Supplemental Digital Content is available in the text.
Keywords: test negative design, respiratory syncytial virus (RSV), chronic lung disease of prematurity, prematurity, case–control study
Abstract
Background:
Infants with premature birth ≤35 weeks gestational age, chronic lung disease of prematurity and congenital heart disease are at an increased risk for lower respiratory tract infections and hospitalization from respiratory syncytial virus (RSV), which has been shown in randomized trials to be prevented by palivizumab. However, palivizumab effectiveness (PE) has not been studied in a large clinical setting.
Methods:
A multicenter study among high-risk US and Canadian children younger than 24 months hospitalized with lower respiratory tract infection and whose nasopharyngeal aspirates were tested for human metapneumovirus (HMPV) and RSV were the subjects of the trial. We conducted a test-negative case–control study in these subjects to determine PE. We used an inverse propensity score weighted (IPSW) multiple logistic regression model to adjust PE.
Results:
Palivizumab was used in 434 (51%) of 849 eligible children. RSV was identified in 403 (47%) children. The unadjusted PE was 43% [95% confidence interval (CI), 34%–51%)]. After IPSW adjustment, the adjusted PE was 58% (95% CI, 43%–69%). Palivizumab prevented intensive care unit admissions (PE, 62%; 95% CI, 35%–78%). PE for 29–35 weeks gestational age and ≤6 months of chronologic age without chronic lung disease of prematurity or congenital heart disease was 74% (95% CI, 56%–85%).
Conclusions:
Using a test-negative case–control design with RSV molecular detection, palivizumab is shown to prevent RSV hospitalizations and intensive care unit admissions in high-risk infants.
Respiratory syncytial virus (RSV) has been estimated to cause between 50,000 and 125,000 annual hospitalizations of US children younger than 5 years.1,2 Risk factors for developing severe RSV disease [hospitalization, intensive care unit (ICU) admission] in young children include preterm birth ≤35 weeks gestational age (wGA), chronic lung disease of prematurity (CLD), hemodynamically significant congenital heart disease (hsCHD) and young chronologic age (<6 months).3–6 Daycare attendance and young siblings are additional risk factors in 32–35 wGA infants.7–10 Palivizumab is an RSV-specific monoclonal antibody licensed for the prevention of serious lower respiratory tract infection (LRTI) caused by RSV in high-risk children. Several prospective clinical trials have demonstrated an efficacy of 45%–82% against RSV-related hospitalizations in high-risk infants.7,11,12 However, the effectiveness of palivizumab in clinical practice using a test-negative controlled study design has not been conducted.
In 2014, the American Academy of Pediatrics recommended restriction of palivizumab prophylaxis to infants born at <29 weeks and 0 days of gestation or those with hsCHD, CLD or other high-risk conditions.13 This recommendation was based on an assessment that in preterm infants 29–35 weeks gestation, palivizumab has “limited effect on RSV hospitalizations on a population basis, no measurable effect on mortality and a minimal effect on subsequent wheezing.”13 This change from previous recommendations14 leaves many of these at-risk premature infants and children without an option for RSV prevention.15
Vaccine effectiveness (VE) studies based on polymerase chain reaction (PCR)-confirmed infections and vaccination history are essential for evaluating how well a vaccine works in preventing disease outside the tightly controlled setting of a randomized controlled clinical trial. These studies provide important information on the public health value of vaccines and help in developing vaccination policies. Test-negative controls in acute respiratory VE studies are used to control for significant differences that might exist due to access to care.16–19 Because palivizumab effectiveness (PE) has not been evaluated in clinical practice, we used a rigorous test-negative study design from a multicenter study conducted to determine the prevalence of respiratory pathogens to answer this question.20 We enrolled children younger than 24 months at high risk for severe respiratory disease who were hospitalized with acute LRTI between 2002 and 2006. As a part of this study, PCR for RSV was performed, and epidemiologic and clinical data, including administration of palivizumab within the preceding 30 days, were collected. We analyzed the data collected during this study to determine PE in preventing RSV hospitalizations. To address potential confounding and bias,21 we used an IPSW, test-negative case–control study design to estimate the protective effect of palivizumab.
MATERIALS AND METHODS
Study Design
A large, prospective, international, multicenter study was conducted between 2002 and 2006 to determine the prevalence of human metapneumovirus (HMPV) in high-risk children younger than 24 months during the fall to spring seasons at 24 sites in the northern hemisphere (the United States, Canada, Italy and the Netherlands) and at 3 sites in the southern hemisphere (Australia).20 Local institutional review boards approved the study before subject enrollment. Eligibility included children 12 months of age or younger who were born prematurely (<36 weeks gestation), children younger than 24 months of age with hsCHD and/or CLD of prematurity who required medical intervention (eg, supplemental oxygen, corticosteroids, bronchodilators or diuretics within the previous 6 months) who were hospitalized with acute LRTI (eg, bronchiolitis, bronchitis, pneumonia or cardiac decompensation associated with respiratory infection). Demographic data, medical history, use of certain medications and use of palivizumab within 30 days of enrollment were recorded. Routine PCR testing for respiratory viruses (eg, HMPV, RSV, parainfluenza and influenza) was performed. This study enrolled 1,126 children across all the study sites, detailed elsewhere.20
We compared the odds of palivizumab administration within the preceding 30 days between RSV cases and controls. Cases were defined as subjects hospitalized with LRTI whose nasopharyngeal aspirates or endotracheal aspirates collected within 2 days of admission tested positive for RSV by reverse transcription (RT)-PCR. Controls were defined as subjects hospitalized for LRTI who tested negative for RSV. For the analyses of the subgroups of patients (1) treated in the ICU and (2) requiring mechanical ventilation (MV), cases and controls were defined as described earlier and analyzed within their respective subgroups.
Because the decision to hospitalize and the use of palivizumab differ substantially between countries and because we were primarily interested in evaluating palivizumab administration during high-risk RSV periods, we limited the analysis to subjects enrolled in the United States and Canada from November 1 to April 30.
Laboratory Methods
Nasopharyngeal aspirates or endotracheal aspirates were obtained from subjects on enrollment, and endotracheal aspirates were collected within 2 days of initiation of MV. Samples were stabilized in viral transport medium and frozen before being tested at Focus Diagnostics, Inc. (Cypress, CA). A multiplex RT-PCR enzyme hybridization assay (Hexaplex; Prodesse, Inc., Waukesha, WI) was used for detection of RSV A and B; influenza virus A and B and parainfluenza virus type 1, 2 and 3.20 Samples with a negative RSV result were retested for RSV B at Cogenics, Inc. (Houston, TX) to optimize recovery. HMPV was detected by a real-time RT-PCR assay.20
Statistical Analysis
Univariate Analysis
Univariate analysis was used to identify factors that could confound the relationship between palivizumab administration and RSV outcome. Categorical variables were summarized by the number and percentage of subjects in each category. Continuous variables were summarized by descriptive statistics including mean, standard deviation, median and range. Mean values were compared using Student’s t test only when test assumptions were met. Otherwise the median values were compared using the Wilcoxon Rank-Sum test. Proportions between 2 groups were compared using Fisher’s exact test, and proportions between more than 2 groups were compared using χ2 test. All statistical analyses were conducted using SAS v.9.4 (SAS Institute, Inc., Cary, NC).
To identify potential confounding factors, we examined statistical associations between potential confounders and palivizumab administration. Potential confounders included age, sex, race/ethnicity, wGA, birthweight, single versus multiple birth, family composition (eg, presence and number of children <6, 6–12 and >12–18 years), daycare attendance, breastfeeding, maternal education level, maternal age, maternal and paternal employment, family atopy status, history and duration of neonatal ICU (NICU) admission, other medical conditions, household smoking, timing of admission relative to disease onset, timing from beginning of the RSV season to admission (as the disease risk may vary during the season) and the year.
Propensity Score
Given that subjects who are treated with palivizumab are more likely to have a number of risk factors (eg, earlier gestational age, exposure to daycare, hsCHD) known to be associated with hospitalization, we planned to reduce selection biases by developing an inverse propensity score weight (IPSW) using the significant (P < 0.05) risk factors associated with palivizumab administration.22 Proc general linear model (GLM; SAS v.9.4) was used to compare the palivizumab group with the control group to identify covariate significance before adjustment. We then created the propensity scores using Proc Logistic with these covariates, followed by taking the inverse of the propensity score to derive the weight.23 Proc GLM was run on the adjusted (IPSW) values to ensure that the treatment and control groups were balanced. Finally, we performed an IPSW multiple logistic regression model to adjust PE. In addition to significance level, we used the Hosmer and Lemeshow goodness-of-fit test to select the best predictive model.24
Multivariate Analysis
We compared the odds of palivizumab administration within the 30 days before disease onset between RSV cases and controls using a multiple logistic regression (SAS Proc Logistic) model, to calculate an adjusted odds ratio, using the IPSW. RSV PE (%) was estimated as (1 − adjusted odds ratio) × 100.18 Outcomes were assessed related to the PE in prevention of ICU admissions and MV after adjustment with an IPSW multiple logistic regression model. The ideal model was considered to have palivizumab treatment status as the only predictor and was weighted by the IPSW, to predict RSV outcome. However, if the IPSW adjustment was insufficient to remove all potential confounding, then other significant predictors were included in the final model. When there was colinearity evident among significant predictors, the predictor with a lesser significant effect was removed from the model.
Sensitivity analyses included restriction to cases hospitalized within 7 days of symptom onset, to address concerns that RSV may be a concurrent infection and not the cause of LRTI in subjects with a longer duration of symptoms before presentation. An additional sensitivity analysis was performed among those who tested negative for RSV but positive for HMPV to assess for the specificity of the effect of PE.
Subgroups were analyzed [preterm infants without hsCHD or CLD with separate analysis for infants <3 versus 3 to <6 months chronologic age overall, <29 wGA and 29–35 wGA for infants < 6 months, children with hsCHD overall (separate analyses of those with chronologic age <12 months on November 1 vs. age of 12 to <24 months on November 1), children with CLD overall (separate analyses of those with chronologic age <12 months on November 1 vs. 12 to <24 months on November 1)]. Interactions between subgroup and palivizumab administration were examined.
RESULTS
Of 1162 children enrolled in the original study, 849 (73.1%) met inclusion criteria for this analysis (Fig. 1). The most common reasons for exclusion were residence in the Southern hemisphere (165, 14.2%) and enrollment outside the RSV season (93, 8.0%). Overall, 58.4% were male, 45.8% were white, 42.7% had a gestational age between 32 and <36 weeks, 33.7% had CLD (without hsCHD), 18.1% had hsCHD and 83.0% (656/790 with known data) had a previous NICU admission. The subjects’ baseline demographics and medical history are presented in Table, Supplemental Digital Content 1, http://links.lww.com/INF/C674.
FIGURE 1.

Flow of study participants.
Two subjects, 1 in the treatment and 1 in the control, missing gestational age were excluded from the GLM analysis of potential confounders and subsequent analyses (Table 1). Compared with the 414 (48.9%) participants who did not receive palivizumab, the 433 (51.1%) participants who had received palivizumab were more likely to have medical and social risk factors for RSV infection. They were more often white than Hispanic, were older (median age, 6.2 vs. 3.8 months, P < 0.0001), had an earlier gestational age (median, 29 vs. 34 weeks, P < 0.0001), had lower birthweight (median, 1300 vs. 2150 g, P < 0.0001), were more frequently admitted to the NICU at birth, had a longer NICU stay (median, 64 vs. 18 days, P < 0.0001), had more chronic medical conditions (75.6% vs. 36.6%, P < 0.0001), were less frequently breastfed (10.6% vs. 17.6%, P = 0.0026) and had older and more educated mothers. Despite these significant potential confounders, the unadjusted PE was 43.3% [95% confidence interval (CI), 34.1%–51.2%] and did not differ when limiting the cases to hospitalization <7 days after symptom onset [44.3% (95% CI, 35.1%–52.3%)].
TABLE 1.
Unadjusted and Adjusted Demographic and Baseline Measure Results for GLM of Potential Confounding Factors Association with Palivizumab Treatment
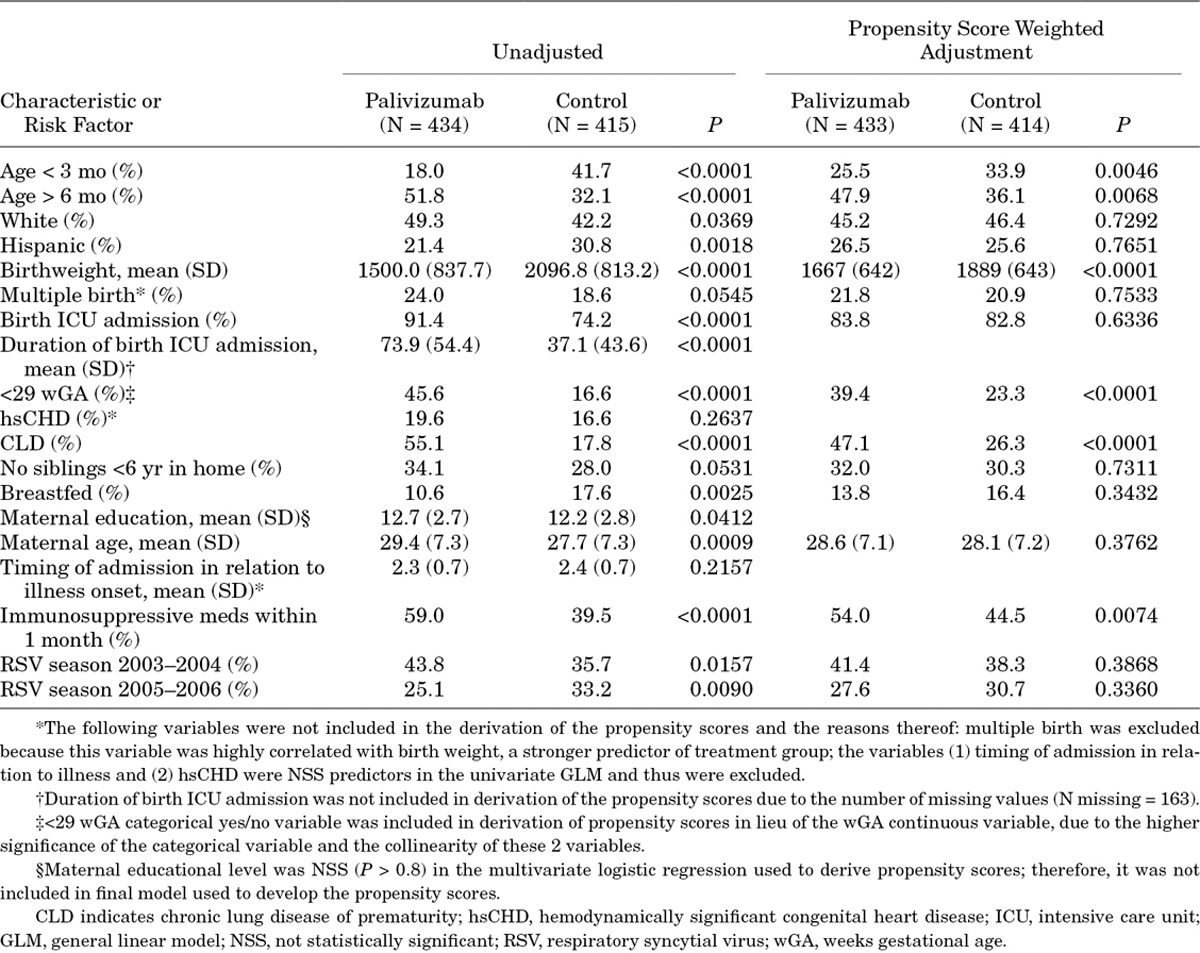
After IPSW adjustment, the differences between the groups resolved except for the percentage of children who were older than 6 months (Table 2). PE was determined to be 58% (95% CI, 43.1%–69%) with a Hosmer and Lemeshow goodness-of-fit test P value of 0.34 (Table 2), indicating an adequate fit of the data to the model. Subgroup analyses of PE are shown in Figure 2. The point estimates and 95% CI in many of the subgroups were wide because of small numbers, particularly in those <29 wGA and in children with hsCHD. The PE for prevention of ICU admissions was 62.1% (95% CI, 35.1%–77.9%; Fig. 2), but PE was not observed in the MV subgroup (31.5%; 95% CI, −41.2%–66.8%). PE for children born at 29–35 wGA and <6 months of chronologic age was statistically significant at 74.1% (95% CI, 56.2%–84.7%) but not in those <29 wGA and <6 months of chronologic age. Palivizumab had no significant effectiveness against HMPV hospitalization [34.7% (95% CI, −12.9% to 62.2%)].
TABLE 2.
Propensity Score Weighted Logistic Regression Models to Compute Adjusted PE for RSV Outcome*
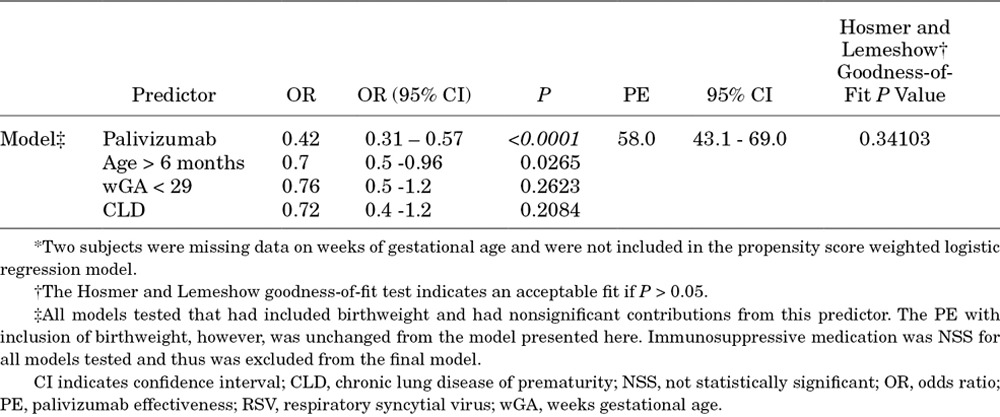
FIGURE 2.

Adjusted palivizumab effectiveness derived using the IPSW. CLD indicates chronic lung disease of prematurity; hsCHD, hemodynamically significant congenital heart disease; ICU, intensive care unit; IPSW, inverse propensity score weight; LCL%, lower confidence limit percent; mo, month; MV, mechanical ventilation; PE, Palivizumab effectiveness; RSV, respiratory syncytial virus; UCL%, upper confidence limit percent; wGA, weeks of gestational age.
DISCUSSION
Children who receive palivizumab have more medical and social risk factors for RSV-related hospitalization than those who do not,25,26 an expected bias amply illustrated in our study (see Table 1 and Table, Supplemental Digital Content 1, http://links.lww.com/INF/C674). Without any attempt to adjust for these potential confounders, the unadjusted PE was 43.3% (95% CI, 34.1%–51.2%) in the prevention of RSV-related hospitalizations. Given the expected imbalance in risk factors in the group receiving palivizumab prophylaxis, we planned a priori to adjust for potential biases between the groups, in PE estimates using IPSW multiple logistic regression. The final model accounted for these potential confounders except for a residual chronologic age-related difference (Table 2) and showed a PE of 58% (95% CI, 43%–69%). Three previous clinical trials have evaluated palivizumab efficacy. The IMpact-RSV trial demonstrated an RSV hospitalization rate of 10.6% in those who received placebo versus 4.8% among high-risk infants who received palivizumab (55% relative reduction, P < 0.001); in this trial, efficacy estimates were higher (78%) in preterm infants (32–35 wGA) without CLD (8.1% vs. 1.8%).11 Gestational age did not predict RSV hospitalization in the logistic regression analysis, and the palivizumab effect remained significant (P < 0.001). A second randomized, double-blind, placebo controlled trial in 1287 children with hsCHD demonstrated an RSV hospitalization rate of 9.7% in those who received placebo versus 5.3% among recipients of palivizumab (45% relative reduction, P < 0.003).12 Finally, a Dutch randomized trial in preterm infants 33–35 wGA demonstrated 82% efficacy against RSV hospitalization (5.1% placebo vs. 0.9% palivizumab, P = 0.01) as well as 80% efficacy against nonhospitalized medically attended RSV disease (4.7% placebo vs. 0.9% palivizumab, P = 0.02).7 Our estimated PE of 58% (95% CI, 43.1–69%) closely approximated that observed in prospective clinical trials of palivizumab efficacy in prevention of RSV-related hospitalizations. This assuages concerns of the generalizability of randomized controlled trial findings to the real world of palivizumab use.13
A recent retrospective analysis using Medicaid administrative claims data in children 29–32 wGA found reduced RSV hospitalization rates in subjects who received palivizumab (3.1% vs. 5.0%, P = 0.04).27 Of interest, in children who received palivizumab had increased hospitalizations for bronchiolitis without an RSV diagnosis (3.3% vs. 1.9%, P = 0.05). These findings are similar to our original, unadjusted data in which the unadjusted PE was 43.3% (95% CI, 34.1%–51.2%) and children infected with HMPV were more likely to have received palivizumab in the month before admission (P < 0.001).20 This study was a database analysis that did not account for known RSV risk factors (eg, demographic, medical, social data) differences between children who receive palivizumab and those who do not (indication bias). Given the very low rate of palivizumab use in the 33–36 wGA group (3.7%) presumably those recipients would have been those most likely to be hospitalized. Not correcting for these biases was a serious flaw in the study analysis.
In contrast, we used several techniques to try and minimize bias, such as limiting the population to “at-risk” children with indications for palivizumab prophylaxis, having a very specific outcome (laboratory-confirmed RSV hospitalizations), limiting analyses to the “RSV season” (November to April), limiting the data to the United States and Canada and using IPSW adjustment and multivariable logistic regression modeling in an attempt to adjust for the inevitable confounding by indication. We also performed 2 sensitivity analyses to test for potential biases: (1) restricting the definition of a “case” to those children with symptoms of <8 days duration and (2) limiting the analysis to children who tested negative for RSV, but positive for HMPV to assess for the specificity of the effect of PE.
Test-negative controls have been used to estimate VE theoretically and also through retrospective reanalysis of prospectively collected data of vaccine efficacy from randomized controlled trials.16–19 Importantly, a retrospective reanalysis of data from IMpact-RSV and the congenital heart disease trial of palivizumab found that PE with a test-negative design (with censoring) approximated the vaccine efficacy identified by the randomized controlled trial.19 Application of this methodology to PE is thus reasonable. Details on the verification of palivizumab administration are limited, which could result in exposure misclassification, but this likely would result in a bias toward the null (eg, parents remembering palivizumab receipt but this having occurred >30 days before hospitalization, or parents not remembering palivizumab receipt). Importantly, children were enrolled into this study from 2002 to 2006, and molecular results were not available to study teams enrolling these children. Because clinical diagnostics at the time were primarily rapid antigen tests, shell vial culture and viral culture, it is unlikely that bias was introduced by clinical test results because of the lack of availability in real time.
We also found that palivizumab was 62.1% effective in prevention of RSV-related ICU admission (95% CI, 35.1%–77.9%), confirming the 57% efficacy observed in the IMpact-RSV trial (P = 0.026).11 Notably, PE was similar among important subgroups such as the 29–35 wGA infants <6 months of chronologic age without underlying hsCHD or CLD and all infants <6 months of chronologic age without underlying hsCHD or CLD. To assess for study bias, we evaluated PE against HMPV because palivizumab should have no activity against HMPV. We did not identify statistically significant PE against HMPV in either the unadjusted or the adjusted analyses. The inclusion of an analysis of PE versus HMPV was done to help provide reassurance that residual confounding was not overlooked and that the effect we measured was specific for RSV. Had the effect we demonstrated been due to uncorrected residual confounding, one would have expected to see the same or slightly lower efficacy against a nonspecific endpoint (in this case HMPV hospitalization). With the sample size (N = 437: 84 HMVP+ and 353 HMPV−) used in the HMPV analysis, we would have nearly 100% power to detect the 58% efficacy against RSV hospitalization using α = 0.05. Further, we would have 83% power to detect an efficacy of 37%. This suggests that the PE that we found was specific to RSV and not because of study biases.
Our study has some limitations. Although we attempted to control during the analysis for the substantial baseline differences that existed in those who received versus those who did not receive palivizumab, residual confounding still exists. Data on some potential risk factors (eg, birthweight, previous NICU admission, breastfeeding) were not collected on children initially enrolled into the study. Point estimates for many of the subgroups (eg, CLD, hsCHD, <29 wGA and <6 months chronologic age) were imprecise with wide CIs because of small numbers of children. Because limited data were collected on CLD and hsCHD severity, additional unresolved bias in palivizumab administration might exist within these cohorts. It is also possible that some infants were misclassified as having hsCHD because of lack of a standard definition. Because of concerns about differences in the use of palivizumab, differences in hospitalization and the small number of children enrolled from non-US and non-Canada sites, we limited our analysis to only US and Canadian sites. This could reduce the generalizability of these data. Finally, we could not assess for impact of palivizumab administration beyond the hospitalization (eg, recurrent wheezing) that others have described.7
Our data should be seriously considered by the United States and global decision makers as they consider the role of palivizumab in the prevention of RSV-related hospitalizations in premature and other high-risk infants and young children. The American Academy of Pediatrics now recommends against universal palivizumab administration in prematures born at ≥29 weeks of gestation unless they have congenital heart disease, CLD or another condition.13 The rationale for this recommendation was partly related to an assessment that palivizumab had limited effect on RSV hospitalizations on a population basis. Pediatric societies in several other countries have similarly revised recommendations toward more restrictive use of palivizumab in preterm children.28–30 In contrast, we found that palivizumab had a 58% (95% CI, 43.1%–69%) effectiveness in the prevention of RSV-related hospitalizations after adjusting PE for underlying differences that exist in those who receive palivizumab. Importantly, the point estimate of PE for those 29–35 wGA and ≤6 months of chronologic age without hsCHD or CLD was 74.1% (95% CI, 56.2%–84.7%). We also identified that palivizumab provided protection against ICU admissions. Recent data suggest that 29–32 wGA infants who have not received palivizumab prophylaxis are at particularly a heightened risk of RSV-related hospitalizations, ICU admissions and MV in comparison to 35wGA infants, particularly at younger chronologic ages.31 In addition, the median cost of hospitalization was $27,461, but was substantially higher in those requiring ICU admission and MV.31 Because we were able to demonstrate the effectiveness of palivizumab in the prevention of RSV-related hospitalizations in premature infants without CLD or hsCHD of these gestational ages, we urge that groups reconsider implementation of restrictive policies on palivizimab use in these infants, particularly given the substantial morbidity associated with RSV hospitalizations.
In conclusion, we analyzed PE against PCR-diagnosed RSV LRTI hospitalizations using a test-negative case–controlled study, the preferred design for such studies.16–18 We found an unadjusted PE of 43.3% (95% CI, 34.1%–51.2%) in the prevention of RSV-related hospitalizations in these high-risk infants. Performing IPSW adjustment and multivariable logistic regression modeling achieved balancing between the groups on all risk factors (except for chronologic age). This resulted in an estimated PE of 58% (95% CI, 43.1%–69%). We also identified PE in several important subgroups (eg, 29–35 wGA and ≤6 months of chronologic age without underlying hsCHD or CLD) and in prevention of ICU admissions. Our data suggest that PE in real life is similar to the efficacy observed in prospective clinical trials.
Supplementary Material
Footnotes
The original HMPV study was conducted between December 2002 and June 2006 and sponsored by MedImmune. As investigators in the HMPV study, the authors received access to the original study data. The study was designed and data were analyzed and interpreted by the authors. The protocol, analysis, results, and conclusions presented here are solely those of the named authors. No assistance was provided by MedImmune.
E.J.A., E.A.F.S. and R.Y. received financial remuneration to their institutions for the original conduct of this study and for ongoing clinical trial work on other studies from MedImmune and/or AstraZeneca. E.J.A. has served as a consultant for Abbvie regarding RSV. E.J.A. has received financial remuneration to his institution for an unrelated clinical trial. This work was unfunded.
Presented in part at IDWeek 2015; October 7–11, 2015; San Diego, CA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pidj.com).
REFERENCES
- 1.Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among U.S. children, 1980–1995. JAMA. 1999;282:1440–1447.. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce TG, Mellen BG, Mitchel EF, Jr, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137:865–870.. [DOI] [PubMed] [Google Scholar]
- 4.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143(5 suppl):S133–S141.. [DOI] [PubMed] [Google Scholar]
- 5.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30:510–517.. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348.. [DOI] [PubMed] [Google Scholar]
- 7.Blanken MO, Rovers MM, Molenaar JM, et al. ; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799.. [DOI] [PubMed] [Google Scholar]
- 8.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. ; IRIS Study Group. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27:788–793.. [DOI] [PubMed] [Google Scholar]
- 9.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23:806–814.. [DOI] [PubMed] [Google Scholar]
- 10.Ambrose CS, Anderson EJ, Simões EA, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J. 2014;33:576–582.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537.. [PubMed] [Google Scholar]
- 12.Feltes TF, Cabalka AK, Meissner HC, et al. ; Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540.. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420.. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Infectious Diseases. From the American Academy of Pediatrics: policy statements—modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701.. [DOI] [PubMed] [Google Scholar]
- 15.Yogev R, Krilov LR, Fergie JE, et al. Re-evaluating the new committee on infectious diseases recommendations for palivizumab use in premature infants. Pediatr Infect Dis J. 2015;34:958–960.. [DOI] [PubMed] [Google Scholar]
- 16.Foppa IM, Haber M, Ferdinands JM, et al. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31:3104–3109.. [DOI] [PubMed] [Google Scholar]
- 17.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–2168.. [DOI] [PubMed] [Google Scholar]
- 18.Orenstein EW, De Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–631.. [DOI] [PubMed] [Google Scholar]
- 19.De Serres G, Skowronski DM, Wu XW, et al. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013:18. [DOI] [PubMed] [Google Scholar]
- 20.Anderson EJ, Simões EA, Buttery JP, et al. Prevalence and characteristics of human metapneumovirus infection among hospitalized children at high risk for severe lower respiratory tract infection. J Pediatric Infect Dis Soc. 2012;1:212–222.. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281.. [DOI] [PubMed] [Google Scholar]
- 23.Leslie S, Thiebaud P. Using Propensity Scores to Adjust For Treatment Selection Bias 2007. Available at: http://www2.sas.com/proceedings/forum2007/184–2007.pdf Accessed January 6, 2016.
- 24.Hosmer DWJ, Lemeshow S. Applied Logistic Regression. 20002nd ed New York, NY: John Wiley & Sons. [Google Scholar]
- 25.Chan P, Li A, Paes B, et al. ; CARESS Investigators. Adherence to palivizumab for respiratory syncytial virus prevention in the Canadian Registry of Palivizumab. Pediatr Infect Dis J. 2015;34:e290–e297.. [DOI] [PubMed] [Google Scholar]
- 26.Escobar GJ, Gebretsadik T, Carroll K, et al. Adherence to immunoprophylaxis regimens for respiratory syncytial virus infection in insured and Medicaid populations. J Pediatric Infect Dis Soc. 2013;2:205–214.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farber HJ, Buckwold FJ, Lachman B, et al. Observed effectiveness of palivizumab for 29-36-week gestation infants. Pediatrics. 2016;138:e20160627. [DOI] [PubMed] [Google Scholar]
- 28.Bollani L, Baraldi E, Chirico G, et al. ; Italian Society of Neonatology. Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV). Ital J Pediatr. 2015;41:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson JL, Le Saux N; Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing hospitalizations for respiratory syncytial virus infection. Paediatr Child Health. 2015;20:321–333.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Aql F, Al-Hajjar S, Bin Mahmoud L, et al. Guidelines for palivizumab prophylaxis in infants and young children at increased risk for respiratory syncytial virus infection in Saudi Arabia. Int J Pediatr Adolesc Med. 2016;3:38–42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson EJ, Krilov LR, DeVincenzo JP, et al. SENTINEL1: an observational study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks’ gestational age not receiving immunoprophylaxis. Am J Perinatol. 2017;34:51–61.. [DOI] [PubMed] [Google Scholar]


