Abstract
We have previously shown that local heating or leg fidgeting can prevent prolonged sitting-induced leg endothelial dysfunction. However, whether physical activity prevents subsequent sitting-induced leg endothelial dysfunction remains unknown. Herein, we tested the hypothesis that sitting-induced leg endothelial dysfunction would be prevented by prior exercise. We also examined if, in the absence of exercise, standing is an effective alternative strategy to sitting for conserving leg endothelial function. Fifteen young healthy subjects completed three randomized experimental trials: 1) sitting without prior exercise; 2) sitting with prior exercise; and 3) standing without prior exercise. Following baseline popliteal artery flow-mediated dilation (FMD) measurements, subjects maintained a supine position for 45 min in the sitting and standing trials, without prior exercise, or performed 45 min of leg cycling before sitting (i.e., sitting with prior exercise trial). Thereafter, subjects were positioned into a seated or standing position, according to the trial, for 3 hours. Popliteal artery FMD measures were then repeated. Three hours of sitting without prior exercise caused a significant impairment in popliteal artery FMD (baseline: 3.8±0.5%, post-sitting: 1.5±0.5%, p<0.05), which was prevented when sitting was preceded by a bout of cycling exercise (baseline: 3.8±0.5%, post-sitting: 3.6±0.7%, p>0.05). Three hours of standing did not significantly alter popliteal artery FMD (baseline: 4.1±0.4%, post-standing: 4.3±0.4%, p>0.05). In conclusion, prolonged sitting-induced leg endothelial dysfunction can be prevented by prior aerobic exercise. In addition, in the absence of exercise, standing represents an effective substitute to sitting for preserving leg conduit artery endothelial function.
Keywords: Aerobic exercise, blood flow, shear stress, sitting, endothelial function
INTRODUCTION
Peripheral artery disease preferentially develops in the vasculature of the lower extremities (1, 2, 3, 4); however, factors contributing to the increased predisposition of the leg vasculature to atherosclerosis remain largely unknown. It is plausible that excessive sitting and ensuing leg endothelial dysfunction leaves the vasculature of the lower limbs vulnerable to disease. Indeed, we (5, 6, 7) and others (8) argue that repeated but extended episodes of reduced leg blood flow and shear stress during sitting is the culprit that sets the leg vasculature at increased risk for atherogenesis; which manifest when additional risk factors are superimposed (obesity, hyperglycemia, aging, etc.). In this regard, it has been recently shown that prolonged sitting leads to transient endothelial dysfunction in the lower limbs which is likely due to the marked and sustained reduction of leg blood flow and shear stress during sitting (5, 6, 7, 9). Consistent with this hypothesis, we showed that leg endothelial dysfunction following sitting could be nullified by preventing the decrease in shear during sitting with the use of local heating (6). We have also shown that intermittent leg fidgeting during sitting, and concomitant periodic increases in leg blood flow and shear stress, prevents leg endothelial dysfunction caused by sitting (7). There is evidence that the same is true when prolonged sitting is broken up by short bouts of walking (9). However, it remains unknown whether prolonged sitting-induced leg endothelial dysfunction can be prevented with prior exercise. It is also unknown if, in the absence of exercise, standing is an effective alternative strategy to sitting for conserving leg endothelial function.
Accordingly, herein we examined if prolonged sitting-induced leg endothelial dysfunction could be prevented by previous aerobic exercise. Specifically, popliteal artery endothelial function was assessed via flow-mediated dilation (FMD) before and after a 3-hour sitting period under two conditions: where sitting was preceded by a 45-min bout of leg cycling exercise and where sitting was preceded by a time-matched period of supine rest. In a third visit, popliteal artery FMD was assessed before and after a 3-hour period of standing, instead of sitting, without prior exercise. We hypothesized that sitting-induced leg endothelial dysfunction would be attenuated by prior lower limb exercise likely as a result of partial preservation of leg blood flow and shear stress during sitting. Further, we reasoned that, in the absence of exercise, standing would represent an effective substitute to sitting for conserving leg endothelial function due to a greater preservation of leg blood flow and shear stress. In this regard, daily standing time is associated with a lower risk of all-cause mortality (10).
METHODS
Fifteen young healthy subjects (male n = 10, female n = 5) recruited from the University of Missouri campus participated in this study (Age: 26.7 ± 0.5 years; Height: 170.2 ± 1.7 cm; Weight: 75.4 ± 2.6 kg; BMI: 25.6 ± 0.5 kg/m2). All experimental procedures and measurements conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board. Prior to participating in the study, each subject provided written informed consent. Subjects were recreationally active (performing moderate exercise two to three days per week), non-smokers, with no history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease as determined from a medical health history questionnaire. No subjects reported taking medications.
Experimental Procedures
A schematic of the study design is presented in Figure 1 illustrating the sequence of events at each experimental visit and various positions in which measurements were made. The order of experimental visits was randomized and each visit was separated by at least 7 days. The three experimental visits consisted of the following conditions 1) sitting without prior exercise; 2) sitting with prior exercise; and 3) standing without prior exercise. Although we did not control for the menstrual cycle among female subjects, within each subject, the three experimental visits were scheduled around the same time of the menstrual cycle over a three month period. One subject was unable to return to complete the standing trial because of geographic relocation. Accordingly, 14 subjects were included for analysis of the effects of standing. Subjects were instructed to eat a light meal 2 or more hours prior to arriving to the lab. In addition, subjects were asked to refrain from caffeine and alcohol for at least 12 hours as well as from exercise for 24 hours prior to the study visit. All experimental sessions were scheduled in the morning between 8AM and 10AM, and the timing was kept constant among visits. All studies were performed in a temperature-controlled room kept at 22–23°C. Upon arrival to the laboratory, subjects were placed in a supine position and instrumented with an automated sphygmomanometer (SphygmoCor XCEL, AtCor Medical, Itasca, IL) for periodic measurements of mean arterial pressure (MAP) after resting quietly for 10 minutes. All vascular measurements in the popliteal artery were performed in one leg which was randomized between subjects. For each subject, the same leg was assessed in all three visits. Popliteal artery diameter and blood velocity were measured using duplex-Doppler ultrasound (Logiq P5; GE Medical Systems, Milwaukee, WI). An 11MHz linear array transducer was placed over the popliteal artery just distal to the popliteal fossa. Simultaneous diameter and velocity signals were obtained in duplex mode at a pulsed frequency of 5MHz and corrected with an insonation angle of 60 degrees. Popliteal artery FMD was assessed as previously described (5, 6, 7, 11). Briefly, a rapid inflating cuff was placed on the lower leg (midpoint between knee and ankle). Two minutes of baseline hemodynamics were recorded and then the cuff was inflated to a pressure of 220 mmHg for 5 minutes. Continuous diameter and blood velocity measures were recorded for 3 minutes following cuff deflation. Recordings of all vascular variables were analyzed offline using specialized edge-detection software (Cardiovascular Suite, Quipu srl., Pisa, Italy).
Figure 1. Experimental design.
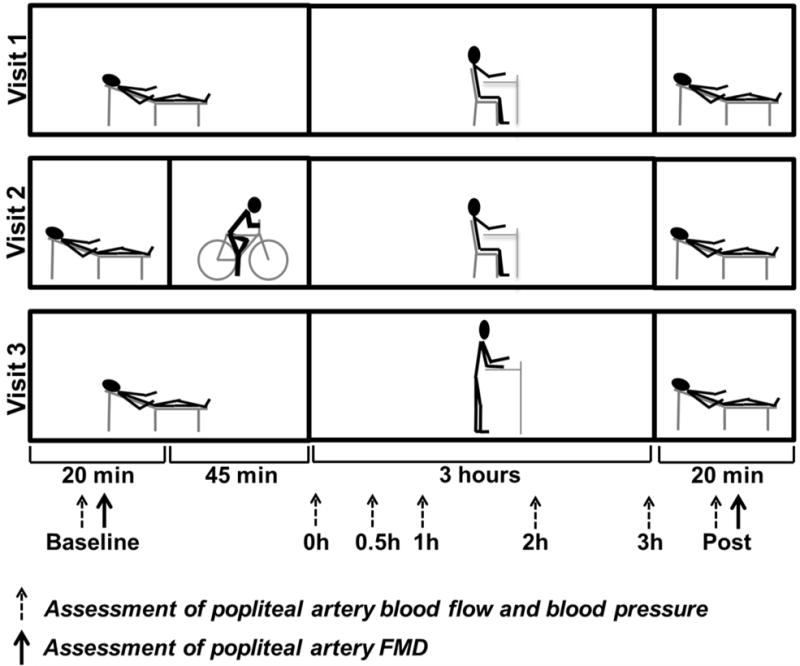
Schematic diagram of experimental protocol and positional changes over the course of the study. Measurements taken at the time points of 0, 0.5, 1, 2, and 3 h were made while the subject was in the seated or standing position, whereas baseline and post-sitting/standing measurements were taken while subject was in the supine position. FMD: flow-mediated dilation.
Following baseline FMD measurements, subjects maintained the supine position for 45 min in the sitting and standing trials without prior exercise; subjects performed 45 min of leg cycling on a stationary bike (Monark 828E, Monark Exercise AB, Vansbro, Sweden) in the sitting with prior exercise trial (Figure 1). During exercise, subjects maintained a cycling cadence of 60 rpm. The workload was individualized for each subject to target a rate of perceived exertion (RPE) of 11–13 (Borg Scale, 6–20). Heart rate (HR) and RPE were monitored throughout the exercise bout. %HRmax was calculated from the age-predicted HRmax ([the average HR during cycling/(220-age)*100]). After 45 min of supine resting or cycling, subjects were positioned into a seated or standing position, according to the trial, for 3 hours. During the sitting period, a study representative monitored the subject to ensure no leg movements or muscle contractions occurred. Subjects were allowed to use a desk for computer work and reading. In the standing trial, subjects were confined within a 60 × 60 cm demarcated area and were also allowed to use a standing desk for computer work and reading. Subjects were allowed only minimal movement during the standing period, monitored by study personnel. Minimal movement included shifting weight between legs; no stepping was permitted. Throughout the course of the sitting and standing periods, MAP, popliteal artery diameter and blood velocity were measured. The chair used allowed sufficient space for the ultrasound probe to access the popliteal artery during sitting so that the subject’s sitting position was not disturbed during ultrasound imaging. During the standing period, the ultrasonographer was positioned behind the subject for popliteal artery measures. Following the 3-hour sitting and standing periods, subjects were manually lifted and placed back into the supine position in order to avoid any leg muscle activity. FMD and MAP assessments were then repeated.
To determine if cycling exercise acutely alters popliteal artery FMD, we repeated the exercise bout in a subset of male subjects (n=5) in which measures of FMD were performed before and within 10 minutes after exercise. Furthermore, in an additional subset of male subjects (n=8), we determined whether hip and knee flexion, inherent to the sitting position, contributes to reduced limb blood flow. Specifically, measurements of popliteal artery blood flow were made with the subject lying down on their side for 5 minutes with the body positioned straight (zero degree angles) versus lying down on their side for 5 minutes with hips and knees bent to ninety degrees (i.e., mimicking the sitting position). The order of experimental measurements was randomized.
Data Analysis
Blood flow was calculated from continuous diameter and mean blood velocity recordings at each of the experimental time points using the following equation: 3.14*(diameter/2)2 * mean blood velocity *60. Popliteal artery FMD percent change was calculated using the following equation: %FMD = (peak diameter-base diameter)/(base diameter) *100. Shear rate, an estimate of shear stress without blood viscosity, was calculated as 4* mean blood velocity/diameter. Hyperemic shear rate area under the curve (AUC) up to peak diameter was calculated as stimulus for FMD, as previously described (11, 12).
Statistical Analysis
A two-way (time × trial) repeated measures analysis of variance (ANOVA) with Tukey posthoc testing was performed on all dependent variables. FMD was also adjusted for hyperemic shear rate AUC via analysis of covariance (ANCOVA) in order to statistically control for the influence of shear stimulus on FMD response. ANCOVA and ANOVA tests were performed using SPSS software (version 23). Significance was accepted at p≤0.05. Data are expressed as means ± SE.
RESULTS
The average workload during cycling exercise was 86.3 ± 4.9 watts (range: 56 – 120 watts) and the relative exercise intensity was 71.2 ± 2.6 %HR max (range: 50.3 – 86.9 %). The average RPE was 11.9 ± 0.2 (range: 10 – 13). Following cycling exercise, while in the seated position, popliteal artery blood flow (Table 1) and shear rate (Figure 2A) were elevated relative to baseline and relative to sitting without prior exercise (p<0.05). However, despite this initial increase in leg blood flow and shear rate caused by exercise, sitting produced a marked reduction in leg blood flow and shear, independent of exercise, such that by 1 hour of sitting there were no differences between trials (Figure 2A). Standing also resulted in a reduction in leg blood flow (Table 1) and shear rate (Figure 3A); however, the magnitude of this reduction was less than that produced by sitting. In fact, the levels of blood flow and shear rate remained higher during standing than during sitting for the entire three-hour period (Table 1 and Figure 3A; p<0.05). Changes in mean shear rate and blood flow were largely driven by changes in antegrade velocity, and not by changes in retrograde velocity (data not shown).
Table 1.
Popliteal artery hemodynamics baseline, during and after sitting or standing for 3 hours.
| Supine Baseline | Duration of sitting or standing
|
Supine Post | ANOVA Sitting without exercsise vs. Sitting with prior exercise | ANOVA Sitting without exercsise vs. Standing | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0h | 0.5h | 1h | 2h | 3h | ||||||
| Basal Diameter (cm) | Sitting without exercsise | 0.540 ± 0.024 | 0.538 ± 0.023 | 0.544 ± 0.022 | 0.548 ± 0.021 | 0.543 ± 0.021 | 0.544 ± 0.022 | 0.559 ± 0.028 | Time: P = 0.4 Trial: P = 0.6 Interaction: P = 0.1 |
Time: P = 0.2 Trial: P = 0.1 Interaction: P = 0.2 |
| Sitting with prior exercise | 0.538 ± 0.023 | 0.541 ± 0.018 | 0.556 ± 0.019 | 0.557 ± 0.021 | 0.550 ± 0.021 | 0.549 ± 0.021 | 0.536 ± 0.025 | |||
| Standing | 0.547 ± 0.024 | 0.530 ± 0.021 | 0.538 ± 0.022 | 0.533 ± 0.025 | 0.524 ± 0.022 | 0.533 ± 0.024 | 0.540 ± 0.025 | |||
|
| ||||||||||
| Blood Flow (ml/min) | Sitting without exercsise | 64.0 ± 9.9 | 35.7 ± 4.3* | 36.0 ± 5.1* | 36.3 ± 4.7* | 30.6 ± 4.9* | 38.9 ± 3.5* | 47.3 ± 10.0 | Interaction: P < 0.001 | Time: P < 0.001 Trial: P = 0.043 Interaction: P = 0.5 |
| Sitting with prior exercise | 66.6 ± 9.6 | 99.7 ± 13.6*† | 51.1 ± 7.2† | 41.1 ± 4.9 | 35.8 ± 5.1* | 36.5 ± 3.7* | 50.1 ± 7.3 | |||
| Standing | 68.9 ± 11.1 | 47.5 ± 6.8*† | 47.3 ± 6.2*† | 48.5 ± 7.8* | 50.9 ± 7.8*† | 56.7 ± 6.5* | 56.7 ± 6.5 | |||
|
| ||||||||||
| Hyperemic Shear Rate AUC (Arbitrary Units) | Sitting without exercsise | 35625 ± 5625 | 19003 ± 3313* | Time: P = 0.022 Trial: P = 0.2 Interaction: P = 0.7 |
Time: P = 0.045 Trial: P = 0.5 Interaction: P = 0.07 |
|||||
| Sitting with prior exercise | 39811 ± 8069 | 25356 ± 4142 | ||||||||
| Standing | 30522 ± 6426 | 28686 ± 5532 | ||||||||
|
| ||||||||||
| ANCOVA corrected FMD (%) | Sitting without exercsise | 4.0 ± 0.6 | 1.2 ± 0.6* | Time: P = 0.013 Trial: P = 0.053 Interaction: P = 0.053 |
Interaction: P = 0.009 | |||||
| Sitting with prior exercise | 4.0 ± 0.6 | 3.5 ± 0.6 | ||||||||
| Standing | 4.1 ± 0.5 | 4.2 ± 0.5† | ||||||||
|
| ||||||||||
| Mean Arterial Pressure (mmHg) | Sitting without exercsise | 87 ± 2 | 93 ± 2* | 92 ± 2* | 94 ± 3* | 96 ± 3* | 95 ± 2* | 93 ± 2* | Interaction: P = 0.008 | Time: P = 0.1 Trial: P = 0.5 Interaction: P = 0.3 |
| Sitting with prior exercise | 90 ± 2 | 96 ± 2*† | 92 ± 2 | 91 ± 2 | 93 ± 2 | 96 ± 2* | 91 ± 2 | |||
| Standing | 87 ± 2 | 91 ± 3 | 95 ± 4* | 96 ± 4* | 96 ± 3* | 98 ± 3* | 91 ± 3* | |||
Mean ± SE.
ANCOVA-corrected flow-mediated dilation (FMD) data are adjusted for hyperemic shear rate area under the curve (AUC).
P < 0.05 vs. Baseline,
P < 0.05 vs Sitting without exercsise.
All baseline and post measuments were collected in the supine position.
Figure 2. Effects of sitting, with and without prior exercise, on popliteal artery shear rate and FMD.
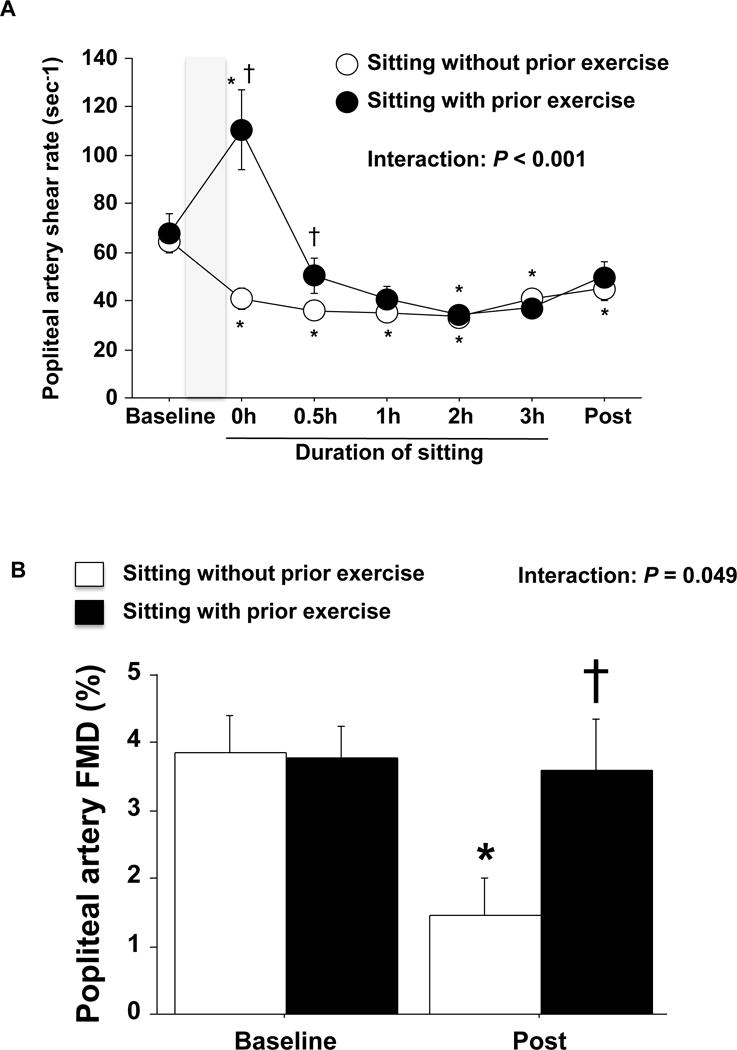
A: Popliteal artery shear rate before, during and after sitting for 3 hours with and without prior exercise. Shaded box indicates a 45 min of cycling exercise or lying down. B: Unadjusted popliteal artery FMD before and after sitting for 3 hours with and without prior exercise. n=15. Data are expressed as means ± SE. *P < 0.05 vs. Baseline, †P < 0.05 vs. sitting without prior exercise.
Figure 3. Effects of sitting and standing on popliteal artery shear rate and FMD.
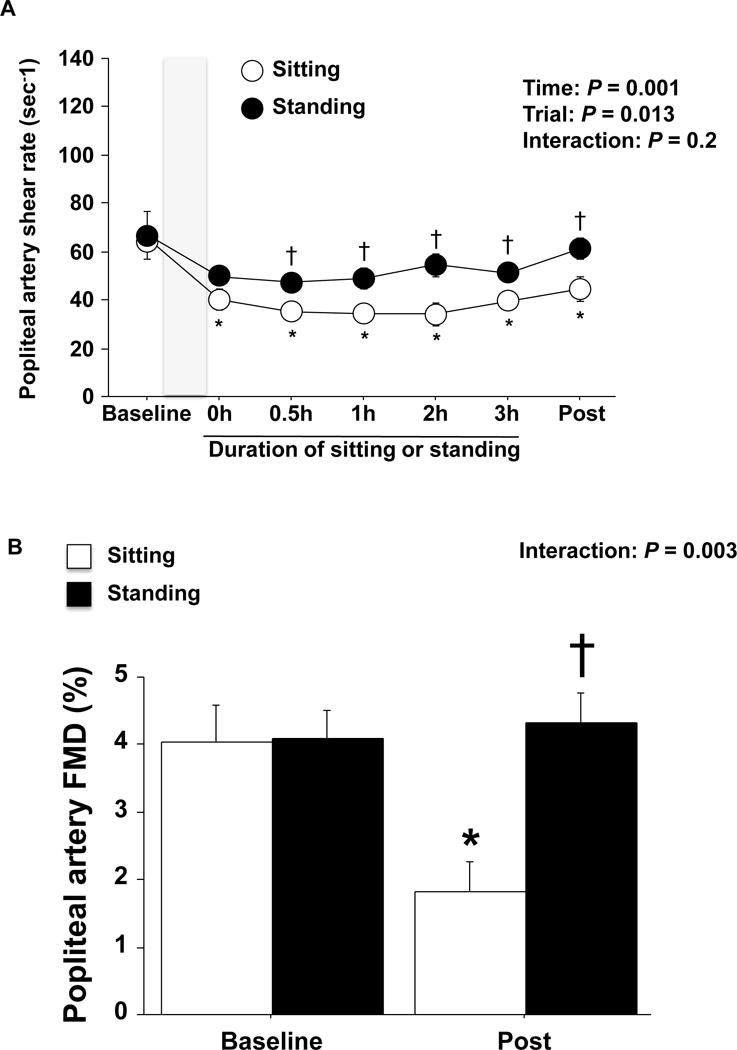
A: Popliteal artery shear rate before, during and after sitting or standing for 3 hours without prior exercise. Shaded box indicates a 45 min bout of lying down B: Unadjusted popliteal artery FMD before and after sitting or standing for 3 hours without prior exercise. n=14. Data are expressed as means ± SE. *P < 0.05 vs. Baseline, †P < 0.05 vs. sitting without prior exercise.
Importantly, three hours of sitting without prior exercise caused a significant impairment in popliteal artery FMD, which was prevented when sitting was preceded by a bout of cycling exercise (Figure 2B, p<0.05). Three hours of standing did not significantly alter popliteal artery FMD (Figure 3B). FMD corrected for hyperemic shear rate AUC did not affect the interpretation of the main findings; that is, only sitting without prior exercise impaired FMD (Table 1). No changes were observed in popliteal artery diameter over time and no differences among the visits were detected across time points (Table 1). MAP was significantly elevated during sitting and standing periods (Table 1, p<0.05).
Popliteal artery FMD was unaffected immediately following cycling exercise (baseline: 4.25 ± 0.67%, post-exercise: 4.22 ± 0.34%, p=0.9, n=5). Under resting lying down conditions, flexing the hips and knees to ninety degrees (i.e., mimicking the sitting position) reduced popliteal artery blood flow by 44.9 ± 9.6%, relative to lying down with the body positioned straight (zero-degree angles) (p=0.0014, n=8).
DISCUSSION
The main findings of the present study are two-fold. First, leg endothelial dysfunction caused by prolonged sitting could be prevented by a prior bout of cycling exercise. Second, in the absence of exercise, standing represented an effective substitute to sitting for maintaining leg endothelial function. Thus, findings reported herein provide evidence of two different behavioral strategies that are effective for combating leg vascular dysfunction associated with prolonged sitting.
Peripheral artery disease preferentially develops in the arteries of the lower extremities (1, 2, 3, 4). The increased propensity of the leg vasculature to atherosclerosis may be attributable to excessive sitting. Indeed, given that shear stress is an important stimulus for maintaining normal vascular health (12, 13, 14, 15, 16, 17, 18), it is conceivable that “knocking down” leg shear stress during the majority of wake hours, which are occupied by sitting increases the susceptibility of the leg vasculature to disease. In this regard, we recently reported that lengthy sitting leads to a transient impairment in popliteal artery FMD (5) and that this impairment could be prevented by increasing limb blood flow and shear stress via local heating (6) or intermittent leg movement (i.e., fidgeting) (7), supporting the notion that reduced leg shear stress during sitting mediates endothelial dysfunction.
In the present investigation, we examined if engaging in aerobic exercise affords vascular protection from subsequent prolonged sitting. In essence, we mimicked the scenario of individuals who actively commute to a sedentary job. Consistent with our hypothesis, we found that one bout of cycling exercise was sufficient to counteract the negative leg vascular effects of 3 hours of sitting. While it remains unknown if vascular protection provided by prior exercise exists beyond 3 hours of uninterrupted sitting, this finding is significant in that it demonstrates that leg endothelial dysfunction caused by sitting is avertible. The factors responsible for this exercise-induced vascular protection from sitting are unknown. One possibility is that leg vascular exposure to increased shear stress during exercise is sufficient to offset the subsequent negative effects of reduced shear stress associated with sitting. There is precedence in the literature supporting the idea that increased shear stress can protect the vasculature from succeeding “insults”. For example, studies in endothelial cell culture demonstrate that pre-exposure of cells to shear stress prevents CD40-induced expression of monocyte chemoattractant protein-1 (19) and angiotensin II-induced apoptosis (20). Studies in isolated perfused arteries also indicate that pretreatment with shear stress prevents TNF alpha-induced inflammation (21). In addition, it has been recently shown that increased forearm blood flow via local heating for 30 min prevents postprandial hyperglycemia-induced brachial artery endothelial dysfunction (22). Based on these findings, it is reasonable to speculate that increased leg shear stress with exercise leads to a window of vascular defense; however, other protective shear stress-independent mechanisms are also likely involved.
Given that only a small fraction of the population actively commutes to work or engages in exercise prior to prolonged sitting, the identification of alternative strategies to escape daily leg vascular dysfunction incited by sitting are needed. An emerging trend in the work place for lessening sitting time is the use of standing desks. In this study, we determined that replacing sitting for standing is another effective behavioral strategy at maintaining leg vascular function. In support of our hypothesis, leg blood flow and shear rate during standing was greater than that during sitting. This is likely due to the fact that sustaining the standing position requires greater skeletal muscle activity compared to sitting. The increased “muscle pump” activity with standing may counteract venous blood pooling associated with sitting. Although not statistically significant, it should be noted that standing also resulted in a small reduction in leg blood flow and shear rate relative to the baseline supine position. This small reduction in leg blood flow may be in part attributable to increased sympathetic nerve activity, hydrostatic pressure-induced myogenic constriction, and/or venous distension-induced arterial constriction during upright posture (23, 24). Importantly, this small reduction in leg blood flow and shear stress during standing was not associated with an impairment in leg endothelial function. The finding that standing is an effective behavioral modification for conserving leg vascular health adds to the recent evidence that substituting sitting for standing holds metabolic benefits. In this regard, Henson et al. (25) found in overweight/obese postmenopausal women that, compared to 7.5 hours of uninterrupted sitting, standing for 5 min every 30 min significantly reduced glucose and insulin areas under the curve throughout the study period. Moreover, current evidence indicates that daily standing time is associated with a lower risk of all-cause mortality (10). Our current findings provide further support that standing is a healthier alternative to sitting. Importantly, standing can be readily incorporated into a variety of settings, including the workplace and domestic environments.
We reasoned that flexion of the hips and knees with sitting, and associated “arterial bending”, may hinder limb blood flow as a result of increased resistance. Consistent with this idea, we found that mimicking the sitting position (hip and knee joints bent to ninety degrees) while lying down on the side markedly reduced popliteal artery blood flow, compared to lying down with the body positioned straight (zero-degree angles). This observation suggests that reduced popliteal artery blood flow and shear stress during sitting may indeed be largely caused by proximal arterial bending. Elimination of these angulations with standing may contribute to the greater popliteal artery blood flow and shear rate compared to sitting. In support of this idea, previous work has demonstrated that femoral artery blood flow can be greatly altered in a cyclic fashion in response to passive knee extension and flexion (26).
Discussion of several experimental considerations is warranted. First, our study included only young healthy subjects and thus future studies need to examine if these findings can be extrapolated to other populations. Second, our small and unequal number of men versus women precludes us from examining if protective effects of prior exercise and standing differ according to sex and phase of the menstrual cycle. In this regard, it is possible that differences in menstrual cycle among women contributed to some variability in our dataset. Last, we opted to use the supine position, instead of sitting, as a time-control condition. One could argue that sitting would have been a more appropriate time-control condition to cycling. The decision of using supine rest as control was made to maintain the duration of sitting equal across trials as we know that prolonged sitting itself produces detrimental leg vascular effects.
In conclusion, this study provides the first evidence that prolonged sitting-induced leg endothelial dysfunction can be prevented by prior aerobic exercise. In addition, in the absence of exercise, standing represents an effective substitute to sitting for preserving leg conduit artery endothelial function. Accordingly, people should be encouraged to engage in aerobic leg exercise before sitting for extended periods of time and, if this is not possible, sitting should be replaced by standing.
Acknowledgments
The authors appreciate the time and effort put in by all volunteer subjects.
GRANTS
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research 16J03636 (to T. Morishima) and National Institutes of Health Grants K01-HL-125503 and R21-DK-105368 (to J. Padilla).
Abbreviations list
- FMD
flow-mediated dilation
- MAP
mean arterial pressure
- RPE
rate of perceived exertion
- HR
heart rate
- ANOVA
analysis of variance
- ANCOVA
analysis of covariance
- AUC
area under the curve
Footnotes
ATHOUR CONTRIBUTION
T.M., R.M.R., L.K.W., J.A.K., and J.P. conception and design of research; T.M. and J.P. performed experiments; T.M. analyzed data; T.M., R.M.R., L.K.W., J.A.K., and J.P. interpreted results of experiments; T.M. and J.P. prepared figures; T.M. and J.P. drafted manuscript; T.M., R.M.R., L.K.W., J.A.K., and J.P. edited and revised manuscript; T.M., R.M.R., L.K.W., J.A.K., and J.P. approved final version of manuscript.
DISCLOSURES OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984;114:79–93. [PMC free article] [PubMed] [Google Scholar]
- 2.Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology. 1999;50:649–654. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 3.Moore WS. A comprehensive review. Philadelphia: W. B. Saunders Company; 2002. Vascular surgery. [Google Scholar]
- 4.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerosis lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 5.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol. 2015;100:829–838. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol. 2016;310:648–653. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel JP, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol. 2016;311:177–182. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinity JD, Groot HJ, Layec G, Rossman MJ, Lves SJ, Richardson RS. Impact of age and body position on the contribution of nitric oxide to femoral artery shear rate: implications for atherosclerosis. Hypetension. 2014;63:1019–1025. doi: 10.1161/HYPERTENSIONAHA.113.02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Medicine and science in sports and exercise. 2015;47:843–849. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT. Standing and mortality in a prospective cohort of Canadian adults. Med Sci Sports Exerc. 2014;46:940–946. doi: 10.1249/MSS.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 11.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. Journal of applied physiology. 2009;115:1519–1525. doi: 10.1152/japplphysiol.00837.2013. 2013 Parker et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 13.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 14.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension. 2013;61:615–621. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD, Wallace JP. Vitamin C prevents the acute decline of flow-mediated dilation after altered shear rate patterns. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2013;38:268–274. doi: 10.1139/apnm-2012-0169. [DOI] [PubMed] [Google Scholar]
- 17.Schreuder TH, Green DJ, Hopman MT, Thijssen DH. Acute impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in humans. Physiol Rep. 2014;2:e00193. doi: 10.1002/phy2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Totosy de Zepetnek JO, Jermey TL, MacDonald MJ. Superficial femoral artery endothelial responses to a short-term altered shear rate intervention in healthy men. PloS one. 2014;9:e113407. doi: 10.1371/journal.pone.0113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbich C, Mallat Z, Tedgui A, Clauss M, Zeiher AM, Dimmeler S. Upregulation of TRAF-3 by shear stress blocks CD40-mediated endothelial activation. J Clin Invest. 2001;108:1451–1458. doi: 10.1172/JCI13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. Angiotensin II induces apotosis of human endothelial cells. Circ Res. 1997;81:970–976. doi: 10.1161/01.res.81.6.970. [DOI] [PubMed] [Google Scholar]
- 21.Yamawaki H, Lehoux S, Berk BC. Chronic physiological shear stress inhibits tumor necrosis factor-induced proinflammatory responses in rabbit aorta perfused ex vivo. Circulation. 2003;30:1619–1625. doi: 10.1161/01.CIR.0000089373.49941.C4. [DOI] [PubMed] [Google Scholar]
- 22.Greyling A, Schreuder TH, Landman T, Draijer R, Verheggen RJ, Hopman MT, Thijissen DH. Elevation in blood flow and shear rate prevents hyperglycemia-induced endothelial dysfunction in healthy subjects and those with type 2 diabetes. J Appl Physiol. 2015;118:579–585. doi: 10.1152/japplphysiol.00936.2014. [DOI] [PubMed] [Google Scholar]
- 23.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. The American journal of physiology. 1993;264:H1–7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- 24.Kitano A, Shoemaker JK, Ichinose M, Wada H, Nishiyasu T. Comparison of cardiovascular responses between lower body negative pressure and head-up tilt. Journal of applied physiology. 2005;98:2081–2086. doi: 10.1152/japplphysiol.00563.2004. [DOI] [PubMed] [Google Scholar]
- 25.Henson J, Davies M, Bodicoat DH, Edwardson CL, Gill JM, Stensel DJ, Tolfrey K, Dunstan DW, Khunti K, Yates T. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes care. 2016;39:130–138. doi: 10.2337/dc15-1240. [DOI] [PubMed] [Google Scholar]
- 26.McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol. 2012;112:560–565. doi: 10.1152/japplphysiol.01223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


