Abstract
Background
The aim of this study was to elucidate the role of Krüppel-Like factor 4 (KLF4) in cisplatin resistance in esophageal squamous cell carcinoma (ESCC) cells, which may eventually help to improve the treatment efficacy.
Material/Methods
Human esophageal squamous cell carcinoma (ESCC) cell line CaEs-17, TE-1, EC109, KYSE510, KYSE140, KYSE70, and KYSE30 were selected to detect their sensitivity to cisplatin. 5-Azacytidine-2′-deoxycytidine (5′-Aza-CdR) treatment and methylation-specific PCR (MS-PCR) were used to detect the methylation status for KLF4. Cell viability, apoptosis, and cell cycle were measured using methyl thiazolyl tetrazolium (MTT) assay, Annexin V affinity assay, and flow cytometry, respectively.
Results
The sensitivity to cisplatin was different in the seven ESCC cell lines, with TE-1 having the lowest sensitivity and KYSE140 having the highest sensitivity. Interestingly, the level of KLF4 was relatively low in TE-1 cells; while it was high in KYSE140 cells. These results suggested that KLF4 may be involved in cisplatin resistance. The promoter region was mostly unmethylated in KYSE140 cells; while it was hypermethylated in TE-1 cells. After treatment with demethylation reagent 5-Aza-CdR, cisplatin sensitivities were significantly increased after upregulation of KLF4, as the IC50 values were significantly decreased in the TE-1 cell treated with 5-Aza-CdR. Furthermore, upregulation of KLF4 induced cell apoptosis and cell cycle arrest at S phase.
Conclusions
KLF4 enhances the sensitivity of cisplatin to ESCC cells through apoptosis induction and cell cycle arrest. Our data provided a novel insight to the mechanism of cisplatin resistance; overexpression of KLF4 may be a potential therapeutic strategy for cisplatin resistance in human ESCC.
MeSH Keywords: Antineoplastic Agents, Cisplatin, Esophageal Neoplasms
Background
Esophageal squamous cell carcinoma (ESCC), accounts for 80% of esophageal cancers, and is one of the most common causes of cancer death worldwide [1–3]. The treatment for ESCC depends on its etiology; treatment options include surgical resection, neoadjuvant therapy, and adjuvant therapy [4]. Despite optimization of surgical treatment, the clinical outcomes for ESCC remain unsatisfactory for patients treated solely with resection [5]. For more advanced stages, chemotherapy has shown favorable results. Combinations of cisplatin and other drugs, such as paclitaxel, docetaxel, and 5-fluorouracil (CF), are standard chemotherapy regimens for advanced ESCC [4–6]. Cisplatin, a molecule that can enter the cell, interferes with the process of DNA synthesis, resulting in inhibition of DNA replication and cell cycle arrest, DNA damage, and subsequent apoptosis and necrosis [7 8]. However, due of intrinsic or acquired nonresponse to chemotherapy, the 5-year survival rate of human ESCC is still unsatisfactory. Therefore, in order to improve therapeutic efficacy and clinical outcomes, there is an urgent need to elucidate the mechanisms underlying cisplatin resistance in human ESCC.
Krüppel-like factor 4 (KLF4), a eukaryotic zinc-finger transcription factor, plays an important role in various essential processes such as cell proliferation, differentiation, and apoptosis [9,10]. Recent studies have shown that KLF4 is expressed in numerous human cancers, and whether the role of KLF4 is tumor suppressive or oncogenic depends on the type of cancer. KLF4 seems to function as an oncogene in oral cancer, head and neck cancer, skin cancer, and pancreatic cancer [11–13]. On the other hand, KLF4 acts as a tumor suppressor in gastric carcinoma, colorectal cancer, and cervical cancer [14–16]. In esophageal squamous cell carcinoma (ESCC), KLF4 is decreased; and KLF4 deletion in mice leads to squamous cell dysplasia [17]. Downregulation of KLF4 in human ESCC suggests that KLF4 may act as a tumor suppressor in ESCC [18–20]. The KLF4 gene has been shown to be epigenetically inactivated in human cervical cancer and lung cancer [21]. Moreover, KLF4 enhances the sensitivity of cisplatin to lung cancer cells [21]. However, whether KLF4 plays a role in cisplatin resistance of human ESCC remains unknown.
In this study, we aimed to investigate the role of KLF4 in cisplatin resistance in human ESCC cells. Two human ESCC cell lines (TE-1 and KYSE140) were selected from seven cell lines (CaEs-17, TE-1, EC109, KYSE510, KYSE140, KYSE70, and KYSE30) according to the cell line sensitivity of cisplatin. KYSE140 cells were highly sensitive to cisplatin, while TE-1 cells were less sensitivity to cisplatin, compared with other cell lines. We detected the level of KLF4 expression and promoter methylation status. Cell viability, cell apoptosis, and cell cycle were also measured.
Material and Methods
Cell culture
Human ESCC cell line CaEs-17, TE-1, EC109, KYSE510, KYSE140, KYSE70, and KYSE30 were cultured in RPMI1640 (Gibco) supplemented with 10% (v/v) fetal calf serum (FBS, Gibco) at 37°C in a humidified 5% CO2 incubator.
5-Azacytidine-2′-deoxycytidine (5-Aza-CdR) treatment and methylation analysis
Cells were cultured in medium containing 1 μmol/L 5-Aza-CdR (the DNA methylation inhibitor) for five days and the medium was replace every 24 hours. Genomic DNA was extracted and modified with sodium bisulfite using a DNA sulfite-sensitive modification kit, according to manufacturer’s instructions. Methylation-specific PCR (MS-PCR) was then performed as described previously [22]. Specific primers for un-methylated KLF4 (U) were as follows: forward, 5′-GGT TGA TTA TTT GAG GTT AGG TGT TT-3′ and reverse, 5′-CCC AAA TAA CAA AAA TTA CAA ACAT-3′; primers for methylated KLF4 (M) were 5′-GTT GAT TAT TTG AGG TTA GGT GTTC-3′ (forward) and 5′-CGA ATA ACG AAA ATT ACA AAC GTA-3′. Conditions for MS-PCR were 95°C for 10 minutes, 94°C for 30 seconds, 52.4°C for 30 seconds to anneal, 72°C for one minute followed by 40 cycles. The MS-PCR products were examined by gel electrophoresis in 1% agarose. The experiments were repeated five times.
RT-PCR
Total RNA was extracted using TRIzol reagent and 2 μg total RNA was used for reverse transcription. The obtained cDNA was then used as the template for RT-PCR. The primers for KLF4 were as follows: sense, 5′-ACC CAC ACT TGT GAT TAC GC-3′ and anti-sense, 5′-CCG TGT GTT TAC GGT AGT GC-3′. The conditions for RT-PCR were as follows: 95°C for 10 minutes, 94°C for 30 seconds, 50°C for 30 seconds to anneal, 72°C for one minute followed by 28 cycles. The products were analyzed after RT-PCR using 1% agarose gel electrophoresis, and the results observed by Bio-Rad Electrophoresis Imaging System. The experiments were repeated five times.
Cisplatin treatment
Cells were cultured in medium containing cisplatin at a final concentration of 0.1 mg/L, 0.5 mg/L, 1 mg/L, 2.5 mg/L, 5 mg/L, and 10 mg/L for 24 hours. After cisplatin treatment, subsequent analysis was carried out accordingly.
Methyl thiazolyl tetrazolium (MTT) assay
Cells were seeded at a density of 4×103 cells per well in a 96-well plate and incubated for 24 hours. Then cultured with refresh medium with RPMI1640 containing cisplatin wat a final concentration of 0 mg/L, 0.1 mg/L, 0.5 mg/L, 1 mg/L, 2.5 mg/L, 5 mg/L, and 10 mg/L, and incubated for 24 hours. After 24 hours, we add 20 μL of 5 g/L MTT and incubated cells at 37°C for four hours. Then we remove the supernatants carefully. We add 150 μL of dimethyl sulfoxide (Sigma) to each well and mix thoroughly for 10 minutes. The absorbance value (OD) at 490 nm was measured using an ELISA reader system. The inhibition rate was calculated as follows: inhibition rate (%)=(1−ODtest group)/ODcontrol group×100%. All experiments were performed six times.
Annexin V affinity assay
In order to detect cell apoptosis, we used the Annexin V affinity assay following the manufacturer’s guidance. Briefly, we collected cells after cisplatin treatment. Then we add PI and Annexin-V-FITC and incubated for 15 minutes in the dark. The experiment was repeated for five times.
Cell cycle analysis
After incubation with cisplatin for 24 hours, cells were fixed overnight using 70% precooled ethanol at 4°C and stained with propidium iodide (PI, 1 mg/mL).
Statistical analysis
Data were presented as mean ±SD (standard deviation), and statistical significance was analyzed by SPSS 19.0. Significant differences were calculated using one-way ANOVA followed by Newman-Keuls post-hoc tests. The probability value p < 0.05 was considered to be of significant difference.
Results
Sensitivity to cisplatin of different ESCC cell lines
The sensitivity to cisplatin of the seven human ESCC cell lines was detected by MTT assay. Our results showed that the inhibition rate was relatively low in TE-1 and KYSE510 cells; while the inhibition rate was relatively high in KYSE140 and EC109 cells (Figure 1). The sensitivity to cisplatin of KYSE140 was relatively high compared to the other five cell lines; whereas TE-1 was the relative less sensitive to cisplatin as compared with the other five. However, it should be noted that a significant difference was not found in TE-1 and KYSE140 compared with all the other five cell lines.
Figure 1.
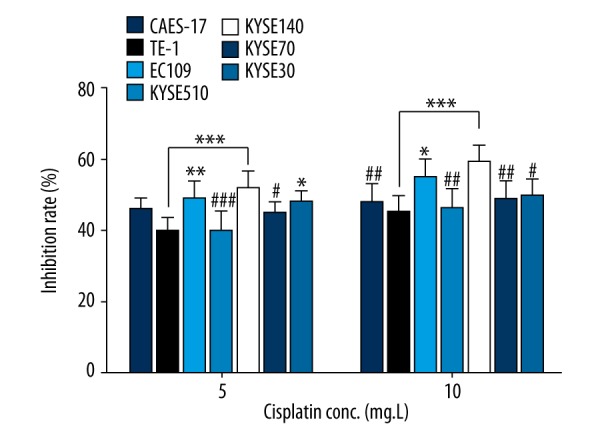
Sensitivity to cisplatin of different ESCC cell lines at final concentration of 5 mg/L and 10 mg/L. Compare with TE-1 cells: * p < 0.05, ** p<0.01, and *** p<0.001; compared with KYSE140 cells: # p<0.05, ## p<0.01, and ### p<0.001.
Cisplatin sensitivity of TE-1 was significantly lower than KYSE140, EC109, and KYSE30 cells under 5 mg/L cisplatin; and significantly lower than KYSE140 and EC109 cells under 10 mg/L cisplatin. Additionally, cisplatin sensitivity of KYSE140 was significantly higher than TE-1, KYSE510, and KYSE70 under 5 mg/L cisplatin; and significantly higher than TE-1, CaEs-17, KYSE510, KYSE70, and KYSE30 under 10 mg/L cisplatin (Figure 1).
The level of KLF4 and the methylation status of KLF4 promoter in different ESCC cell lines
The expression levels of KLF4 were detected by RT-PCR. Relative high levels of KLF4 were found in ESCC cell line CaEs-17, EC109, KYSE140, KYSE70, and KYSE30; while relative low levels of KLF4 were found in TE-1 and KYSE510 (Figure 2A). It has been reported that KLF4 was downregulated in ESCC typically by hypermethylation [20]. In our study, we detected the methylation status of KLF4 promoter by MS-PCR. Our results showed that the promoter region was mostly unmethylated in CaEs-17, EC109, KYSE140, KYSE70, and KYSE30 cells; whereas, relatively hypermethylated in TE-1 and KYSE510 (Figure 2B). Our results agree with the previous studies and suggest that hypermethylation in the promoter region leads to downregulation of KLF4 in ESCC cells. Moreover, combining our results of cisplatin sensitivity of the seven ESCC cell lines, we found that a high level of KLF4 was associated with high sensitivity to cisplatin. Our results suggest that KLF4 may be involved in the cisplatin resistance of ESCC cells.
Figure 2.
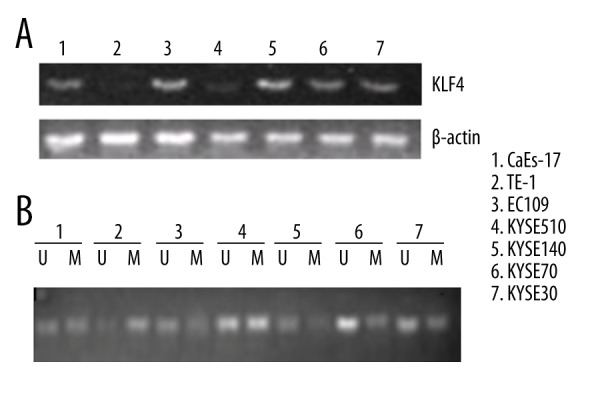
The level of KLF4 and the methylation status of KLF4 promoter in different ESCC cell lines. (A) The expression level of KLF4 was detected by RT-PCR in the seven human ESCC cell lines. (B) The methylation status of KLF4 promoter was measured by MS-PCR. U – indicates unmethylation; M – indicates methylation.
KLF4 enhances sensitivity to cisplatin of ESCC cells
We selected TE-1 (low level of KLF4 and promoter region hypermethylated) and KYSE140 (high level of KLF4; promoter region hypomethylated) to further investigate the role of KLF4 in cisplatin resistance. To further confirm the role of methylation in the level of KLF4, we treated TE-1 and KYSE140 cells with the demethylation agent 5-Aza-CdR; this agent could cause DNA demethylation or hemi-demethylation through inhibition of DNA methyltransferase activity. We found that significant upregulation of the expression level of KLF4 was found in TE-1 cells; while no obvious change was found in KYSE140 cells (Figure 3A). This result suggested that low level of KLF4 in TE-1 cells may be caused by hypermethylation of the promoter. Then, we detected cell growth by MTT assay when the cells were treated with different doses of cisplatin. We found that the elevated KLF4 could significantly inhibit cell growth. Moreover, cisplatin sensitivities were significantly increased after upregulation of KLF4, as the IC50 values were significantly decreased in TE-1 cell treated with 5-Aza-CdR (Figure 3B). These results imply that restored KLF4 expression could significantly inhibit cell growth and increase the chemosensitivity for cisplatin in human ESCC cells.
Figure 3.
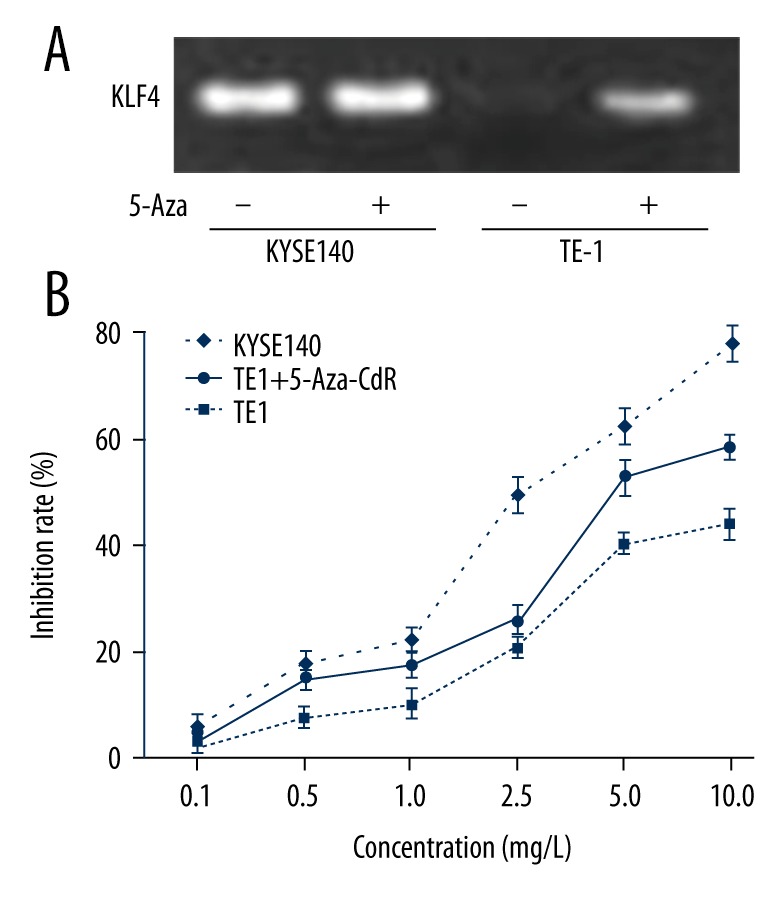
KLF4 enhances sensitivity to cisplatin of ESCC cells. (A) Level of KLF4 in TE-1 and KYSE140 cells after demethylation reagents 5-Aza-CdR. (B) Cell viability of TE-1 cells, TE-1 cell treated with 5-Aza-CdR, and KYSE140 cell were detected by MTT assay.
Upregulation of KLF4 induces apoptosis and cell cycle arrest
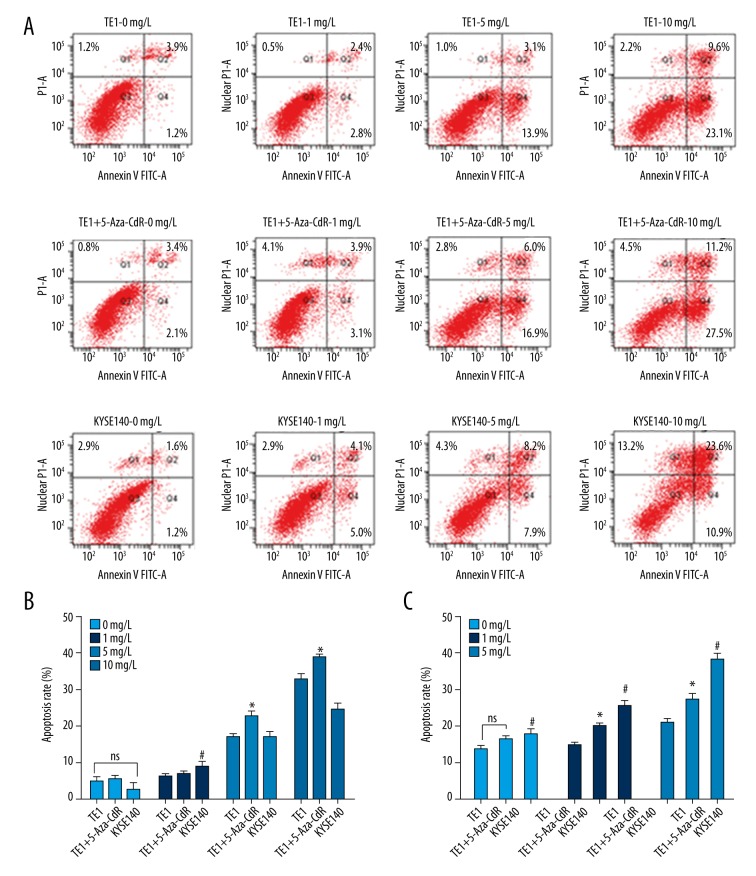
In order to detect the effect of KLF4 overexpression on ESCC cell apoptosis with the treatment of cisplatin, TE-1, TE-1-5-Aza, and KYSE140 cells were treated with cisplatin at the final concentrations of 0 mg/L, 1 mg/L, 5 mg/L, and 10 mg/L. Apoptosis was detected by flow cytometry. We found that apoptosis rate was similar without cisplatin treatment in TE-1 (5.1±1.2), TE-1-5-Aza (5.6±0.9) and KYSE140 cells (2.8±1.7), with no significant difference (Figure 4A, 4B). With the increase in cisplatin doses, the apoptosis rate in TE-1-5-Aza was greatly increased. When we treated cells with 5 mg/L cisplatin, the apoptosis rate in TE-1 cells (17.0±0.7) was significantly different (p=0.001) from that in TE-1-5-Aza cells (22.9±1.1). When the final concentration of cisplatin increased to 10 mg/L, the apoptosis rate in TE-1 (32.7±1.6), TE-1-5-Aza (38.7±1.2) and KYSE140 cells (24.5±1.8) was all greatly increased, and a significant difference was found in 5-Aza-CdR treated TE-1 cells compared with TE-1 cells (p=0.007) (Figure 4A, 4B). Our results suggested that upregulation of KLF4 induced apoptosis in human ESCC cells.
Figure 4.
Upregulation of KLF4 induces apoptosis and cell cycle arrest. Cell apoptosis (A, B) and cell cycle (C) was detected by flow cytometry. NS, no significant difference in TE-1 cell after 5-Aza-CdR treatment compared with TE-1 cells; #, significant difference in TE-1 cells compared with KYSE140 cells (p<0.05); *, significant difference in TE-1 cell after 5-Aza-CdR treatment compared with TE-1 cells (p<0.05).
Cell cycle was detected by flow cytometry. The results showed that the percentage of cells at S phase was 27.20±2.1%, 32.71±1.9%, and 35.02±2.9% without cisplatin treatment. There was a significant difference in the percentage of cells at S phase in TE-1 cells compared with KYSE140 cells. At the final concentration of 0.1 mg/L of cisplatin, a significant difference was found in the percentage of cells at S phase in TE-1 cells (29.03±2.3%) compared with 5-Aza-CdR treated TE-1 cells (39.51±1.7%) (Figure 4C). Similar results was obtained at the final concentration of 0.5 mg/L of cisplatin, were a significant difference was found in the percentage of cells at S phase in TE-1 cells (41.68±2.5%) compared with 5-Aza-CdR treated TE-1 cells (54.17±3.6%) (Figure 4C). Taken together, these results suggested that increased level of KLF4 could cause cell cycle arrest at S phase in human ESCC cells.
Discussion
Krüppel-like factor 4 (KLF4) can act as a tumor suppressor or an oncogene depending on the type of tumor. KLF4 might function as a tumor suppressor through activating the promoter of p21 leading to cell cycle arrest at the border of G1/S and G2/M, which further inhibits cell proliferation and induces cell apoptosis [8]. KLF4 can also inhibit tumor invasiveness and metastasis by regulation of EMT [23]. However, the role of KLF4 is controversial in the development and metastasis of ESCC. Yang et al. reported that although the loss of KLF expression was important for initial tumor growth in ESCC, the restoration of its expression enhanced tumor spread and KLF expression was inversely correlated with survival [20]. KLF4 expression was reported to be associated with histological types, but not an independent prognostic factor, as compared with Tir Na Nog (NANOG) [24]. In our previous study, through enrollment of 98 cases of esophageal carcinoma patients, we found that KLF4 was downregulated in esophageal squamous cell carcinoma (ESCC) patients, and was associated with poor prognosis of ESCC patients [25]. Our results suggested that KLF4 might act as a tumor suppressor in ESCC; as the expression status of KLF4 has been shown to be closely related with the tumor stage and prognosis for ESCC patients [25].
The mechanism underlying downregulation of KLF4 includes promoter methylation, loss of heterozygosity and genetic mutation [26]. In the present study, we selected seven human ESCC cell lines. The level of KLF4 expression and the methylation status of KLF4 promoter were measured. The results of MSP and RT-PCR showed that different levels of KLF4 promoter methylation existed in the seven ESCC cell lines, with the most obvious hypermethylation in TE-1 cells. Inversely, the level of KLF4 was relatively low in TE-1 cells, and the level of KF4 was restored in TE-1 cells after 5-Aza-CdR treatment. Our results further verified that hypermethylation of KLF4 in its promoter region may play a crucial role in the downregulation of KLF4, which was consistent with the results reported by Yang et al. [20]. However, it should be noted that besides the downregulation of KLF4 through the aforementioned mechanisms, splicing variants of KLF4 also play an important role in tumor formation and progression [27,28]. KLF4α, one of the main isoforms of KLF4, has been reported to be oncogenic in pancreatic and breast cancers [29,30]. However, the role of KLF4α in the development and progression of human ESCC has been rarely reported, and relative studies should be carried out in the future.
Cisplatin plays an important role in chemotherapy of human ESCC, serving as the first-line treatment in platinum-based chemotherapy. The main role of cisplatin is to interfere with the process of DNA synthesis causing inhibition of DNA replication, cell cycle arrest, DNA damage, and subsequent apoptosis and necrosis [7,8]. However, any factors that influence the binding of DNA to cisplatin and interfere with cell apoptosis may result in the insensitivity or even resistance to cisplatin. In cervical cancer, methylation in the promoter region could inhibit expression of KLF4 and restoring KLF4 expression using demethylating agent 5-Azacytidine (5-Aza) could enhance the sensitivity to cisplatin of cervical cancer cells [20]. In addition, Liu et al. reported that overexpression of KLF4 may improve sensitivity of cisplatin to lung cancer cells [21]. In the present study, under the final concentration of 0.5 mg/L, 0.1 mg/L, 2.5 mg/L, 5 mg/L, and 10 mg/L of cisplatin, cell viability was significantly decreased after treatment of demethylation reagents 5-Aza-CdR in TE-1 cells. These results suggested that demethylation in the promoter region and the restored expression of KLF4 could inhibit cell proliferation and increase the sensitivity to cisplatin. In the meanwhile, the cell viability of KYSE140 was significantly decreased compared with TE-1 cells, which suggested that hypomethylation in promoter and high level of KLF4 could inhibit the proliferation of human ESCC cells.
In oral squamous cell carcinoma, overexpression of KLF4 has been reported to promote cell cycle arrest in vitro and induce apoptosis in vivo [10]. He and colleges reported that KLF4 could inhibit the cell cycle transition from G1 phase to S phase [31]. Consistent with these findings, the results of flow cytometry assay showed that the apoptosis rate was significantly increased in KYSE140 cells when cells were treated with 1 mg/L cisplatin, compared with TE-1 cells, suggesting that high levels of KLF4 with promoter hypomethylation could induce cell apoptosis in human ESCC cells. Moreover, when TE-1 cells were treated with cisplatin at a final concentration of 5 mg/L and 10 mg/L, the apoptosis of TE-1 cells was significantly increased after 5-Aza-CdR treatment, suggesting enhanced sensitivity to cisplatin of human ESCC cells by high level of KLF4. It has been reported that KLF4 inhibits cell cycle progression by activating p21 or p27, and by repressing CCNB1 and CCND1 [23,32]. Moreover, the function of KLF4 is often context-dependent based on the tissue, tumor type, or cancer stage, which may be mediated by molecular switches such as BMP4, p21, p53, and SIN3A [33,34]. We found that in KYSE140 cell line with high levels of KLF4, the percentage of cells arrested at S phase was significantly higher than TE-1 cells. After TE-1 cells were treated with demethylation reagent 5-Aza-CdR, the percentage of cells arrest at S phase was significantly elevated. Taken together, these results suggested that overexpression of KLF4 could promote cell apoptosis, induce cell cycle arrest and enhance the sensitivity to cisplatin of human ESCC cells.
Conclusions
Our findings showed that KLF4, acting as a tumor suppressor in human ESCC cells, was downregulated in human ESCC cells by hypermethylation in the promoter region. KLF4 could enhance the sensitivity of cisplatin through inhibiting cell proliferation, promoting cell apoptosis, and inducing cell cycle arrest. Our results provide novel insight into the mechanism underlying cisplatin-resistance, and overexpression of KLF4 may serve as a potential therapeutic strategy for human ESCC treatment, especially for patients with cisplatin-resistant. However, it should be noted that due to the contradictory data on the role of KLF4, more studies should be carried out before the therapeutic use of KLF4.
Footnotes
Source of support: This work was support by the National Nature Science Foundation of China (Grant 81071981) and Science & Technology Development Fund of Tianjin Education Commission for Higher Education (Grant 20130121)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia – the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540–52. doi: 10.1158/1055-9965.EPI-12-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima M, Kato H. Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother. 2013;14:1345–54. doi: 10.1517/14656566.2013.801454. [DOI] [PubMed] [Google Scholar]
- 5.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: Role of surgery and other modalities. Lancet Oncol. 2007;8:545–53. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura Y, Hiraoka M, Koike R, et al. Long-term follow-up of a randomized Phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer (KROSG0101/JROSG021) Jpn J Clin Oncol. 2012;42:807–12. doi: 10.1093/jjco/hys112. [DOI] [PubMed] [Google Scholar]
- 7.Scarpace SL. Metastatic squamous cell non-small-cell lung cancer (NSCLC): Disrupting the drug treatment paradigm with immunotherapies. Drugs Context. 2015;4:212289. doi: 10.7573/dic.212289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon HS, Chen X, Yang VW. Krüppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–5. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaira V, Faversani A, Martin NM, et al. Regulation of lung cancer metastasis by Klf4-Numb-like signaling. Cancer Res. 2013;73:2695–705. doi: 10.1158/0008-5472.CAN-12-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Liu M, Su Y, et al. The Janus-faced roles of Krüppel-like factor 4 in oral squamous cell carcinoma cells. Oncotarget. 2015;6:44480–94. doi: 10.18632/oncotarget.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YJ, Wu CY, Chang CC, et al. Nuclear Krüppel-like factor 4 expression is associated with human skin squamous cell carcinoma progression and metastasis. Cancer Biol Ther. 2008;7:777–82. doi: 10.4161/cbt.7.5.5768. [DOI] [PubMed] [Google Scholar]
- 12.Abrigo M, Alvarez R, Paparella ML, et al. Impairing squamous differentiation by Klf4 deletion is sufficient to initiate tongue carcinoma development upon K-Ras activation in mice. Carcinogenesis. 2014;35:662–69. doi: 10.1093/carcin/bgt349. [DOI] [PubMed] [Google Scholar]
- 13.Shi M, Cui J, Du J, et al. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin Cancer Res. 2014;20:4370–80. doi: 10.1158/1078-0432.CCR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang WT, Zheng PS. Krüppel-like factor 4 functions as a tumor suppressor in cervical carcinoma. Cancer. 2012;118:3691–702. doi: 10.1002/cncr.26698. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Chen Y, Min L, et al. Augmented miR-10b expression associated with depressed expression of its target gene KLF4 involved in gastric carcinoma. Int J Clin Exp Pathol. 2015;8:5071–79. [PMC free article] [PubMed] [Google Scholar]
- 16.Ghaleb AM, Elkarim EA, Bialkowska AB, Yang VW. KLF4 suppresses tumor formation in genetic and pharmacological mouse models of colonic tumorigenesis. Mol Cancer Res. 2016;14:385–96. doi: 10.1158/1541-7786.MCR-15-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tetreault MP, Yang Y, Travis J, et al. Esophageal squamous cell dysplasia and delayed differentiation with deletion of Krüppel-like factor 4 in murine esophagus. Gastroenterology. 2010;139:171–81e9. doi: 10.1053/j.gastro.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo A, Kong J, Hu G, et al. Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 2004;23:1291–99. doi: 10.1038/sj.onc.1207218. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Liu ZH, Ding F, et al. Down-regulation of gut-enriched Krüppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966–70. doi: 10.3748/wjg.v8.i6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Katz JP. KLF4 is downregulated but not mutated during human esophageal squamous cell carcinogenesis and has tumor stage-specific functions. Cancer Biol Ther. 2016;17:422–29. doi: 10.1080/15384047.2016.1156260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Yang H, Chen Y, et al. Krüppel-like factor 4 enhances sensitivity of cisplatin to lung cancer cells and inhibits regulating epithelial-to-mesenchymal transition. Oncol Res. 2016;24:81–87. doi: 10.3727/096504016X14597766487717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nojima M, Suzuki H, Toyota M, et al. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene. 2007;26:4699–713. doi: 10.1038/sj.onc.1210259. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Wang J, Xiao W, et al. Epigenetic alterations of Krüppel-like factor 4 and its tumor suppressor function in renal cell carcinoma. Carcinogenesis. 2013;34:2262–70. doi: 10.1093/carcin/bgt189. [DOI] [PubMed] [Google Scholar]
- 24.Shimada Y, Okumura T, Sekine S, et al. Expression analysis of iPS cell-inductive genes in esophageal squamous cell carcinoma by tissue microarray. Anticancer Res. 2012;32:5507–14. [PubMed] [Google Scholar]
- 25.Ma MQ, Zhang HD, Tang P, et al. Association of Krüppel-like factor 4 expression with the prognosis of esophageal squamous cell carcinoma patients. Int J Clin Exp Pathol. 2014;7:6679–85. [PMC free article] [PubMed] [Google Scholar]
- 26.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–81. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Magnen C, Bubendorf L, Ruiz C, et al. Klf4 transcription factor is expressed in the cytoplasm of prostate cancer cells. Eur J Cancer. 2013;49:955–63. doi: 10.1016/j.ejca.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Camacho-Vanegas O, Till J, Miranda-Lorenzo I, et al. Shaking the family tree: Identification of novel and biologically active alternatively spliced isoforms across the KLF family of transcription factors. FASEB J. 2013;27:432–36. doi: 10.1096/fj.12-220319. [DOI] [PubMed] [Google Scholar]
- 29.Wei D, Wang L, Kanai M, et al. KLF4α up-regulation promotes cell cycle progression and reduces survival time of patients with pancreatic cancer. Gastroenterology. 2010;139:2135–45. doi: 10.1053/j.gastro.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferralli J, Chiquet-Ehrismann R, Degen M. KLF4alpha stimulates breast cancer cell proliferation by acting as a KLF4 antagonist. Oncotarget. 2016;7:45608–21. doi: 10.18632/oncotarget.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He H, Li S, Hong Y, et al. Krüppel-like factor 4 promotes esophageal squamous cell carcinoma differentiation by up-regulating keratin 13 expression. J Biol Chem. 2015;290:13567–77. doi: 10.1074/jbc.M114.629717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz JP, Perreault N, Goldstein BG, et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–45. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Tetreault MP, Yang Y, Katz JP. Krüppel-like factors in cancer. Nat Rev Cancer. 2013;13:701–13. doi: 10.1038/nrc3582. [DOI] [PubMed] [Google Scholar]
- 34.Yan W, Zhang H, Li J, et al. BMP4 promotes a phenotype change of an esophageal squamous epithelium via up-regulation of KLF4. Exp Mol Pathol. 2016;101:259–66. doi: 10.1016/j.yexmp.2016.09.007. [DOI] [PubMed] [Google Scholar]