthol with total suspended particulates in ambient air in municipal middle-school students in Korea. Arch Environ Health. 2002;57:377–382. doi: 10.1080/00039890209601425. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention (CDC) Vital signs: nonsmokers’ exposure to secondhand smoke--United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59:6. [PubMed] [Google Scholar]
- 60.Suwan-ampai P, Navas-Acien A, Strickland PT, Agnew J. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol Biomark Prev. 2009;18:884–893. doi: 10.1158/1055-9965.EPI-08-0939. [DOI] [PubMed] [Google Scholar]
- 61.Yoon HS, Lee KM, Lee KH, Kim S, Choi K, Kang D. Polycyclic aromatic hydrocarbon (1-OHPG and 2-naphthol) and oxidative stress (malondialdehyde) biomarkers in urine among Korean adults and children. Int J Hyg Environ Health. 2012;215:458–464. doi: 10.1016/j.ijheh.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 62.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106:148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srogi K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett. 2007;5:35. doi: 10.1007/s10311-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vyskocil A, Fiala Z, Chenier VV, Krajak L, Ettlerova E, Bukac J, et al. Assessment of multipathway exposure of small children to PAH. Environ Toxicol Pharmacol. 2000;8:111–118. doi: 10.1016/s1382-6689(00)00032-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.