Abstract
Background
Renal cell carcinoma (RCC) is among the most common malignant cancers of males worldwide. For advanced RCC patients, there still is no effective therapy. Immune checkpoint blockade therapies have shown benefits for many cancers, but previous clinical trials of immune checkpoint blockade therapies in RCC patients achieved only modest results.
Material/Methods
We explored the effects of combining chemotherapy with immune checkpoint blockade therapy in RCC xenograft mouse models. We also studied the potential mechanisms by which chemotherapy might enhance the efficacy of immune checkpoint blockade therapy, both in vitro and in vivo.
Results
Our results showed that many commonly used chemotherapy agents can induce immunogenic marker release in RCC cell lines. Importantly, the RCC xenograft mouse model mice who received the combination treatment of 5-fluorouracil (5-FU) and anti-programmed cell death-ligand 1 (PD-L1) antibodies (Abs) had longer survival times compared to those who received 5-FU or anti-PD-L1 Abs alone. Also, increased key cytokines that promote tumor immunity, such as IL-2, IFN-γ, and TNF-α, as well as tumor-infiltrating cytotoxic T cells, were also increased after the combination treatment.
Conclusions
We conclude that 5-FU can sensitize RCC to anti-PD-L1 treatment by releasing the immune suppression in the tumor microenvironment.
MeSH Keywords: Antineoplastic Agents, Drug Resistance, Kidney Neoplasms, Tumor Escape
Background
Renal cell carcinoma (RCC) is the ninth most common malignant cancer in males worldwide [1]. The incidence of RCC usually varies geographically and developed countries tend to have higher incidence [2]. Most RCC patients are at early stages (localized diseases), and about one-third of RCC patients are at advanced stages with metastasis [3]. Although surgical strategies are still major therapeutic methods for RCC patients, they are not effective for advanced RCC patients. In recent decades, many new treatments have been developed for RCC, such as targeted therapies and novel chemotherapeutic strategies [4,5]. However, the median survival of RCC patients with 1 or more adverse features, such as hypercalcemia and anemia, is still less than 3 years [6,7]. Thus, new treatments need to the be developed for RCC patients.
Immune checkpoint blockade molecules are a class of proteins that negatively regulate the immune response at multiple levels [8,9]. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein1 (PD-1), and programmed death-ligand 1 (PD-L1) are representatives. Under some pathophysiological circumstance, such as a tumor microenvironment that is beneficial for tumor development, immune checkpoints are highly expressed to suppress anti-tumor immunity [8–12]. Therefore, immune checkpoint blockade therapies have been developed and are effective in treating several cancers. However, previous studies of immune checkpoint blockade therapies in RCC showed limited objective response and activity [7,13]. Therefore, there is a great need to increase the sensitivity of RCC patients to immune checkpoint blockade therapy.
Considering that effective anti-tumor immunity relies on sufficient antigen exposure and a positive immune-regulating microenvironment, which can be achieved by chemotherapies, we hypothesized that certain chemotherapies might promote the anti-tumor immunity and synergize immune checkpoint blockade therapies in RCC. Here, we explored the effects of commonly used chemotherapies and the anti-PD-L1 combination treatment in RCC preclinical models.
Material and Methods
Cell culture
Murine RCC cell line Renca (ATCC® CRL-2947™) and human RCC cell line 769-P (ATCC® CRL-1933™) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, non-essential amino acids (NEAA) (0.1 mM extra only for Renca), 100 U/mL penicillin, and 100 μg/mL streptomycin. Caki-1 cell line (ATCC® HTB-46™) was cultured in McCoy’s 5a medium modified by adding 10% fetal bovine serum. All cells were maintained in an incubator with 95% humidified atmosphere and 5% CO2 at 37°C. Subculture was performed when the cells were 80–100% confluent. All the cells were purchased from ATCC (VA, USA). Different treatment groups contained: saline, cyclophosphamide (Cytoxan, 200 μM), 5-fluorouracil (5-FU, 15 μM), and paclitaxel (8 nM). Each drug was added to incubate for 24 h.
Fluorescence-activated cell sorter analysis
Fluorescence-activated cell sorter (FACS) analysis was performed to measure the number of CD8+ cells and CD11b+Ly6G+Ly6Clow myeloid-derived suppressor cells (MDSC) from the RCC tumor tissue of animal models. Fluorescence-labeled primary antibodies (Abs), including anti-CD8, anti-CD11b, anti-Ly6G, and anti-Ly6C Abs, were purchased from Biolegend. Tumor tissues were chopped and minced to small pieces and disassociated into suspended single cells by collagenase type I. Primary antibodies were then added to the cells to incubate for 30 min at 4°C, followed by washing with PBS 3 times, analysis using a FACSCanto II machine (Becton Dickinson, NJ, USA), and visualization by Flow Jo software.
Cytokine assay
The levels of cell cytokines of RCC tumor tissues from the animal model, including IFN-γ, TNF-α, IL-2, high mobility group box 1 (HMGB1), and perforin, were measured by an enzyme-linked immunosorbent assay (ELISA) kit (ThermoFisher Scientific, CA, USA) following the manufacturer’s instructions.
Animal model
An animal model of RCC was established using Balb/c mice (female, 6-week old, 19–21 g) and Renca cells. All mice were purchased from the Experimental Animal Division, Peking University Health Science Center (Beijing, China). A total of 0.5×106 Renca cells were subcutaneously inoculated into the flanks of mice. All mice were maintained in a specific-pathogen-free environment with access to clean water and standard food. Treatments started on the mice bearing visible tumors 1 week after the inoculation. Each treatment group contained 10 mice. The treatment groups included the control groups (IgG), 5-FU (20 mg/kg), anti-PD-L1 Abs (200 μg/dose), and anti-PD-L1 (200 μg/dose) +5-FU (20 mg/kg) groups. Each mouse was treated once a week. The tumor volume (equals width2×length×π/6) was measured and recorded every week and the survival status was checked every day. Tumor tissue was harvested for further study after the mice were sacrificed due to large tumor volume or at the observation end point.
Statistical analysis
All statistical analysis and data visualization were performed using Graph Pad software (CA, USA). One-way ANOVA and Bonferroni pairwise comparisons were used to analyze the difference of means between different groups. Kaplan-Meier method was used to perform survival analysis. Differences with two-tailed P<0.05 were considered as statistically significant.
Result
RCC mouse model was resistant to anti-PD-L1 treatment
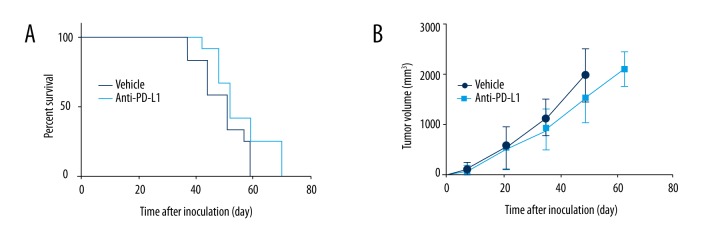
Many advanced RCC patients are resistant to immune checkpoint blockade therapy. To mimic this drug resistance phenotype, we created the RCC subcutaneous mouse model using Renca cells and treated them with anti-PD-L1 Abs. As shown in Figure 1A, the anti-PD-L1 treatment barely inhibited the RCC tumor development. The mice treated by anti-PD-L1 Abs showed a survival curve similar to that of the control group. Consistently, the tumor volume growth of the anti-PD-L1 Abs-treated group and the control group were also similar (Figure 1B). These data suggested that this RCC mouse model was resistant to anti-PD-L1 treatment. Thus, we used it for further study to explore the new treatment of RCC.
Figure 1.
Anti-PD-L1 treatment in a RCC subcutaneous mouse model. A total of 0.5×106 Renca cells were subcutaneously inoculated into the flanks of mice to establish the xenograft RCC model. Anti-PD-L1 treatment did not achieve significant effects in this mouse model, which was similar to the drug resistance phenotype of RCC patients to anti-PD-L1 treatment. (A) The survival curve of mice receiving anti-PD-L1 treatment was close to the survival curve of mice in the control group. (B) Tumor volume growth of the mice who received anti-PD-L1 treatment was slightly slower than in the control group, but no significant difference was observed.
Chemotherapy promoted the effects of anti-PD-L1 treatment in RCC mouse model
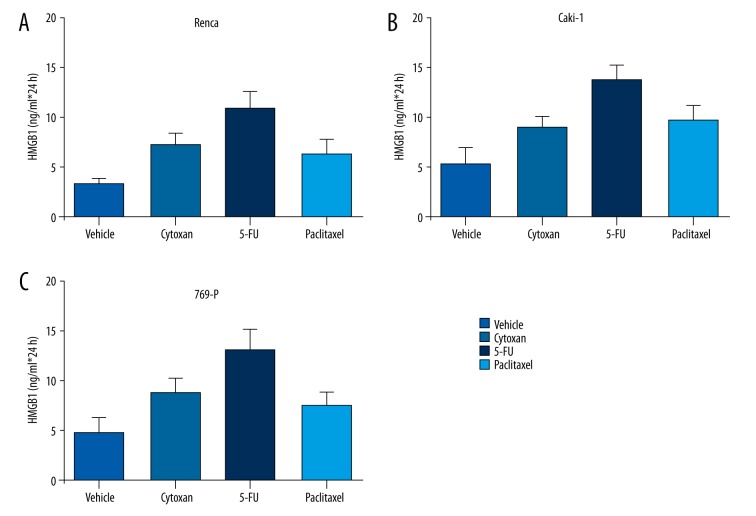
In an in vitro study, we explored the ability of multiple chemotherapies, such as Cytoxan, 5-FU and paclitaxel, to induce tumor immunity. Interestingly, we found that these chemotherapies induced the release of HMGB1 from RCC cell lines Renca, Caki-1, and 769-P (Figure 2). Among these chemotherapies, 5-FU achieved the strongest effects in inducing HMGB1 release, which suggested that chemotherapy might be a supportive factor that enhances that effects of immune checkpoint blockade therapy in RCC.
Figure 2.
The HMBG1 level in RCC cell lines after chemotherapies. Multiple RCC cell lines were treated with various chemotherapeutic agents, including Cytoxan, 5-FU, and paclitaxel. The HMGB1 level was measured by ELISA analysis. Increased HMGB1 levels were detected in these RCC cell lines: Renca (A), Caki-1 (B) and 769-P (C).
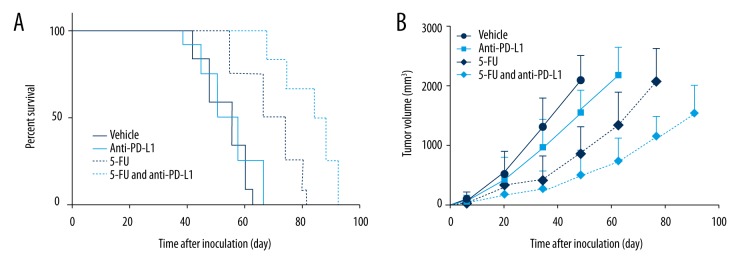
Then we further explored the function of 5-FU in an RCC subcutaneous mouse model in combination with anti-PD-L1 treatment. As shown in Figure 3A, the RCC mouse models treated with 5-FU and anti-PD-L1 Abs had the longest survival time and highest survival compared to other treatment groups, such as the anti-PD-L1 Abs single-treatment group and the 5-FU single-treatment group. The plot of the tumor volume increase also tracked similar trends: the mice receiving anti-PD-L1 and 5-FU combination treatment grew slower compared with other treatment groups and the control group (Figure 3B).
Figure 3.
Combination therapy of anti-PD-L1 and 5-FU in RCC subcutaneous mouse model. Mice were randomly allocated to different groups to accept different treatments: IgG, anti-PD-L1 Abs, 5-FU, and 5-FU and anti-PD-L1 Abs. (A) The survival curves indicated that the mice treated with 5-FU and anti-PD-L1 Abs had longer survival times compared to the mice receiving 5-FU or anti-PD-L1 Abs single treatment. (B) The tumor volume increase of mice receiving 5-FU and anti-PD-L1 combination treatment was the slowest among all the treatment groups.
Chemotherapy and anti-PD-L1 combination therapy enhanced cytotoxic cytokines level in RCC tumor tissue
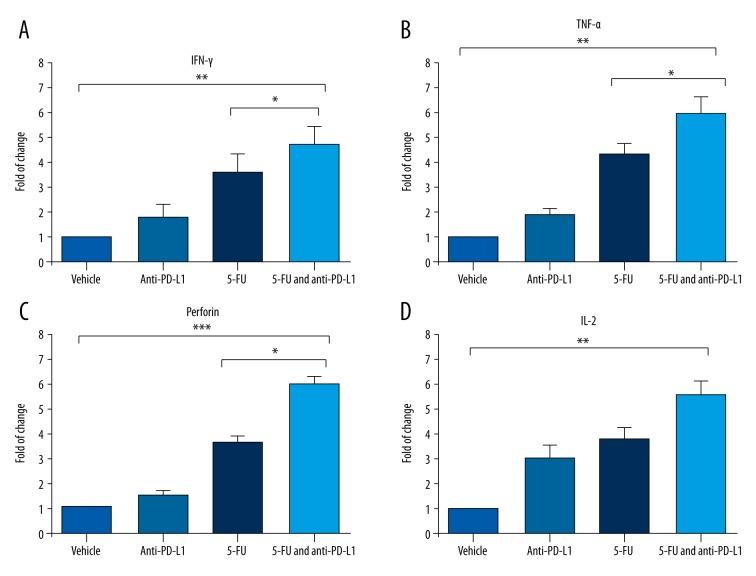
To further study the mechanism that the combination therapy of anti-PD-L1 and 5-FU in RCC model, we collected the subcutaneous tumor tissue and measured their key cytokines. As shown in Figure 4, we found many cytotoxic cytokines whose expression had been significantly increased by the combination treatment, including IFN-γ, TNF-α, and perforin. Notably, we also found that the level of IL-2 in the tumor tissue was also increased, although no statistically significant difference was observed between the combination treatment group and 5-FU single-treatment group (Figure 4D).
Figure 4.
Enhanced release of cytokines in mouse RCC tumor tissue induced by chemotherapy and anti-PD-L1 therapy. The RCC tumor tissues were collected from the Renca subcutaneous mouse model mice to measure the alterations of cytokines. (A–C): Many cytotoxic cytokines, including IFN-γ, TNF-α, and perforin, were significantly enhanced in the RCC tumor tissue of the mice treated with 5-UF and anti-PD-L1 compared to mice treated with 5-FU single treatment. (D) IL-2 level, which is critical for T cell proliferation, was also increased in the RCC tumor tissue of mice treated with 5-FU and anti-PD-L1 combination treatment. * P value less than 0.05; ** P value less than 0.01; *** P value less than 0.001.
ICBT combining with chemotherapy promoted the tumor immunity in RCC tissue
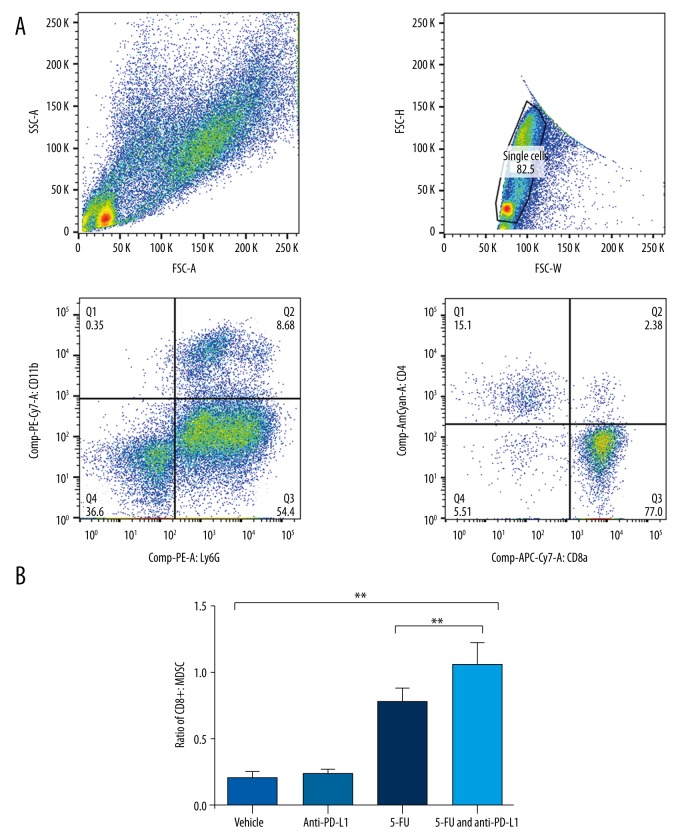
As cytotoxic immune cells are the direct killers of tumor cells, we measured the level of tumor immune cells and immunosuppressive cells in the tumor tissue of the RCC subcutaneous model mice. The FACS analysis result indicated that 5-FU and anti-PD-L1 combination treatment significantly enhanced the ratio of CD8+ immune cells and CD11b+Ly6G+Ly6Clow MDSC compared with the 5-FU and anti-PD-L1 Abs single-treatment groups (Figure 5). This result suggested that the combination of chemotherapy and anti-PD-L1 treatment successfully induced the tumor immunity, which inhibited the RCC development in the mouse model.
Figure 5.
Combination treatment with 5-FU and anti-PD-L1 suppressed myeloid-derived suppressive cells. FACS analysis was performed to the measure the key immune cells infiltrating to the tumor tissue of RCC subcutaneous mouse model mice. (A) the representative picture of gating strategy of FACS analysis. (B) The ratio of CD8+ T cells vs. CD11b+Ly6G+Ly6Clow MDSC in tumor tissue of RCC mouse model mice. The mice receiving the 5-FU and anti-PD-L1 combination treatment had significantly increased ratio of CD8+ T cells and MDSC cells. **** P value less than 0.0001.
Discussion
Under physiological conditions, immune checkpoint molecules work cooperatively to protect the hosts from autoimmunity or immune collateral damage by inhibiting T cell signals. However, in many tumors, these suppressive molecules are abnormally activated, which results in depressed tumor immunity and allows the tumor cells to escape from tumor immunity [14,15]. This provided a rationale for blocking these molecules to promote cancer treating. Recent studies have shown that immune checkpoint blockade benefited some cancer patients, even though limitations in response rate still exists [16,17].
In our study, we primarily examined the efficacy of anti-PD-L1 treatment alone in a RCC xenograft mouse model and found that these mice were resistant to the anti-PD-L1 treatment. This drug-resistant phenotype was consistent with the modest activity observed in a previous clinical trial of immune checkpoint blockade treatment of metastatic RCC [13]. Then, we used this RCC mouse model to explore the effects of combining immune checkpoint blockade therapy with chemotherapy. Notably, we observed the significantly increased efficacy of the combination treatment compared to either of the other 2 single treatments. The RCC mice who underwent 5-FU and anti-PD-L1 treatment had longer survival times compared with that of mice treated with 5-FU alone or anti-PD-L1 alone. This result was also consistent with a previous study in non-small cell lung cancer, which showed enhanced benefits of combination treatment of CTLA-4 antibody, carboplatin, and paclitaxel compared with the patients who received carboplatin and paclitaxel alone [17].
To further explore the mechanisms behind the obvious benefits of 5-FU and anti-PD-L1 combination treatment in the RCC mouse model, we tested levels of key cytokines that regulate tumor immunity. We found the several important cytokines were apparently increased in the tumor tissue of mice that received the combination treatment, including IFN-γ, TNF-α, and perforin. IFN-γ is well known as a key activator of macrophages, a cytotoxic cytokine of targeting cells, and a stimulator of the expression of class II major histocompatibility complex (MHC) molecules [18]. TNF-α primarily comes from various immune cells, such as macrophages and NK cells, and plays important roles in regulating immune response [19]. Thus, the increase in IFN-γ and TNF-α suggests that the immune response intensity was enhanced by the combination treatment, and the increased level of perforin also suggests enhanced cytotoxic T cell activities. More importantly, our data from the FACS analysis of the tumor-infiltrating immune cells of the RCC mouse model indicated an increased ratio of CD8+ cell versus MDSC cells, suggesting that the combination treatment enhances tumor immunity via depleting immune-suppressive components.
Conclusions
In summary, based on the data from the RCC animal model and analyses of possible mechanisms, we conclude that 5-FU chemotherapy can sensitize RCC to anti-PD-L1 treatment by releasing immune suppression in the tumor microenvironment. This study provides important evidence showing that combination of chemotherapy and anti-PD-L1 treatment might benefit advanced RCC patients.
Acknowledgements
We thank Beijing Luhe Hospital, Capital Medical University for providing an internal grant and technical support.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Kidney cancer incidence statistics. 2013. [Google Scholar]
- 3.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–79. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson BJ, Kalra S, Wang X, et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol. 2014;191:611–18. doi: 10.1016/j.juro.2013.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mickisch G, Garin A, Van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 7.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber J. Immune checkpoint proteins: A new therapeutic paradigm for cancer – preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–39. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Allison James P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, He Y, Chen H. Autophagic tumor stroma: Mechanisms and roles in tumor growth and progression. Int J Cancer. 2013;132:1–8. doi: 10.1002/ijc.27664. [DOI] [PubMed] [Google Scholar]
- 12.Michaud M, Martins I, Sukkurwala AQ, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–77. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 13.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nirschl CJ, Drake CG. Molecular pathways: Coexpression of immune checkpoint molecules: Signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917–24. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 18.Peng W, Liu C, Xu C, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants–past, present and future. Cytokine Growth Factor Rev. 2014;25:453–72. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]