Abstract
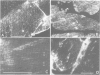
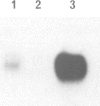
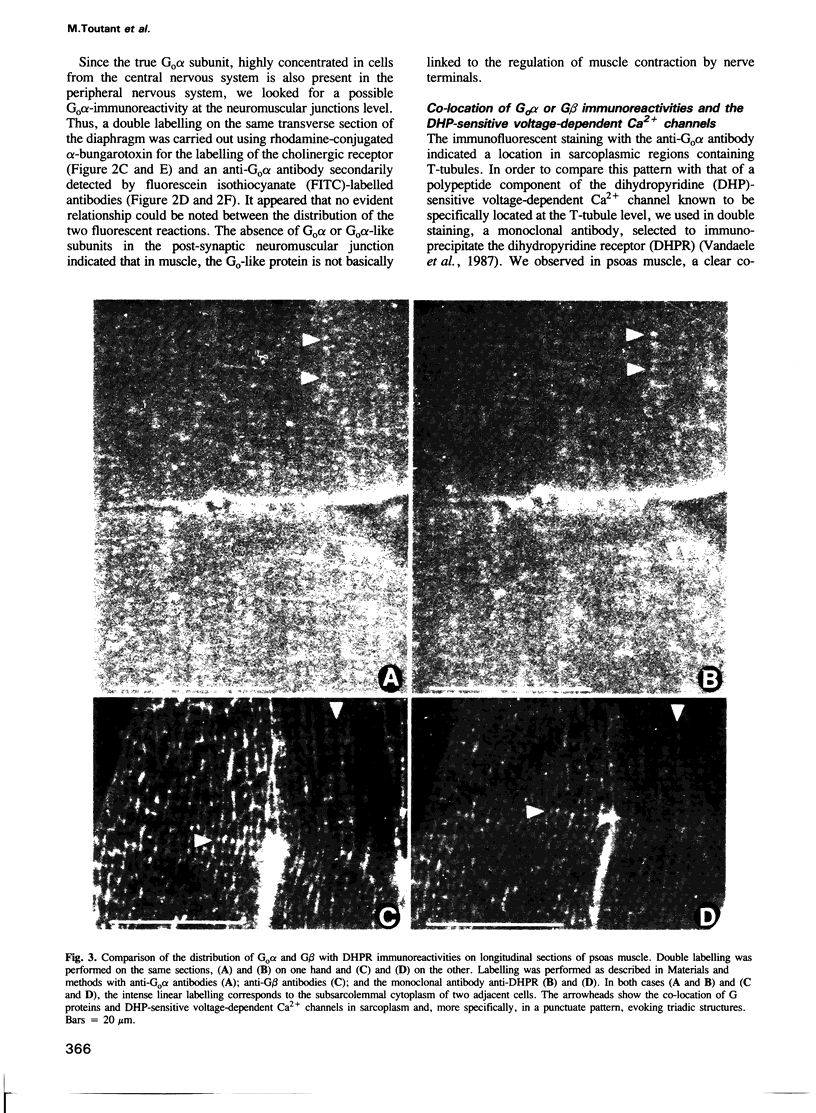
GTP binding proteins have been proposed to play a role in excitation--contraction coupling. In a precedent study [Toutant et al., (1988), Biochem. J., 405-409], we determined that Bordetella pertussis toxin is able to catalyse ADP-ribosylation of two substrates in the detergent soluble fraction of total muscle extracts. Purified fractions of transverse tubule membranes (T-tubule membranes), a key element of the excitation--contraction coupling, were shown to exhibit a major ADP-ribosylated substrate at 40 kd and an immunoreactivity with antisera raised against purified bovine brain Go alpha or G beta. In the present study, we have investigated the cellular distribution of G protein subunits in comparison with that of the voltage-dependent Ca2+ channels by immunofluorescence on transverse and longitudinal sections of fast and slow muscles. With affinity-purified antibodies against G beta subunits, a fluorescent labelling underlined the myofibrils and sarcolemma, whereas a strong immunoreaction in a dotted pattern evoked the presence of the subunit in repetitive triadic structures. With anti-Go alpha antibodies, the immunofluorescence was more clearly focussed on a dotted pattern and the co-location with the voltage-dependent Ca2+ channel immunoreactivity indicates that both proteins were located in very close subcellular structures. Immunoblot analysis and PTX ADP-ribosylation of the purified light sarcoplasmic reticulum (LSR), heavy sarcoplasmic reticulum (HSR) and T-tubule subcellular fractions indicate the discrete presence of G proteins in LSR, an unambiguous labelling of the HSR fraction, while T-tubule membranes clearly appear very rich in a Go-like protein, confirming the observed preferential immunocytochemical distribution of G protein subunits.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barhanin J., Coppola T., Schmid A., Borsotto M., Lazdunski M. The calcium channel antagonists receptor from rabbit skeletal muscle. Reconstitution after purification and subunit characterization. Eur J Biochem. 1987 May 4;164(3):525–531. doi: 10.1111/j.1432-1033.1987.tb11158.x. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Homburger V., Rouot B. GTP binding proteins: a key role in cellular communication. Biochimie. 1987 Apr;69(4):329–338. doi: 10.1016/0300-9084(87)90024-1. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Brabet P., Dumuis A., Sebben M., Pantaloni C., Bockaert J., Homburger V. Immunocytochemical localization of the guanine nucleotide-binding protein Go in primary cultures of neuronal and glial cells. J Neurosci. 1988 Feb;8(2):701–708. doi: 10.1523/JNEUROSCI.08-02-00701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Caswell A. H., Brandt N. R. Does muscle activation occur by direct mechanical coupling of transverse tubules to sarcoplasmic reticulum? Trends Biochem Sci. 1989 May;14(5):161–165. doi: 10.1016/0968-0004(89)90265-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Salviati G., Pozzan T., Volpe P. Is a guanine nucleotide-binding protein involved in excitation-contraction coupling in skeletal muscle? EMBO J. 1986 Feb;5(2):259–262. doi: 10.1002/j.1460-2075.1986.tb04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Fukada Y., Ohguro H., Saito T., Yoshizawa T., Akino T. Beta gamma-subunit of bovine transducin composed of two components with distinctive gamma-subunits. J Biol Chem. 1989 Apr 5;264(10):5937–5943. [PubMed] [Google Scholar]
- Gabrion J., Brabet P., Nguyen Than Dao B., Homburger V., Dumuis A., Sebben M., Rouot B., Bockaert J. Ultrastructural localization of the GTP-binding protein Go in neurons. Cell Signal. 1989;1(1):107–123. doi: 10.1016/0898-6568(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Gao B., Mumby S., Gilman A. G. The G protein beta 2 complementary DNA encodes the beta 35 subunit. J Biol Chem. 1987 Dec 25;262(36):17254–17257. [PubMed] [Google Scholar]
- Goldsmith P., Backlund P. S., Jr, Rossiter K., Carter A., Milligan G., Unson C. G., Spiegel A. Purification of heterotrimeric GTP-binding proteins from brain: identification of a novel form of Go. Biochemistry. 1988 Sep 6;27(18):7085–7090. doi: 10.1021/bi00418a062. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Hammond C., Paupardin-Tritsch D., Homburger V., Rouot B., Bockaert J., Gerschenfeld H. M. An alpha 40 subunit of a GTP-binding protein immunologically related to Go mediates a dopamine-induced decrease of Ca2+ current in snail neurons. Neuron. 1988 Mar;1(1):27–32. doi: 10.1016/0896-6273(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburger V., Brabet P., Audigier Y., Pantaloni C., Bockaert J., Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987 Apr;31(4):313–319. [PubMed] [Google Scholar]
- Horgan D. J., Kuypers R. Isolation of transverse tubules by fractionation of sarcoplasmic reticulum preparations in ion-free sucrose density gradients. Arch Biochem Biophys. 1987 Mar;253(2):377–387. doi: 10.1016/0003-9861(87)90191-3. [DOI] [PubMed] [Google Scholar]
- Horne W. A., Abdel-Ghany M., Racker E., Weiland G. A., Oswald R. E., Cerione R. A. Functional reconstitution of skeletal muscle Ca2+ channels: separation of regulatory and channel components. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3718–3722. doi: 10.1073/pnas.85.11.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Milligan G., Tanfin Z., Goureau O., Unson C., Harbon S. Identification of both Gi2 and a novel, immunologically distinct, form of Go in rat myometrial membranes. FEBS Lett. 1989 Feb 27;244(2):411–416. doi: 10.1016/0014-5793(89)80574-5. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Péraldi S., Nguyen Than Dao B., Brabet P., Homburger V., Rouot B., Toutant M., Bouille C., Assenmacher I., Bockaert J., Gabrion J. Apical localization of the alpha subunit of GTP-binding protein Go in choroidal and ciliated ependymocytes. J Neurosci. 1989 Mar;9(3):806–814. doi: 10.1523/JNEUROSCI.09-03-00806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouot B., Carrette J., Lafontan M., Lan Tran P., Fehrentz J. A., Bockaert J., Toutant M. The adipocyte Go alpha-immunoreactive polypeptide is different from the alpha subunit of the brain Go protein. Biochem J. 1989 May 15;260(1):307–310. doi: 10.1042/bj2600307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer N. M., Toro M. J., Entman M. L., Birnbaumer L. G-protein distribution in canine cardiac sarcoplasmic reticulum and sarcolemma: comparison to rabbit skeletal muscle membranes and to brain and erythrocyte G-proteins. Arch Biochem Biophys. 1987 Dec;259(2):431–440. doi: 10.1016/0003-9861(87)90509-1. [DOI] [PubMed] [Google Scholar]
- Suki W. N., Abramowitz J., Mattera R., Codina J., Birnbaumer L. The human genome encodes at least three non-allellic G proteins with alpha i-type subunits. FEBS Lett. 1987 Aug 10;220(1):187–192. doi: 10.1016/0014-5793(87)80900-6. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Toutant M., Barhanin J., Bockaert J., Rouot B. G-proteins in skeletal muscle. Evidence for a 40 kDa pertussis-toxin substrate in purified transverse tubules. Biochem J. 1988 Sep 1;254(2):405–409. doi: 10.1042/bj2540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandaele S., Fosset M., Galizzi J. P., Lazdunski M. Monoclonal antibodies that coimmunoprecipitate the 1,4-dihydropyridine and phenylalkylamine receptors and reveal the Ca2+ channel structure. Biochemistry. 1987 Jan 13;26(1):5–9. doi: 10.1021/bi00375a002. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Salviati G., Di Virgilio F., Pozzan T. Inositol 1,4,5-trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature. 1985 Jul 25;316(6026):347–349. doi: 10.1038/316347a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Codina J., Hamilton S. L., Brown A. M., Birnbaumer L. The stimulatory G protein of adenylyl cyclase, Gs, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem. 1988 Jul 15;263(20):9887–9895. [PubMed] [Google Scholar]