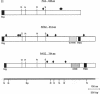
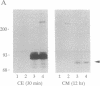
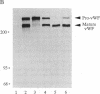
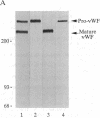
Abstract
Intracellular proteolytic processing of precursor polypeptides is an essential step in the maturation of many proteins, including plasma proteins, hormones, neuropeptides, and growth factors. Most frequently, propeptide cleavage occurs after paired basic amino acid residues. To date, no mammalian propeptide processing enzyme with such specificity has been purified or cloned and functionally characterized. We report the isolation and functional expression of a cDNA encoding a propeptide-cleaving enzyme from a human liver cell line. The encoded protein, called PACE (paired basic amino acid cleaving enzyme), has structural homology to the well-characterized subtilisin-like protease Kex2 from yeast. The functional specificity of PACE for mediating propeptide cleavage at paired basic amino acid residues was demonstrated by the enhancement of propeptide processing of human von Willebrand factor when coexpressed with PACE in COS-1 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Hormone processing and membrane-bound proteinases in yeast. EMBO J. 1985 Jan;4(1):173–177. doi: 10.1002/j.1460-2075.1985.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathurst I. C., Brennan S. O., Carrell R. W., Cousens L. S., Brake A. J., Barr P. J. Yeast KEX2 protease has the properties of a human proalbumin converting enzyme. Science. 1987 Jan 16;235(4786):348–350. doi: 10.1126/science.3541206. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Cromlish J. A., Seidah N. G., Marcinkiewicz M., Hamelin J., Johnson D. A., Chrétien M. Human pituitary tryptase: molecular forms, NH2-terminal sequence, immunocytochemical localization, and specificity with prohormone and fluorogenic substrates. J Biol Chem. 1987 Jan 25;262(3):1363–1373. [PubMed] [Google Scholar]
- Dickerson I. M., Dixon J. E., Mains R. E. Biosynthesis and posttranslational processing of site-directed endoproteolytic cleavage mutants of pro-neuropeptide Y in mouse pituitary cells. J Biol Chem. 1990 Feb 15;265(5):2462–2469. [PubMed] [Google Scholar]
- Docherty K., Carroll R., Steiner D. F. Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3245–3249. doi: 10.1073/pnas.80.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Kaufman R. J. Analysis of synthesis, processing, and secretion of proteins expressed in mammalian cells. Methods Enzymol. 1990;185:577–596. doi: 10.1016/0076-6879(90)85046-q. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Garcia P. D., Rutter W. J. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988 May 15;263(14):6810–6815. [PubMed] [Google Scholar]
- Fay P. J., Kawai Y., Wagner D. D., Ginsburg D., Bonthron D., Ohlsson-Wilhelm B. M., Chavin S. I., Abraham G. N., Handin R. I., Orkin S. H. Propolypeptide of von Willebrand factor circulates in blood and is identical to von Willebrand antigen II. Science. 1986 May 23;232(4753):995–998. doi: 10.1126/science.3486471. [DOI] [PubMed] [Google Scholar]
- Foster D. C., Sprecher C. A., Holly R. D., Gambee J. E., Walker K. M., Kumar A. A. Endoproteolytic processing of the dibasic cleavage site in the human protein C precursor in transfected mammalian cells: effects of sequence alterations on efficiency of cleavage. Biochemistry. 1990 Jan 16;29(2):347–354. doi: 10.1021/bi00454a007. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A. J., Thorner J. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science. 1989 Oct 27;246(4929):482–486. doi: 10.1126/science.2683070. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A., Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Stephans J. C., Crawford K., Okino K., Barr P. J. Ligand-affinity cloning and structure of a cell surface heparan sulfate proteoglycan that binds basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6985–6989. doi: 10.1073/pnas.87.18.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Yeast KEX2 genes encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem Biophys Res Commun. 1988 Oct 14;156(1):246–254. doi: 10.1016/s0006-291x(88)80832-5. [DOI] [PubMed] [Google Scholar]
- Moehle C. M., Tizard R., Lemmon S. K., Smart J., Jones E. W. Protease B of the lysosomelike vacuole of the yeast Saccharomyces cerevisiae is homologous to the subtilisin family of serine proteases. Mol Cell Biol. 1987 Dec;7(12):4390–4399. doi: 10.1128/mcb.7.12.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Takami N., Fujiwara T., Misumi Y., Ikehara Y. Proalbumin is processed to serum albumin in COS-1 cells transfected with cDNA for rat albumin. Biochem Biophys Res Commun. 1989 Aug 30;163(1):194–200. doi: 10.1016/0006-291x(89)92120-7. [DOI] [PubMed] [Google Scholar]
- Pan L. C., Price P. A. The propeptide of rat bone gamma-carboxyglutamic acid protein shares homology with other vitamin K-dependent protein precursors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6109–6113. doi: 10.1073/pnas.82.18.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish D. C., Tuteja R., Altstein M., Gainer H., Loh Y. P. Purification and characterization of a paired basic residue-specific prohormone-converting enzyme from bovine pituitary neural lobe secretory vesicles. J Biol Chem. 1986 Nov 5;261(31):14392–14397. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Bussemakers M. J., van Heerikhuizen H., Onnekink C., Debruyne F. M., Bloemers H. P., Van de Ven W. J. Characterization of human c-fes/fps reveals a new transcription unit (fur) in the immediately upstream region of the proto-oncogene. Mol Biol Rep. 1986;11(2):117–125. doi: 10.1007/BF00364823. [DOI] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Leunissen J. A., Onnekink C., Bloemers H. P., Van de Ven W. J. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 1986 Sep;5(9):2197–2202. doi: 10.1002/j.1460-2075.1986.tb04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schild D., Brake A. J., Kiefer M. C., Young D., Barr P. J. Cloning of three human multifunctional de novo purine biosynthetic genes by functional complementation of yeast mutations. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2916–2920. doi: 10.1073/pnas.87.8.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Sleep D., Belfield G. P., Goodey A. R. The secretion of human serum albumin from the yeast Saccharomyces cerevisiae using five different leader sequences. Biotechnology (N Y) 1990 Jan;8(1):42–46. doi: 10.1038/nbt0190-42. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Steimer K. S., Higgins K. W., Powers M. A., Stephans J. C., Gyenes A., George-Nascimento C., Luciw P. A., Barr P. J., Hallewell R. A., Sanchez-Pescador R. Recombinant polypeptide from the endonuclease region of the acquired immune deficiency syndrome retrovirus polymerase (pol) gene detects serum antibodies in most infected individuals. J Virol. 1986 Apr;58(1):9–16. doi: 10.1128/jvi.58.1.9-16.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B. S., Engleman E. G. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J Biol Chem. 1990 Feb 15;265(5):2640–2649. [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L., Hansen M. T., Norris K., Hoegh I., Boel E., Forstrom J., Ammerer G., Fiil N. P. Secretion and processing of insulin precursors in yeast. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6766–6770. doi: 10.1073/pnas.83.18.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Hruby D. E. Gene transfer techniques to study neuropeptide processing. Annu Rev Physiol. 1988;50:323–332. doi: 10.1146/annurev.ph.50.030188.001543. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Thorne B. A., Caton L. W., Thomas G. Expression of mouse proopiomelanocortin in an insulinoma cell line. Requirements for beta-endorphin processing. J Biol Chem. 1989 Feb 25;264(6):3545–3552. [PubMed] [Google Scholar]
- Warren T. G., Shields D. Expression of preprosomatostatin in heterologous cells: biosynthesis, posttranslational processing, and secretion of mature somatostatin. Cell. 1984 Dec;39(3 Pt 2):547–555. doi: 10.1016/0092-8674(84)90461-6. [DOI] [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., van Duijnhoven H. L., Keizer G. D., Dorssers L. C., Van de Ven W. J. Structural homology between the human fur gene product and the subtilisin-like protease encoded by yeast KEX2. Nucleic Acids Res. 1990 Feb 11;18(3):664–664. doi: 10.1093/nar/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]