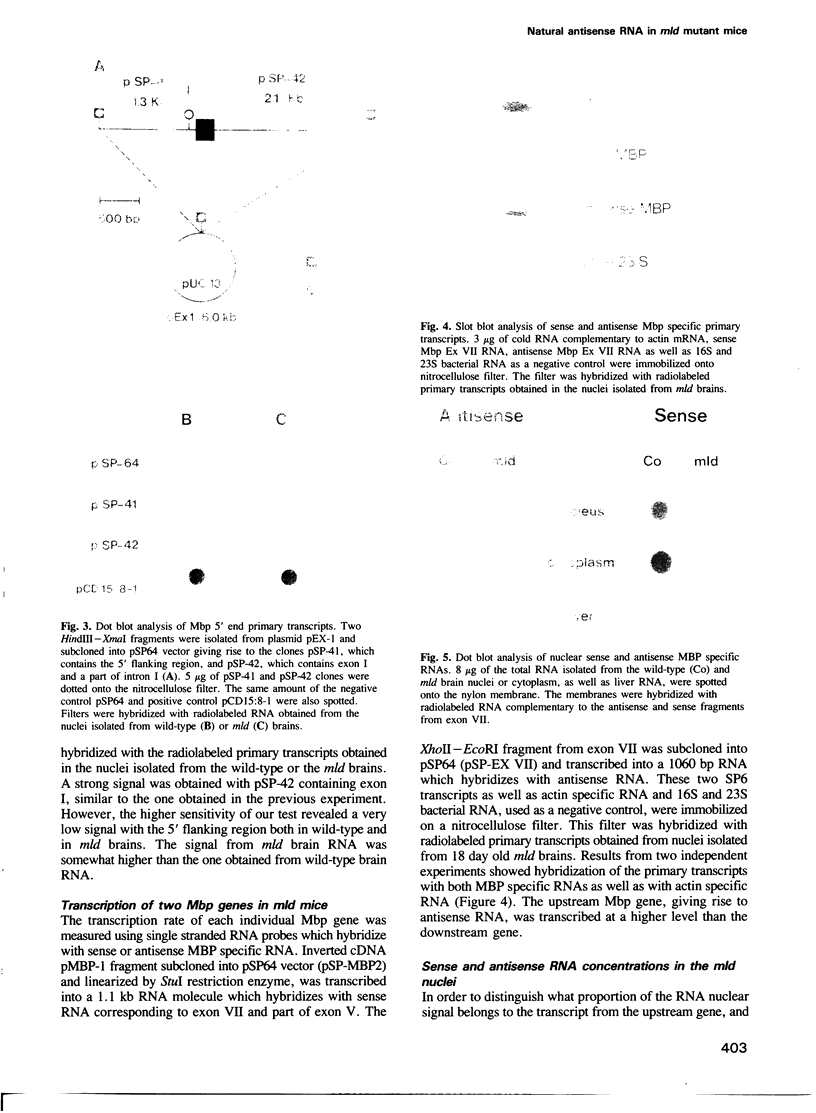
Abstract
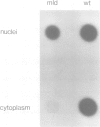
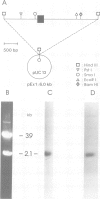
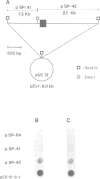
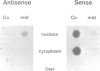
Myelin deficient mice (mld) are characterized by tandem duplication of the gene coding for myelin basic protein (MBP). The upstream gene contains a large inversion of the 3' region which includes exons 3-7, and therefore it cannot give rise to mature mRNA and functional protein. MBP and MBP mRNA concentrations in mld brains constitute only approximately 2% of the concentrations present in normal mice. The overall transcription rate of the Mbp gene is normal. In order to explain the discrepancy between mRNA concentration and transcription rate, we studied transcription of each individual gene. The two genes were transcribed independently, although some uninterrupted transcription could not be excluded. The rate of transcription of the upstream gene was higher than that of the downstream gene. This difference was reflected in the concentration of sense and antisense RNA found in nuclei. Our results indicate that the low concentration of the mature mRNA cannot be caused by transcriptional interference. High concentration of nuclear antisense RNA strongly suggests that post-transcriptional regulation occurs in mld mice through formation of double stranded RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akowitz A. A., Barbarese E., Scheld K., Carson J. H. Structure and expression of myelin basic protein gene sequences in the mld mutant mouse: reiteration and rearrangement of the MBP gene. Genetics. 1987 Jul;116(3):447–464. doi: 10.1093/genetics/116.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourre J. M., Jacque C., Delassalle A., Nguyen-Legros J., Dumont O., Lachapelle F., Raoul M., Alvarez C., Baumann N. Density profile and basic protein measurements in the myelin range of particulate material from normal developing mouse brain and from neurological mutants (Jimpy; quaking; Trembler; shiverer and its mld allele) obtained by zonal centrifugation. J Neurochem. 1980 Aug;35(2):458–464. doi: 10.1111/j.1471-4159.1980.tb06287.x. [DOI] [PubMed] [Google Scholar]
- Campagnoni A. T., Campagnoni C. W., Bourre J. M., Jacque C., Baumann N. Cell-free synthesis of myelin basic proteins in normal and dysmyelinating mutant mice. J Neurochem. 1984 Mar;42(3):733–739. doi: 10.1111/j.1471-4159.1984.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R. A semi-dominant allele, niv-525, acts in trans to inhibit expression of its wild-type homologue in Antirrhinum majus. EMBO J. 1988 Apr;7(4):877–883. doi: 10.1002/j.1460-2075.1988.tb02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Farnham P. J., Abrams J. M., Schimke R. T. Opposite-strand RNAs from the 5' flanking region of the mouse dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3978–3982. doi: 10.1073/pnas.82.12.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garner I., Minty A. J., Alonso S., Barton P. J., Buckingham M. E. A 5' duplication of the alpha-cardiac actin gene in BALB/c mice is associated with abnormal levels of alpha-cardiac and alpha-skeletal actin mRNAs in adult cardiac tissue. EMBO J. 1986 Oct;5(10):2559–2567. doi: 10.1002/j.1460-2075.1986.tb04535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginalski-Winkelmann H., Almazan G., Matthieu J. M. In vitro myelin basic protein synthesis in the PNS and CNS of myelin deficient (mld) mutant mice. Brain Res. 1983 Oct 31;277(2):386–388. doi: 10.1016/0006-8993(83)90952-6. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Heywood S. M. tcRNA as a naturally occurring antisense RNA in eukaryotes. Nucleic Acids Res. 1986 Aug 26;14(16):6771–6772. doi: 10.1093/nar/14.16.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Katsuki M., Sato M., Kimura M., Yokoyama M., Kobayashi K., Nomura T. Conversion of normal behavior to shiverer by myelin basic protein antisense cDNA in transgenic mice. Science. 1988 Jul 29;241(4865):593–595. doi: 10.1126/science.2456614. [DOI] [PubMed] [Google Scholar]
- Kidd S., Young M. W. Transposon-dependent mutant phenotypes at the Notch locus of Drosophila. Nature. 1986 Sep 4;323(6083):89–91. doi: 10.1038/323089a0. [DOI] [PubMed] [Google Scholar]
- Kimura M., Inoko H., Katsuki M., Ando A., Sato T., Hirose T., Takashima H., Inayama S., Okano H., Takamatsu K. Molecular genetic analysis of myelin-deficient mice: shiverer mutant mice show deletion in gene(s) coding for myelin basic protein. J Neurochem. 1985 Mar;44(3):692–696. doi: 10.1111/j.1471-4159.1985.tb12870.x. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Almazan G., Waehneldt T. V. Intrinsic myelin proteins are normally synthesized in vitro in the myelin-deficient (mld) mutant mouse. Dev Neurosci. 1983;6(4-5):246–250. doi: 10.1159/000112351. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M., Tamura T., Aoyama A., Mikoshiba K. The promoter elements of the mouse myelin basic protein gene function efficiently in NG108-15 neuronal/glial cells. Gene. 1989 Jan 30;75(1):31–38. doi: 10.1016/0378-1119(89)90380-6. [DOI] [PubMed] [Google Scholar]
- Molineaux S. M., Engh H., de Ferra F., Hudson L., Lazzarini R. A. Recombination within the myelin basic protein gene created the dysmyelinating shiverer mouse mutation. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7542–7546. doi: 10.1073/pnas.83.19.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H., Ikenaka K., Mikoshiba K. Recombination within the upstream gene of duplicated myelin basic protein genes of myelin deficient shimld mouse results in the production of antisense RNA. EMBO J. 1988 Nov;7(11):3407–3412. doi: 10.1002/j.1460-2075.1988.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H., Miura M., Moriguchi A., Ikenaka K., Tsukada Y., Mikoshiba K. Inefficient transcription of the myelin basic protein gene possibly causes hypomyelination in myelin-deficient mutant mice. J Neurochem. 1987 Feb;48(2):470–476. doi: 10.1111/j.1471-4159.1987.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Okano H., Tamura T., Miura M., Aoyama A., Ikenaka K., Oshimura M., Mikoshiba K. Gene organization and transcription of duplicated MBP genes of myelin deficient (shi(mld)) mutant mouse. EMBO J. 1988 Jan;7(1):77–83. doi: 10.1002/j.1460-2075.1988.tb02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B., Puckett C., Hood L. A novel mutation in myelin-deficient mice results in unstable myelin basic protein gene transcripts. Neuron. 1988 May;1(3):221–225. doi: 10.1016/0896-6273(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Popko B., Puckett C., Lai E., Shine H. D., Readhead C., Takahashi N., Hunt S. W., 3rd, Sidman R. L., Hood L. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell. 1987 Feb 27;48(4):713–721. doi: 10.1016/0092-8674(87)90249-2. [DOI] [PubMed] [Google Scholar]
- Roach A., Boylan K., Horvath S., Prusiner S. B., Hood L. E. Characterization of cloned cDNA representing rat myelin basic protein: absence of expression in brain of shiverer mutant mice. Cell. 1983 Oct;34(3):799–806. doi: 10.1016/0092-8674(83)90536-6. [DOI] [PubMed] [Google Scholar]
- Roach A., Takahashi N., Pravtcheva D., Ruddle F., Hood L. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 1985 Aug;42(1):149–155. doi: 10.1016/s0092-8674(85)80110-0. [DOI] [PubMed] [Google Scholar]
- Roch J. M., Brown-Luedi M., Cooper B. J., Matthieu J. M. Mice heterozygous for the mld mutation have intermediate levels of myelin basic protein mRNA and its translation products. Brain Res. 1986 Nov;387(2):137–144. doi: 10.1016/0169-328x(86)90005-7. [DOI] [PubMed] [Google Scholar]
- Roch J. M., Cooper B. J., Ramirez M., Matthieu J. M. Expression of only one myelin basic protein allele in mouse is compatible with normal myelination. Brain Res. 1987 Dec;427(1):61–68. doi: 10.1016/0169-328x(87)90045-3. [DOI] [PubMed] [Google Scholar]
- Roch J. M., Tosic M., Roach A., Matthieu J. M. The duplicated myelin basic protein gene in mld mutant mice does not impair transcription. Brain Res. 1989 Jan 16;477(1-2):292–299. doi: 10.1016/0006-8993(89)91417-0. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Gietz R. D., Hodgetts R. B. Overlapping transcription units in the dopa decarboxylase region of Drosophila. Nature. 1986 Jul 17;322(6076):279–281. doi: 10.1038/322279a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Roach A., Teplow D. B., Prusiner S. B., Hood L. Cloning and characterization of the myelin basic protein gene from mouse: one gene can encode both 14 kd and 18.5 kd MBPs by alternate use of exons. Cell. 1985 Aug;42(1):139–148. doi: 10.1016/s0092-8674(85)80109-4. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]