Abstract
Improving biodiversity predictions is essential if we are to meet the challenges posed by global change. As knowledge is key to feed models, we need to evaluate how debated theory can affect models. An important ongoing debate is whether environmental constraints limit the number of species that can coexist in a community (saturation), with recent findings suggesting that species richness in many communities might be unsaturated. Here, we propose that biodiversity models could address this issue by accounting for a duality: considering communities as unsaturated but where species composition is constrained by different scale-dependent biodiversity drivers. We identify a variety of promising advances for incorporating this duality into commonly applied biodiversity modelling approaches and improving their spatial predictions.
Trends
The majority of biodiversity modelling approaches do not explicitly address the question of saturation.
Theoretical and methodological implications of saturation or unsaturation in biodiversity modelling.
Addressing saturation or unsaturation is vital to produce more reliable conservation strategies.
Integrative community modelling frameworks may be the way forward.
Taking a Modelling Perspective on an Old Debate: Are Communities Saturated?
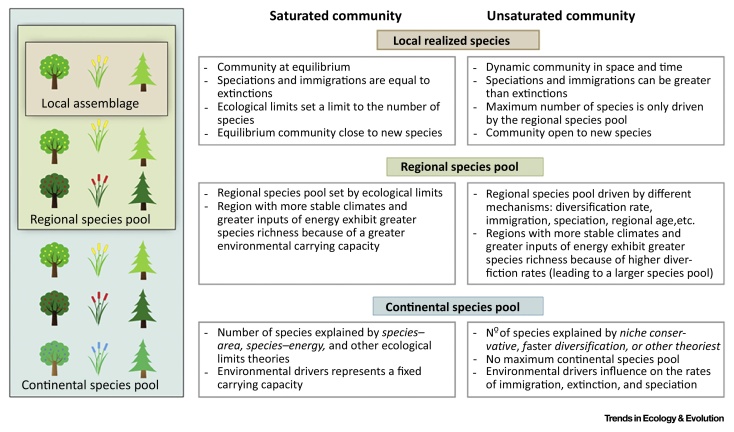
A recent debate 1, 2 has revived an old discussion in ecology: are communities ‘saturated’? Is there a limit to the number of species that a community (see Glossary) can support? Whether saturation plays a role in shaping communities remains a fundamental issue widely assessed in ecology and evolution, but no formal consensus has been reached (see Figure I in Box 1). If saturation occurs, is it caused by ecological limits or by geographic area? In particular, are communities constrained by an ‘environmental carrying capacity’ (ECC) that limits the number of coexisting species?
Figure I.
Main Theoretical Implications for Saturation and Unsaturation. The main theoretical differences and implications in community ecology assuming communities as saturated or unsaturated.
Box 1. Are Communities Saturated?
In the past, a large body of literature aimed at identifying the ecological limits of species richness concluded that strong control is exerted by environmental factors, especially climate conditions and environmental heterogeneity 29, 61 (and references therein). In this context, a central method used has been the regression of local on regional richness [62]; saturating or nonlinear functions indicate that an upper limit of local diversity has been set by ecological limits [63]. However, important methodological limitations, such as pseudoreplication, limit the use of this graphical approach 30, 64. More recently, ecological analyses of seed addition experiments 65, 66, contemporary biotic exchanges [66], fossil records [67], invasion processes 66, 68, 69, 70, the packing and filling of functional space considering functional trait data [71] (but see [72]), diversification models [73], and molecular phylogenies 1, 39, 74 have suggested that ecological systems might rarely be saturated and instead remain open to new species (Figure I).
The relevance of saturation might therefore be relative to the scale 31, 34, 75. Thus, some authors suggest that communities might not be saturated [76] or might only be saturated at very small scales (e.g., <1 m2 for plant communities [69]). At such scales, stochasticity (e.g., disturbances such as fires, neutral processes) 7, 50 is an important element in understanding biodiversity, with purely physical parameters becoming more important, such as how many individuals can occupy the total area. At coarser resolutions, according to several authors 1, 66, communities are dynamic in space and time and are virtually always accessible to new species (with speciation and migration occurring more often than extinction), and community diversity is not set by ecological limits. Nevertheless, richness would remain correlated with variables that are related to productivity or energy within a geographic space [77], though this correlation might be explained by alternative hypotheses 1, 38 (Boxes 2 and 3).
Although we take into consideration that communities might be unsaturated, at local scales and for high values of richness, the invasion of a community by new species is likely to become progressively more arduous [66]. This resistance might result from more intense biotic interactions at a higher species richness [66] and the inhibitory properties of established species [50]. Therefore, at fine spatial resolution, locally dominant species, organism body size, and disturbances are important factors [7].
Alt-text: Box 1
Answering this overarching saturation question is not only crucial from a fundamental perspective, but also constitutes a major challenge for the biodiversity models used to predict current and future patterns of species composition and richness. Here, we do not intend to continue or solve the saturation debate per se, but we aim instead to assess the key implications that this question has for modelling communities (see Outstanding Questions), particularly taking a spatial and temporal perspective.
Explicitly considering potential for unsaturation or saturation in biodiversity models can have drastic effects on their predictions as well as on their interpretation, yet this issue is rarely addressed. Predictions under global change or following biological invasions might differ markedly depending on the approaches applied and whether saturation is assumed or not. This is especially important if we consider that, under global change, the flux of species in and out of communities (i.e., the species turnover) is likely to increase [3]. The level to which communities are saturated is therefore likely to have a very large influence on the potential for species to shift their distributions and track changing climates, given inertia in the composition of communities, founder priority effects, and limits on space resources 4, 5. Hence, improving our capacity to predict species richness and community composition by considering saturation or unsaturation is key for using models to produce more reliable conservation strategies in response to global environmental change [6].
Biodiversity Modelling and Community Saturation
Tremendous effort has been expended to explain biodiversity patterns [7], including high-profile theories [8] (Box 2) and an increasing number of spatiotemporal biodiversity modelling techniques [9] (Table 1, Key Table). These theories and models focus on different questions, biodiversity drivers, contexts, and scales, with corresponding variety in the ways in which community saturation is considered. Here, we focus primarily on modelling approaches developed for constructing spatially explicit predictions of species richness and community composition across large regions and over changing environmental conditions. We emphasize correlative modelling approaches (and not stochastic ones, like neutral models; see [9] and references therein) because they are the techniques most commonly applied to address conservation issues [10] and predict future patterns of biodiversity in the face of global change [6], such as for global biodiversity assessments [11].
Box 2. Towards a Reinterpretation of Some Macroecological Theories?
The relationship between available energy and biodiversity is well known [78]. This species–energy relationship was suggested by Hutchinson [79] and has been interpreted by some authors as a limit on the number of species based on the available energy [80]. Consequently, fifty years ago, many ecologists assumed that communities were saturated [34]. Later, Currie [29] theorized that a measure of energy availability ought to enable the species richness in a region to be predicted [2]. This measure is interpreted by some authors as the ECC of a unit and defines the boundaries of community saturation 81, 82.
Nevertheless, the macroecological theories of a direct association between current climate and biodiversity, such as the ‘species–energy’ theory [78], ‘species–area’ theory [83], or ‘ecological limit’ hypothesis [2], have not led to an adequate description of all the drivers of species richness patterns 47, 77; for example, they do not consider evolutionary history factors [47]. At least three alternative theories 1, 7, 38, 39, the ‘niche conservative’ [84], the ‘faster diversification’, and the ‘metabolic’ theories [85], have been proposed in an attempt to explain this correlation between species richness and energy (and habitat heterogeneity) without placing a limit on the number of species. These theories predict that greater values of biodiversity are observed not because of greater energy or space availability, but because more species in the regional species pool are adapted to the ecological conditions in the area. These theories are the most promising for explaining diversity patterns because they are integrative and consider a combination of drivers that affect the fundamental processes underlying species richness patterns (evolution, immigration, extinctions, and ecological interactions), rather than only the influence of environmental variables [7]. For example, these theories note that time and diversification rates, as well as adaptive traits, are important drivers of biodiversity patterns [86] and that mutualistic interactions promote diversification in some groups [73]. More studies might still be needed to explain the role of evolutionary history in community assembly 33, 87.
Recently, Cornell [38] proposed the new ‘damped increase hypothesis’, which attempts to reconcile two opposing hypotheses (saturated vs. unsaturated). Cornell’s theory further predicts that ‘biodiversity generally increases through the time but that its rate of increase is often slowed by ecological constraints’. Under this point of view, the regional species pool remains an important concept, recalling the importance of scale in community assembly (Box 3).
Alt-text: Box 2
Table 1.
| Modelling approach | Predictions and model type | Saturation assumption | Taxonomic scope | Complexity | Methodological solution |
|---|---|---|---|---|---|
| S-SDM [15] | SC and SR from stacked correlative SDMs | No | All except rare species (e.g., <10 occurrences) |
Low | Incorporate constraints as predictors to avoid overpredicting richness |
| Mechanistic S-SDM [25] |
SC and SR from stacked mechanistic SDMs | No | Groups with physiological data for all species | High | Need to incorporate processes of interspecific interactions and other ecological constraints |
| Joint-SDM [21] | SC and SR from a multispecies correlative model | Yes (implicitly) | Small communities (computational limits) | High | Same as S-SDM but including interactions and allowing rare species |
| Correlative MEM [29] |
Only SR (or other whole community properties) from correlative model | Yes (explicitly) | Total richness based on all species (no limitation), no composition | Low | Implicitly assume saturation New, multiscale and probabilistic solutions will be needed to account for unsaturation |
| Simulation MEM [47] | Only SR from MEM based on (non-niche-based) range simulations | No | Total richness based on all species (no limitation), no composition | High | Through individual range simulations, theoretically includes solutions to account for unsaturation, i.e., account for biogeographic legacies |
| SESAM [22] | SC and SR from integrating correlative S-SDMs, correlative MEMs, species pools, and assembly rules | Flexible | All except rare species (e.g., <10 occurrences) |
Moderate | Saturation can or not be enforced. Possibility of probabilistic SR predictions. Switching ‘on’ or ‘off’ of various eco-evolutionary filters/constraints at different scales |
| DynamicFOAM [23] | SC from correlative MEM and dissimilarity model | Yes (explicitly) | All species | High | Possibility of probabilistic SR predictions. Predictions can be tuned by different drivers and constrains at different scales |
| M-SET [24] | Metacommunity model. SC from processes as well as correlative MEM | Yes (explicitly) | All species | High | Possibility to switch ‘off’ saturation, and to incorporate additional drivers constraining richness without assuming saturation |
| Hierarchical Bayesian [35] | SR using a flexible-scale hierarchical Bayesian framework | No | All species, no composition | High | Explicitly incorporate probability distribution of SR, whose properties depend on different drivers at different scales |
| DVM [28] | Dominant tree and shrub species at local scale from forest dynamic gap models, and thus SR or SC for these species | Not strictly | Suite of dominant species with known ecodemographic parameters | High | Saturation implicitly assumed by the number of species parameterized in the model. Could be alleviated by adding any new species entering the system if parameters available |
| DGVM [26] | Mainly functional plant composition from mechanistic model at large scales. Possibility for some DGVM to model some dominant species | Not really applicable | Plant functional groups, dominant plant species | High | Not a solution directly applicable. Could be coupled with or incorporated into one of the integrative community modelling frameworks (see above) to set constraints (e.g., to available water for the community) |
The table presents identified methodological solutions when considering unsaturation and biodiversity constraints in different modelling approaches to predict SR and SC. The complexity (need for data and computational time) and the taxonomic scope of the model are also provided.
Abbreviations: DGVM, dynamic global vegetation models; DVM, dynamic vegetation model; SC, species composition; SR, species richness.
Two commonly applied biodiversity modelling approaches [12] crystallize the debate on saturation when taken from a modelling perspective, predicting for instance similar or distinct patterns under future climate [13]. The first approach to predicting community composition and diversity has been to model the distributions of many individual species and combine the projected occurrences or probabilities – that is, stacked species distribution models (S-SDM); ‘predict-first, assemble later’ in [14]. This S-SDM approach implicitly assumes unsaturated communities [12], and has been used in situations such as reconstructing patterns of plant diversity along elevation [15]. It has been suggested that the implicit assumption made by S-SDMs of unsaturated communities could help explain their observed tendency to overpredict species richness 12, 15 (but see [16]). One proposed solution is to stack the original probabilistic predictions rather than converting them to binary ones 12, 17.
The second alternative approach, macroecological models (MEMs) [12], predicts the number of species (i.e., species richness) directly as a function of various hypothesized environmental drivers (‘assemble first, predict later’ in [14]), thus assuming species–energy, species–area, and other richness–environment relationships (Box 2) to implicitly define limits to the expected number of species based on some ECC (Box 2) [12].
A variety of other biodiversity modelling approaches also lie in-between these two extremes, which correlate the whole species composition to environmental factors based on the covariance of species, thus allowing simultaneous predictions of the occurrences of multiple species. These approaches thus better incorporate the effect of biotic interactions, but usually do not consider saturation explicitly. Typical examples include ordination-based modelling approaches such as canonical correspondence analysis [18], multivariate regression trees [19], constrained additive ordination [20], or approaches directly accounting for interactions in SDMs, for example, the recent joint SDMs (J-SDMs) [21]. Constrained additive ordination and J-SDMs are, however, currently technically limited to relatively small suites of species, usually a subset of all the species of a taxonomic group present in a region.
Very recently, whole integrative community modelling frameworks have been proposed [9]. For example, the spatially explicit species assemblage modelling (SESAM) [22] approach can (or not) use MEM predictions (in such case assuming saturation), but also other information, such as biotic interactions and regional species pools, to constrain S-SDMs predictions [22]. The recent linked approaches of DynamicFOAM [23] and the M-SET metacommunity model [24] more explicitly consider beta-diversity patterns (which SESAM does not), and also include saturation explicitly [9] (Table 1). While these new approaches have been successful in improving predictions of community composition and diversity [16], the question of the saturation assumption remains crucial, especially if these approaches are to be harnessed for projecting changes into the future.
A range of mechanistic models have also been used to make spatial predictions of communities 8, 24. Mechanistic models of single species (e.g., mechanistic SDMs) [25] are typically only applicable to a small number of species for which the required input parameters are available 26, 27. By contrast, mechanistic models of whole communities or ecosystems often apply to a specific taxonomic or functional group, and those able to predict composition usually only apply to a defined region and limited number of species (e.g., dominant trees and shrubs in forest gap models) [28]. The latter typically assume unsaturated communities, and let species richness and composition emerge from the component processes (e.g., resource use, competition). However, mechanistic models applicable to large spatial extents are usually not informative of species composition and cannot always predict richness, for example, dynamic vegetation models of intermediate complexity (dynamic global vegetation models) [26], and hence do not provide a simple solution in themselves for considering (un)saturation in biodiversity modelling of whole flora and fauna. However, more mechanisms could be added to the modelling of communities by coupling or incorporating dynamic global vegetation models with or into one of the integrative community modelling frameworks (Table 1) – for example, as additional community constraints.
Identifying a Duality: Unsaturation and Constraints in Biodiversity Modelling
Studies that have examined the evidence for or against saturation have yielded contrasting results, and no unanimous conclusion has emerged 1, 2. Yet, one major advance is that unsaturation is increasingly considered a valid and likely assumption. Although certainly not valid for all cases (e.g., not at very fine scale where physical space becomes limiting; Box 1), it might apply to a large majority of communities, especially towards larger resolutions (Box 1). Therefore, biodiversity modelling frameworks [9] should at least partially incorporate an unsaturated perspective, allowing some flexibility in the number of species occurring in a community. Yet, the multitude of MEM predicting species richness from its direct correlation with environmental factors has also shown that variations in richness follow, at least in part, the variations in environmental conditions (Box 2). And accordingly, the mean or maximum number of species in a given location can, in many cases, be predicted with reasonable accuracy from limits in available area, energy, resources, or heterogeneity [29] (Box 2).
This contradiction represents the key challenge for biodiversity modelling stemming from the ongoing saturation debate 1, 2. Here, we suggest that this apparent contradiction underlies a duality: all communities might be unsaturated and yet inherently constrained in their composition by various drivers (i.e., ecological, evolutionary, historical, or biological; Box 3). Biodiversity modelling approaches therefore need to deal with these two dimensions – unsaturation and constraints – simultaneously.
Box 3. On the Significance of Scale for Biodiversity Drivers.
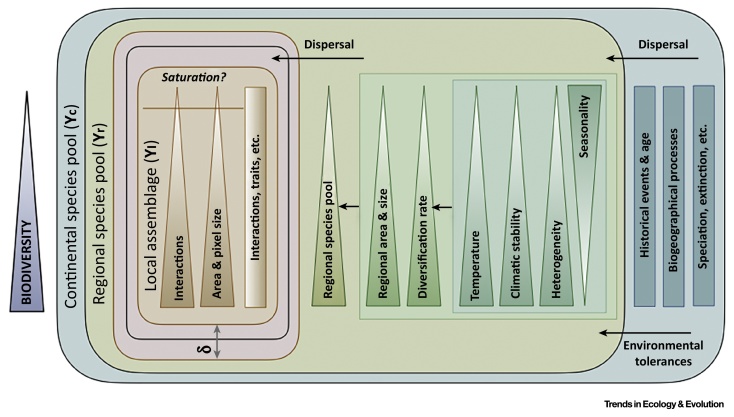
Communities are assembled by drivers that operate over a variety of temporal and spatial scales [31] (Figure I). Local species richness has been reported to be limited or driven by local factors, such as available energy [78] and some authors state that saturation is only possible at this local scale (Box 1). In this view, local environmental factors (mainly energy and habitat heterogeneity) constitute important controls on species richness (Box 2). Other studies and theories have suggested that achieving a comprehensive understanding of biodiversity patterns at local scale also requires information on regional and historical drivers (Box 2). Under this perspective communities are not saturated; local assemblages would not only be governed by local ecological limits, but also by processes at larger scale (e.g., historical contingencies, evolution) [88]. In addition, biotic and abiotic filters [89] might interact to downgrade regional species composition in local sites [89]. Following this reasoning, saturation would not be possible at regional and continental scales. The regional species pool would be mainly shaped by speciation, immigration, range extension, diversification rates, and regional age 38, 39. Diversification rates can potentially be driven by climatic stability, time available for diversification, climatic age, time available for immigration, ambient energy, the size of the regional area, productivity, and habitat heterogeneity 38, 39. Areas with stable climates and greater inputs of energy should exhibit greater species numbers, not because of a greater ECC [90], but because they present higher diversification rates and, consequently, larger regional species pools [38]. At the continental scale, richness patterns are shaped mainly by biogeographical drivers (rates of speciation, immigration and extinction of lineages, and historical processes) and dispersal limitations [39].
To summarize, the maximum species richness at a local site might be more strongly influenced by the regional species pool than by some ECC. Thus, to understand the drivers of local richness, the drivers of regional and continental biodiversity must first be known [76]. An improved understanding of biodiversity drivers is key to building a more robust theory that would improve our ability to predict biodiversity.
Alt-text: Box 3
Communities are assembled by biodiversity drivers that operate over a variety of temporal and spatial scales 30, 31, 32, 33, 34 (Box 3). And although scale can be a complicating factor, an increasing number of hierarchical approaches are now emerging that should allow integrating scale within modelling frameworks [35]. More importantly, most correlative biodiversity modelling approaches have neglected important theoretical advances, especially from evolutionary biology 33, 36, 37. Diversification rates (Box 3), in particular, are expected to be major processes influencing the biodiversity found in a site 33, 36, 38, with major effects on two important aspects: (i) the size of the regional species pool, which also depends on the dispersal history of taxa [39], and (ii) the size of the ‘habitat’ species pool, nested within the regional pool, as defined in [22], that is, the number of species that could successfully evolve adaptations to particular environments [39]. Incorporating constraints such as these into biodiversity modelling, within an unsaturated community perspective, therefore requires a range of innovative modelling solutions.
Confronting the Duality: Accounting for Unsaturation and Constraints in Biodiversity Modelling
Here, we propose that advancing biodiversity modelling requires accounting for the duality of unsaturated communities where species composition is constrained by a variety of scale-dependent biodiversity drivers. An important core requirement in developing more robust biodiversity models under this perspective would be to improve our basic ecological understanding of saturation, and the ways in which drivers and constraints influence community assembly processes at different scales 40, 41. These empirical investigations will however be especially informative where applied at the local community (e.g., 30–1000 m) and regional (e.g., 100–5000 km) extents that match those typical of biodiversity models.
The development of improved biodiversity models should thus benefit from incorporating new information from the increasing number of macroecological studies on systematics, phylogenetics, biogeography, palaeontology, and other approaches [42], which all represent biodiversity drivers and potential constraints on community assemblages. For example, phylogenetic community ecology (ecophylogenetics) [43] is an emerging field that uses phylogenetics to test hypotheses about how ecological communities are assembled by providing temporal and evolutionary dimensions to community ecology 33, 44, 45. New perspectives are also offered by evolutionary models simulating speciation and extinction events through time, or by operationalizing trait-based environmental filtering knowledge into predictive modelling techniques [46]. It would also be important to provide flexibility such that these models could consider complementary or alternative theories than the current mainstream theories (e.g., ‘species–energy’), such as the ‘faster diversification or metabolic’ theories (Box 2), and could include complementary spatial information (i.e., not strictly related to ECC), such as the location of endemism centres, patterns of beta-diversity, species phylogenies, phylogeographical patterns, and species traits. Innovative, more mechanistic approaches 47, 48 will thus be needed to incorporate key processes with generic, easily applicable correlative modelling techniques, including multiple interacting attributes of species (e.g., environmental suitability, functional traits), local properties of assemblages (e.g., resource availability, co-occurrence patterns, disturbance), and regional (e.g., species pool, dispersal barriers) and continental (historical factors, speciation) contexts.
For the widely applied S-SDM approach, we see that it appropriately considers communities as unsaturated, but it ignores many important constraints on community composition (see below), hence it bears the risk of overpredicting the number of species in a location [22]. However, as a way forward, it could be useful here to view the species that are overpredicted as the ‘dark biodiversity’ [49] of the unsaturated local assemblage, or a set of species that could potentially invade the community [50]. Additional constraints could be incorporated into S-SDMs, such as through new multiscale (and potentially more mechanistic) spatial (i.e., remote sensing) layers [51] in the component SDMs that better encapsulate some of the nonabiotic constraints to species occurrence, such as known size of the regional species pool, historical events, site accessibility, or biotic interactions. Regarding the later, further development of J-SDMs or other similar approaches might be beneficial in deriving SDMs that incorporate some elements of interspecific interactions as community constraints [52]. In this regard, an explicit way to incorporate these additional constraints into S-SDMs could involve application of the SESAM approach, which integrates species-level and community-level modelling and information to account for biotic interactions (see below) [53].
By contrast, correlative curve-fitting MEMs can capture spatial and environmental constraints in predicting species richness, with accurate predictions under present conditions (Box 2). However, because these models implicitly tend to consider communities as saturated, their predictions of species richness into the future, under global change and shifting species distributions, are likely to be far less reliable. As with S-SDMs, there could be opportunities for accounting for unsaturation in correlative MEMs through incorporating scalers modifying spatially explicit richness predictions as predictors in the models. Layers describing the likely regional species richness around every grid cell (either now, or in the future; e.g., focal windows approaches) could be used for such a purpose, enabling the predictions of MEMs to be ‘corrected’ by the neighbourhood, for example, in areas likely to experience substantial influx of range-shifting species. Pattern-oriented modelling approaches could also be an interesting alternative [54], where it is possible to use correlative MEMs conceptually based on theories that do not assume saturation (Box 2). An initial set of relevant explanatory variables – not only energy-related, and not assuming saturation – could be used from which to define competing models that best encompass and predict species richness patterns at the targeted scale. For example, Mateo et al. [55] employed a multimodel inference approach within an MEM framework for this purpose. Provided all explanatory variables are spatially explicit, model averaging can then be used to derive spatial predictions. Following this procedure, MEMs would not just limit the number of species by energy drivers, but would represent the statistical correlation between different biodiversity drivers (e.g., historical climatic stability, annual climatic stability, geographical and spatial gradients, historical aspects, evolutionary history, vegetation types, human impact) and species richness patterns, potentially involving other processes than saturation to predict biodiversity patterns.
One recurrent problem of the simple correlative approaches previously described (e.g., one single model for richness or a simple stacking of species models) is that they cannot account explicitly for processes, but can only fit and predict patterns. This limits the capacity for these approaches to fully account for the duality of unsaturated communities where composition is constrained by different drivers. Here, we propose instead that if models have to account for this duality, they should be able to incorporate the different mechanisms behind biodiversity drivers (i.e., ecological, historical, and evolutionary processes) operating across multiple scales. There is thus a challenge to develop modelling approaches incorporating more process-based drivers in generic, easily transferable, and applicable ways.
One way forward is to further develop the new integrative community modelling frameworks, like SESAM [22] and DynamicFOAM [24], or M-SET [56], by enabling these to use mechanistic knowledge as much as possible, relying less on empirically measured (i.e., observed) correlations. In particular, D’Amen et al. [9] suggested the use of such frameworks in a way that different modelling modules are not only more mechanistic but can also be switched ‘on’ or ‘off’ after identifying the importance of different drivers. This could be the perfect setting to account for both unsaturation and constraints in biodiversity modelling. For instance, within the SESAM framework based on stacking predictions from individual species models [16], unsaturation could be simply enforced by not constraining the stack of species predictions by a separate richness prediction model (MEM) while using eco-evolutionary models to define the regional and habitat species pools. The individual species models could then be based on mechanistic approaches (e.g., [25]), and experimentally based (rather than empirical) biotic interactions (e.g., [57]) could, for instance, be used to determine which species might ultimately coexist. Where environmental constraints are particularly strong (e.g., small pixel size at local scale, with tight richness–environment relationships), application of the SESAM, DynamicFOAM, or M-SET frameworks (or the simpler MEM) might then be used as currently.
Finally, a further promising development in such integrated frameworks could be to consider the final predicted composition or species richness as a probability distribution, whose properties (i.e., mean and variance) depend on the different processes at different scales (see Figure I in Box 3). As an unsaturated community does not have a fixed number of species, the probability distribution of species richness values would define the flexibility to capture and predict the composition and species richness at a local scale. One approach to develop such analysis is a Bayesian hierarchical modelling approach [35], where each driver could be considered as a different component in the modelling framework 9, 24. A Bayesian hierarchical approach allows a conceptual approximation and incorporation of different model components, and inclusion of diverse information sources from empirical ecological studies 58, 59, for example, the regional species pool. Bayesian hierarchical modelling offers a flexible approach to incorporate drivers that operate over a variety of temporal and spatial scales (Box 3), though development of generic, easily transferable, and applicable models requires further attention. We suggest exploring the implementation of different hierarchical Bayesian methods frequently applied to spatial–temporal clinical data, for example ‘Joint Models’ [60], and represent useful starting points to develop methodological solutions for the spatial modelling of communities and biodiversity.
Figure I.
Main Biodiversity Drivers across Scales. The main drivers influencing species diversity (purple) across scales, considering theories that reflect communities as unsaturated at local (brown), regional (green), and continental (blue) scales. Triangles express the magnitude of the relationship (the wider part reflects greater magnitude) between the driver and the biodiversity. Integrated biodiversity modelling frameworks could be used to consider the final predicted composition or species richness as a probability distribution (δ), whose properties (i.e., mean and variance) depend on the different drivers and processes at different scales (γl, γr, γc).
Concluding Remarks
Our understanding of community saturation or unsaturation is far from complete, and the ways in which drivers influence community assembly processes at different scales remain one of the most important challenges in ecology and biogeography 40, 41. Substantial effort, including new methodological and conceptual approaches, will be needed in the future to illuminate this research area. These studies should be considered as important conceptual bases for improving biodiversity models that have typically ignored, or insufficiently addressed, the saturation concept to date. Here, we have proposed a number of practical solutions to accounting for a duality of unsaturated communities in biodiversity models, where species richness is constrained by scale-dependent biodiversity drivers, where the final predicted composition or species richness is a probability distribution, whose properties depend on the different processes at different scales.
Outstanding Questions.
New findings suggest that saturation (maximal level of local richness by ecological constraints) in communities might only occur at a very small spatial scale (e.g., a few square meters), while many communities might not be saturated (Box 1). However, most biodiversity modelling approaches do not consider saturation concept. Therefore, a new question emerges for biodiversity modelling: how should modelling proceed if we assume communities are unsaturated? What are the drivers or constraints of community assembly and biodiversity patterns if saturation is not assumed? How do the biodiversity drivers then vary depending on scale? What are the conceptual and methodological implications of unsaturation for biodiversity models? Can unsaturated models still accurately predict current and future patterns in species richness and community composition?
Acknowledgements
R.G.M. was funded by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme (ACONITE, PIEF-GA-2013-622620). A.G. received support from the Swiss National Science Foundation (SESAM’ALP and INTEGRALP projects). Many thanks are due to Paul Craze, David Nogués-Bravo, and one anonymous referee for their constructive comments on the manuscript.
Glossary
- Biodiversity models
here, we consider biodiversity models that use data from most or all the species in a taxonomic group to generate information about the spatial patterns in the distribution of diversity at the community level. The outputs from these biodiversity models comprise predictive mapping of species richness or gradients of compositional variation [14].
- Community
an assemblage of species that co-occur in a location at a given time.
- Community assembly
mechanisms (drivers) that determine the species composition of a community.
- Dark biodiversity
portion of the regional species pool that is absent from the local community [49]. In other words, the species present at a regional scale that can potentially colonize the local community but are absent from it. This concept provides valuable information for conservation applications and for improving knowledge of community assembly processes [49].
- Environmental carrying capacity (ECC) of a community
‘steady-state level of richness specific to a particular site or local ecosystem that is set by resource availability and other local conditions and is maintained despite changes in species composition’ [90]. Here it thus refers to a carrying capacity for the number of species in a community, not to be misled with the ECC that traditionally refers to the number of individuals that a population of a single species can sustain in a given environment.
- Macroecological models
biological data are first classified or aggregated to produce community-level data (e.g., species richness data) that are then modelled as such in relation to environmental predictors. These models have been described as ‘assemble first, predict later’ models by Ferrier and Guisan [14].
- Regional species pool
set of species present in a region that could potentially colonize a local site or community based on the suitability of local ecological conditions.
- Saturation
in a saturated community, local richness exhibits a maximal level that depends on ecological or areal constraints or limits.
- Species richness
number of species present in a community or area. This concept is equivalent to α-diversity.
- Stacked species distribution models
individual species are modelled separately as a function of environmental variables; the model predictions are then stacked to produce a potential richness map. These models have been described as ‘predict first, assemble later’ models by Ferrier and Guisan [14].
References
- 1.Harmon L.J., Harrison S. Species diversity is dynamic and unbounded at local and continental scales. Am. Nat. 2015;185:584–593. doi: 10.1086/680859. [DOI] [PubMed] [Google Scholar]
- 2.Rabosky D.L., Hurlbert A.H. Species richness at continental scales is dominated by ecological limits. Am. Nat. 2015;185:572–583. doi: 10.1086/680850. [DOI] [PubMed] [Google Scholar]
- 3.Alexander J.M. When climate reshuffles competitors: a call for experimental macroecology. Trends Ecol. Evol. 2016;31:831–841. doi: 10.1016/j.tree.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blois J.L. Modeling the climatic drivers of spatial patterns in vegetation composition since the last glacial maximum. Ecography. 2013;36:460–473. [Google Scholar]
- 5.Blonder B. Linking environmental filtering and disequilibrium to biogeography with a community climate framework. Ecology. 2015;96:972–985. doi: 10.1890/14-0589.1. [DOI] [PubMed] [Google Scholar]
- 6.Nogués-Bravo D., Rahbek C. Communities under climate change. Science. 2011;334:1070–1071. doi: 10.1126/science.1214833. [DOI] [PubMed] [Google Scholar]
- 7.Lomolino M.V. Sinauer Associates; 2010. Biogeography. [Google Scholar]
- 8.Cabral J.S. Mechanistic simulation models in macroecology and biogeography: state-of-art and prospects. Ecography. 2017;40:267–280. [Google Scholar]
- 9.D’Amen M. Spatial predictions at the community level: from current approaches to future frameworks. Biol. Rev. 2017;92:169–187. doi: 10.1111/brv.12222. [DOI] [PubMed] [Google Scholar]
- 10.Guisan A. Predicting species distributions for conservation decisions. Ecol. Lett. 2013;16:1424–1435. doi: 10.1111/ele.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (2016) The Methodological Assessment Report on Scenarios and Models of Biodiversity and Ecosystem Services (Ferrier, S., et al., eds), pp. 348, Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services
- 12.Dubuis A. Predicting spatial patterns of plant species richness: a comparison of direct macroecological and species stacking approaches. Divers. Distrib. 2011;17:1122–1131. [Google Scholar]
- 13.Distler T. Stacked species distribution models and macroecological models provide congruent projections of avian species richness under climate change. J. Biogeogr. 2015;42:976–988. [Google Scholar]
- 14.Ferrier S., Guisan A. Spatial modelling of biodiversity at the community level. J. Appl. Ecol. 2006;43:393–404. [Google Scholar]
- 15.Mateo R.G. Do stacked species distribution models reflect altitudinal diversity patterns? PLoS One. 2012;7:e32586. doi: 10.1371/journal.pone.0032586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amen M. Using species richness and functional traits predictions to constrain assemblage predictions from stacked species distribution models. J. Biogeogr. 2015;42:1255–1266. [Google Scholar]
- 17.Calabrese J.M. Stacking species distribution models and adjusting bias by linking them to macroecological models. Glob. Ecol. Biogeogr. 2014;23:99–112. [Google Scholar]
- 18.Guisan A. GLM versus CCA spatial modeling of plant species distribution. Plant Ecol. 1999;143:107–122. [Google Scholar]
- 19.De’Ath G. Multivariate regression trees: a new technique for modeling species-environment relationships. Ecology. 2002;83:1105–1117. [Google Scholar]
- 20.Yee T.W. Constrained additive ordination. Ecology. 2006;87:203–213. doi: 10.1890/05-0283. [DOI] [PubMed] [Google Scholar]
- 21.Pollock L.J. Understanding co-occurrence by modelling species simultaneously with a Joint Species Distribution Model (JSDM) Methods Ecol. Evol. 2014;5:397–406. [Google Scholar]
- 22.Guisan A., Rahbek C. SESAM – a new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J. Biogeogr. 2011;38:1433–1444. [Google Scholar]
- 23.Mokany K., Ferrier S. Predicting impacts of climate change on biodiversity: a role for semi-mechanistic community-level modelling. Divers. Distrib. 2011;17:374–380. [Google Scholar]
- 24.Mokany K. Dynamic macroecology and the future for biodiversity. Glob. Change Biol. 2012;18:3149–3159. doi: 10.1111/j.1365-2486.2012.02760.x. [DOI] [PubMed] [Google Scholar]
- 25.Kearney M., Porter W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 26.Boulangeat I. Improving plant functional groups for dynamic models of biodiversity: at the crossroads between functional and community ecology. Glob. Change Biol. 2012;18:3464–3475. doi: 10.1111/j.1365-2486.2012.02783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallien L. Predicting potential distributions of invasive species: where to go from here? Divers. Distrib. 2010;16:331–342. [Google Scholar]
- 28.Bugmann H. A review of forest gap models. Clim. Change. 2001;51:259–305. [Google Scholar]
- 29.Currie D.J. Energy and large-scale patterns of animal-species and plant-species richness. Am. Nat. 1991;137:27–49. [Google Scholar]
- 30.Srivastava D.S. Using local–regional richness plots to test for species saturation: pitfalls and potentials. J. Anim. Ecol. 1999;68:1–16. [Google Scholar]
- 31.Godfray H.C.J., Lawton J.H. Scale and species numbers. Trends Ecol. Evol. 2001;16:400–404. doi: 10.1016/s0169-5347(01)02150-4. [DOI] [PubMed] [Google Scholar]
- 32.Loreau M. Are communities saturated? On the relationship between α, β and γ diversity. Ecol. Lett. 2000;3:73–76. [Google Scholar]
- 33.Mittelbach G.G., Schemske D.W. Ecological and evolutionary perspectives on community assembly. Trends Ecol. Evol. 2015;30:241–247. doi: 10.1016/j.tree.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Ricklefs R.E., Jenkins D.G. Biogeography and ecology: towards the integration of two disciplines. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:2438–2448. doi: 10.1098/rstb.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kery M., Royle J.A. Academic Press; 2015. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS. [Google Scholar]
- 36.Johnson M.T.J., Stinchcombe J.R. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 2007;22:250–257. doi: 10.1016/j.tree.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Thuiller W. A road map for integrating eco-evolutionary processes into biodiversity models. Ecol. Lett. 2013;16:94–105. doi: 10.1111/ele.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornell H.V. Is regional species diversity bounded or unbounded? Biol. Rev. 2013;88:140–165. doi: 10.1111/j.1469-185X.2012.00245.x. [DOI] [PubMed] [Google Scholar]
- 39.Cornell H.V., Harrison S.P. What are species pools and when are they important? Annu. Rev. Ecol. Evol. Syst. 2014;45:45–67. [Google Scholar]
- 40.Sexton J.P. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 2009;40:415–436. [Google Scholar]
- 41.Loreau M., de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- 42.Pinto-Sánchez N.R. Using historical biogeography to test for community saturation. Ecol. Lett. 2014;17:1077–1085. doi: 10.1111/ele.12310. [DOI] [PubMed] [Google Scholar]
- 43.Mouquet N. Ecophylogenetics: advances and perspectives. Biol. Rev. 2012;87:769–785. doi: 10.1111/j.1469-185X.2012.00224.x. [DOI] [PubMed] [Google Scholar]
- 44.Cavender-Bares J. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 45.Donoghue M.J. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11549–11555. doi: 10.1073/pnas.0801962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Münkemüller T., Gallien L. VirtualCom: a simulation model for eco-evolutionary community assembly and invasion. Methods Ecol. Evol. 2015;6:735–743. [Google Scholar]
- 47.Gotelli N.J. Patterns and causes of species richness: a general simulation model for macroecology. Ecol. Lett. 2009;12:873–886. doi: 10.1111/j.1461-0248.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- 48.Kerr J.T. The macroecological contribution to global change solutions. Science. 2007;316:1581–1584. doi: 10.1126/science.1133267. [DOI] [PubMed] [Google Scholar]
- 49.Pärtel M. Dark diversity: shedding light on absent species. Trends Ecol. Evol. 2011;26:124–128. doi: 10.1016/j.tree.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cord A.F. Remote sensing data can improve predictions of species richness by stacked species distribution models: a case study for Mexican pines. J. Biogeogr. 2014;41:736–748. [Google Scholar]
- 52.Wisz M.S. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. 2013;88:15–30. doi: 10.1111/j.1469-185X.2012.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gavish Y. Accounting for biotic interactions through alpha-diversity constraints in stacked species distribution models. Methods Ecol. Evol. 2017 Published online February 17, 2017. [Google Scholar]
- 54.Grimm V. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science. 2005;310:987–991. doi: 10.1126/science.1116681. [DOI] [PubMed] [Google Scholar]
- 55.Mateo R.G. The mossy North: an inverse latitudinal diversity gradient in European bryophytes. Sci. Rep. 2016;6:25546. doi: 10.1038/srep25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mokany K. Combining α- and β-diversity models to fill gaps in our knowledge of biodiversity. Ecol. Lett. 2011;14:1043–1051. doi: 10.1111/j.1461-0248.2011.01675.x. [DOI] [PubMed] [Google Scholar]
- 57.Keddy P. Relative competitive performance of 63 species of terrestrial herbaceous plants. J. Veg. Sci. 2002;13:5–16. [Google Scholar]
- 58.Golding N., Purse B.V. Fast and flexible Bayesian species distribution modelling using Gaussian processes. Methods Ecol. Evol. 2016;7:598–608. [Google Scholar]
- 59.Talluto M.V. Cross-scale integration of knowledge for predicting species ranges: a metamodelling framework. Glob. Ecol. Biogeogr. 2015;25:238–249. doi: 10.1111/geb.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Held L. Towards joint disease mapping. Stat. Methods Med. Res. 2005;14:61–82. doi: 10.1191/0962280205sm389oa. [DOI] [PubMed] [Google Scholar]
- 61.MacArthur R.H., Wilson E.O. An equilibrium theory of insular zoogeography. Evolution. 1963;17:373–387. [Google Scholar]
- 62.Lawton J.H. Are there general laws in ecology? Oikos. 1999;84:177–192. [Google Scholar]
- 63.Cornell H.V. Unsaturation and regional influences on species richness in ecological communities: a review of the evidence. Ecoscience. 1999;6:303–315. [Google Scholar]
- 64.Hillebrand H. Regressions of local on regional diversity do not reflect the importance of local interactions or saturation of local diversity. Oikos. 2005;110:195–198. [Google Scholar]
- 65.Myers J.A., Harms K.E. Seed arrival, ecological filters, and plant species richness: a meta-analysis. Ecol. Lett. 2009;12:1250–1260. doi: 10.1111/j.1461-0248.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 66.Smith S.A., Shurin J.B. Room for one more? Evidence for invasibility and saturation in ecological communities. In: Cadotte M.W., editor. Conceptual Ecology and Invasion Biology: Reciprocal Approaches to Nature. Springer; 2006. pp. 423–447. [Google Scholar]
- 67.Marcot J.D. Late Cenozoic onset of the latitudinal diversity gradient of North American mammals. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7189–7194. doi: 10.1073/pnas.1524750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stohlgren T.J. The myth of plant species saturation. Ecol. Lett. 2008;11:313–322. doi: 10.1111/j.1461-0248.2008.01153.x. [DOI] [PubMed] [Google Scholar]
- 69.Sax D.F. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Seebens H. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017;8:14435. doi: 10.1038/ncomms14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swenson N.G., Weiser M.D. On the packing and filling of functional space in eastern North American tree assemblages. Ecography. 2014;37:1056–1062. [Google Scholar]
- 72.Swenson N.G. Constancy in functional space across a species richness anomaly. Am. Nat. 2016;187:E83–E92. doi: 10.1086/685083. [DOI] [PubMed] [Google Scholar]
- 73.Lagomarsino L.P. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae) New Phytol. 2016;210:1430–1442. doi: 10.1111/nph.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etienne R.S. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proc. Biol. Sci. 2011;279:1300–1309. doi: 10.1098/rspb.2011.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fridley J.D. The invasion paradox: reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:tiprpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 76.Gaston K.J., Blackburn T.M. Blackwell; 2000. Pattern and Process in Macroecology. [Google Scholar]
- 77.Currie D.J. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004;7:1121–1134. [Google Scholar]
- 78.Wright D.H. Species-energy theory: an extension of species-area theory. Oikos. 1983;41:496–506. [Google Scholar]
- 79.Hutchinson G.E. Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. [Google Scholar]
- 80.Hairston N.G. Community structure, population control, and competition. Am. Nat. 1960;94:421–425. [Google Scholar]
- 81.Boucher-Lalonde V. Does climate limit species richness by limiting individual species’ ranges? Proc. Biol. Sci. 2014;281:20132695. doi: 10.1098/rspb.2013.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hurlbert A.H., Stegen J.C. When should species richness be energy limited, and how would we know? Ecol. Lett. 2014;17:401–413. doi: 10.1111/ele.12240. [DOI] [PubMed] [Google Scholar]
- 83.Hurlbert A.H., Jetz W. More than “more individuals”: the nonequivalence of area and energy in the scaling of species richness. Am. Nat. 2010;176:50–65. doi: 10.1086/650723. [DOI] [PubMed] [Google Scholar]
- 84.Rangel T. Species richness and evolutionary niche dynamics: a spatial pattern-oriented simulation experiment. Am. Nat. 2007;170:602–616. doi: 10.1086/521315. [DOI] [PubMed] [Google Scholar]
- 85.Allen A.P. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. [DOI] [PubMed] [Google Scholar]
- 86.Zobel M. The formation of species pools: historical habitat abundance affects current local diversity. Glob. Ecol. Biogeogr. 2011;20:251–259. [Google Scholar]
- 87.Vanoverbeke J. Community assembly is a race between immigration and adaptation: eco-evolutionary interactions across spatial scales. Ecography. 2015;39:858–870. [Google Scholar]
- 88.Herzog S.K., Kessler M. Local vs. regional control on species richness: a new approach to test for competitive exclusion at the community level. Glob. Ecol. Biogeogr. 2006;15:163–172. [Google Scholar]
- 89.Lortie C.J. Rethinking plant community theory. Oikos. 2004;107:433–438. [Google Scholar]
- 90.Brown J.H. Regulation of diversity: maintenance of species richness in changing environments. Oecologia. 2001;126:321–332. doi: 10.1007/s004420000536. [DOI] [PubMed] [Google Scholar]