Abstract
Elevated level of blood phosphate (Pi) associated with chronic kidney disease (CKD) is a risk factor of aortic valve calcification. Aortic valve interstitial cells (AVICs) display osteogenic responses to high Pi although the underlying mechanism is incompletely understood. Sox9 is a pro-chondrogenic factor and may play a role in ectopic tissue calcification. Circulating and kidney levels of Klotho are reduced in patients with CKD. We hypothesized that Sox9 mediates high Pi-induced osteogenic responses in human AVICs and that Klotho inhibits the responses. Treatment of human AVICs with high Pi increased protein levels of Runt-related transcription factor 2 (Runx2) and alkaline phosphatase (ALP), and a prolonged exposure to high Pi caused calcium deposition. High Pi induced Sox9 upregulation through PKD and Akt activation. Knockdown of Sox9 essentially abolished the effect of high Pi on the osteogenic responses. Lower Klotho levels were observed in calcified aortic valve tissues. Interestingly, high Pi decreased Klotho levels in AVICs from normal valves, and treatment with recombinant Klotho markedly reduced the effect of high Pi on the levels of Sox9, Runx2, and ALP and suppressed calcium deposition. We conclude that high Pi induces human AVIC osteogenic responses through Sox9. Human AVICs express Klotho, and its levels in AVICs are modulated by high Pi and valvular calcification. Importantly, Klotho suppresses the pro-osteogenic effect of high Pi on human AVICs. These novel findings indicate that modulation of Klotho may have therapeutic potential for mitigation of valvular calcification associated with CKD.
Keywords: Phosphate, Aortic valve interstitial cells, Osteogenic responses, Sox9, Klotho
Introduction
Calcific aortic valve disease (CAVD) is the leading cause for valve replacement in Europe and North America and increasingly affects the aging population. Approximately 2.8% of adults over 75 years old have aortic valve calcification [1, 2]. Currently, there is no effective pharmacological therapy for CAVD. Thus, it is important to understand the mechanism responsible for CAVD progression.
There is considerable evidence to suggest that chronic renal disease (CKD) is a risk factor for aortic valve calcification [3]. Aortic valve calcification in CKD patients is linked to the abnormal mineral metabolism characterized by the long-term elevation of serum phosphate (Pi) levels. The osteogenic responses of aortic valvular interstitial cells (AVICs) to high Pi are believed to contribute to the mechanism of aortic valve calcification [4, 5]. However, the mechanisms that mediate high Pi-induced osteogenic responses in human AVICs remain unclear.
In the past decade, increasing studies suggest that CAVD does not result from passive deposition of calcium–phosphate complexes on the injured valve surface but is an actively regulated pathological process mediated by the phenotype changes in AVICs, the most abundant cell type in valve tissue. Chronic stress is thought to cause prolonged activation of AVICs and may result in myofibroblastic as well as osteoblast-like phenotypic transition [6, 7]. Sox9, a master chondrogenesis factor, is upregulated in uremia [8] and participates in the stimulation of chondrogenic gene expression in vascular smooth muscle cells [9]. However, it has been reported that reduced Sox9 function promotes heart valve calcification [10]. In addition, Sox9 has also been found to inhibit bone morphogenetic protein 2 (BMP-2)-induced osteogenic responses in human mesenchymal stem cells [11]. It is unclear whether Sox9 is a pro-osteogenic factor in human aortic valves and whether it plays a role in high Pi-induced osteogenic responses in human AVICs.
Klotho was identified as an anti-aging protein [12], and its level is highest in the kidney [13]. The extracellular domain of this transmembrane protein can be cleaved off and released into the blood [14]. Klotho levels in the kidney and blood were lower in patients with CKD [15]. Klotho-deficient mice suffer from a wide variety of age-related disorders as well as tissue calcification, including aortic valve calcification [16, 17]. However, it remains unknown whether Klotho suppresses high Pi-induced osteogenic responses in human AVICs.
We hypothesized that high Pi induces the osteogenic responses in human AVICs through upregulation of Sox9 and that Klotho suppresses high Pi-induced AVIC osteogenic responses. The aim of the present study was to determine (1) how high Pi induces osteogenic responses in human AVICs (2) and whether Klotho had effect on high Pi-induced AVIC osteogenic responses and (3) the mechanism by which Klotho exerts its effect.
Materials and methods
Chemicals and reagents
Antibodies against Klotho and recombinant human Klotho protein (expressed by mouse myeloma cell line; endotoxin free) were purchased from Abcam (Cambridge, MA). Antibodies against Runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), Sox9, phosphorylated protein kinase D (PKD, also termed PKCµ), total PKD, phosphorylated protein kinase B (Akt), total Akt, and GAPDH were purchased from Cell Signaling, Inc. (Beverly, MA). The two antibodies against phosphorylated PKD recognize PKD ser916 and ser744/748, respectively. Specific PKD inhibitor CID755673 and specific Akt inhibitor MK2206 were purchased from Selleckchem (Houston, TX). Lentiviral short hairpin RNA (shRNA) constructs for Sox9 and Pit 2 were from the Functional Genomics Facility of University of Colorado. Lentiviral non-targeting shRNA expression construct was used as control. HiPerFect Transfection Reagent and other transfection-related reagents were purchased from Qiagen, Inc. (Valencia, CA). Medium 199 was purchased from Lonza (Walkersville, MD). Lipopolysaccharide (LPS), collagenase, and other reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Isolation and culture of AVICs
This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of University of Colorado. All patients provided written informed consent. Calcified aortic valve leaflets were obtained intraoperatively from patients undergoing aortic valve replacement at University of Colorado Hospital due to aortic stenosis. Normal aortic valve leaflets were collected from the explanted hearts of patients with cardiomyopathy and undergoing heart transplantation at the University of Colorado Hospital. Patients with a history of infective endocarditis, rheumatic heart disease, or a genetic syndrome were excluded. All valves were tricuspid. The valve leaflets from explanted hearts were thin and had no histological abnormality.
AVICs were isolated from normal and calcified valves by collagenase I digestion method as previously described [18, 19]. Briefly, valvular leaflets were digested in essential medium containing 1 mg/ml collagenase (type I) at 37 °C for 30 min. After removing endothelial cells by vortex, the leaflets were further digested with a fresh solution of 1 mg/ml collagenase for 4–6 h at 37 °C. After vortex and repeated aspirating to break up the tissue mass, the suspension was spun at 1000 rpm for 10 min to precipitate cells. Cells were resuspended and cultured in M199 growth medium, supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% fetal bovine serum, in an incubator with 5% CO2 at 37 °C. Cells of passages 3–6 were used for the experiments. When grown to 80–90% confluence, AVICs were stimulated with Pi of varying concentrations for different time periods. When needed, cells were pretreated with pharmacological reagents, including CID755673 (40 µmol/l), MK2206 (5 µmol/l), and recombinant Klotho 1 h prior to the addition of Pi. All experiments were repeated using cells isolated from four different donor valves.
Immunofluorescence staining
Detection of phospho-PKD, phospho-Akt, and Klotho proteins in valve tissues was performed using immunofluorescence staining. Frozen sections (5 µm thick) were prepared and dried at room temperature for 30 min. After permeabilization with a methanol/acetone mixture, frozen sections were fixed in 4% paraformaldehyde and then were incubated with primary antibodies against phospho-PKD ser916 (1:50 dilution), phospho-Akt (1:50 dilution), or Klotho (1:50 dilution) overnight at 4 °C. After washing with PBS, sections were incubated with a Cy3-tagged secondary antibody (imaged on red channel). 4′,6-Diamidino-2-phenylindole (DAPI) was used for nucleus counterstaining (imaged on blue channel). Alexa 488-tagged wheat germ agglutinin (WGA, green) was used to outline plasma membrane when needed.
AVICs were seeded in 8-well chamber slides and treated with or without high Pi (3 mmol/l) for indicated time period. If needed, recombinant Klotho (0.5 µg/ml) was added 1 h prior to the addition of Pi. Immunofluorescence staining was performed as previously described to localize Sox9 in normal human AVICs [20].
Microscopy was performed with a Leica CTR5500 digital microscope (Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany).
Immunoblotting
Immunoblotting was applied to analyze Runx2, ALP, Sox9, Klotho, phosphorylated and total PKD, phosphorylated and total Akt, and GAPDH. Human aortic valve tissues and cells were homogenized or lysed in commercial sample buffer according to the manufacturer’s instructions. The protein samples were resolved on 4 to 20% SDS-PAGE gels and then transferred onto nitrocellulose membranes using a wet transfer system. After blocking with 5% skim milk at room temperature for 2 h, membranes were incubated with primary antibodies at 4 °C overnight, followed by incubation with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody diluted in 5% skim milk for 1 h. Protein bands were detected using the enhanced chemiluminescence system. Band density was analyzed using the National Institutes of Health ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Alizarin red staining
For analysis of calcium deposition, AVICs were seeded on 24-well plates. When reaching 80% confluence, cells were treated with indicated interventions in a conditioning medium (growth medium supplemented with 10 mmol/l β-glycerophosphate, 10 nmol/l dexamethasone, 4 µg/ml cholecalciferol, and 8 mmol/l CaCl2) for 14 days. The medium was changed every 3 days. At the end of experiments, alizarin red staining and quantification of calcium deposition were performed as previously described [21].
Gene knockdown
Gene knockdown was performed as previously described [22]. AVICs were treated with recombinant lentivirus expressing control shRNA, Sox9 shRNA, or Pit 2 shRNA in TransDux™ transduction reagent according to the manufacturer’s instructions. Three days later, cells were harvested for validation of knockdown or stimulated with high Pi for 72 h to determine the effect of knockdown on AVIC osteogenic responses.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences, version 17.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard error (SE). After confirming that all variables were normally distributed using the Kolmogorov–Smirnov test, Student’s t test was applied for comparisons between two groups, and one-way ANOVA was used to analyze differences between multiple groups. P < 0.05 was accepted as statistically significant.
Results
High Pi induces osteogenic responses in human AVICs through the PKD and Akt pathways
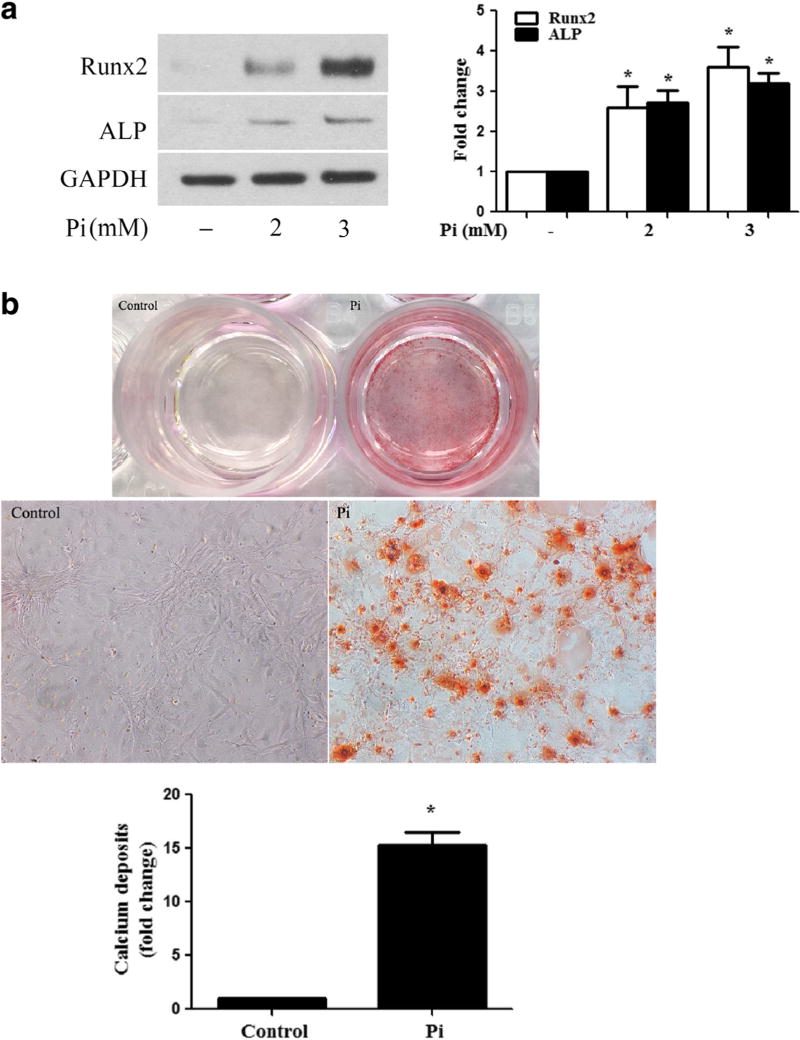
To determine that the effect of high Pi has an effect on AVIC osteogenic responses, we simulated human AVICs of normal valves with high Pi (2 or 3 mmol/l) for 72 h and analyzed protein levels of Runx2 and ALP. As shown in Fig. 1a, high Pi increased cellular levels of Runx2 and ALP in a dose-dependent manner. To determine whether high Pi stimulation induces calcium deposition, we stimulated cells with high Pi for 14 days in a conditioning medium. Figure 1b shows that greater levels of calcium deposition were induced by high Pi. Thus, high Pi elevates the pro-osteogenic activity in human AVICs.
Fig. 1. High Pi induces osteogenic responses in human AVICs.
a Human AVICs were treated with high Pi (2 or 3 mmol/ l) for 72 h. Representative immunoblots and densitometric data show that high Pi upregulates the levels of Runx2 and ALP in human AVICs in a dose-dependent manner. b Human AVICs were treated with high Pi (3 mmol/l) for 14 days in the conditioning medium (growth medium supplemented with 10 mmol/l of β-glycerophosphate, 10 nmol/l of dexamethasone, and 8 mmol/l of CaCl2). Representative images of alizarin red staining and spectrophotometric data show that cells exposed to high Pi form a greater amount of calcium deposits (×10 objective for original magnification in the higher power images). Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. untreated control
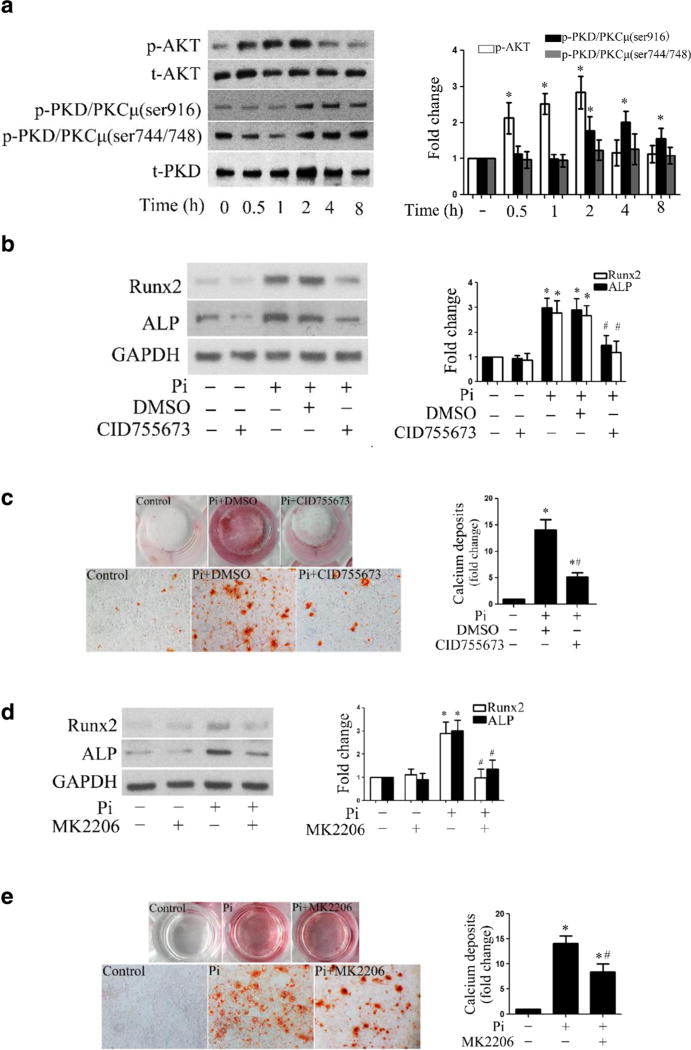
The PKD and Akt pathways have been found to play a critical role in vascular cell calcification [23, 24]. As shown in Supplemental Fig. 1, diseased aortic valves had higher levels of phosphorylated PKD and Akt. To address the role of PKD and Akt in mediating the osteogenic responses to high Pi, we stimulated cells with high Pi (3 mmol/l) for 0.5 to 8 h and observed that high Pi induces the phosphorylation of both Akt and PKD (Fig. 2a). However, no changes in the levels of phosphorylated PKD and Akt were observed in the same time course when high Pi is absent (Supplemental Fig. 2). Then, we applied specific pan inhibitors of PKD (CID755673) and Akt (MK2206) to verify the roles of these signaling pathways. As shown in Fig. 2b, inhibition of PKD markedly reduced the protein levels of Runx2 and ALP in cells treated with high Pi. To determine whether PKD plays a role in high Pi-induced calcium deposit formation, we stimulated cells with high Pi, in the presence or absence of CID755673 for 14 days in conditioning medium. Figure 2c shows that high Pi-induced calcium deposit formation was abolished by inhibition of PKD. Similarly, inhibition of Akt with MK2206 significantly reduced the expression of Runx2 and ALP and attenuated calcium deposition induced by high Pi (Fig. 2d, e). Taken together, these data reveal a critical role of PKD and Akt in mediating high Pi-induced osteogenic responses in human AVICs.
Fig. 2. PKD and Akt mediate the osteogenic responses induced by high Pi.
a Human AVICs were treated with high Pi (3 mmol/l) for 0.5 to 8 h. Representative immunoblots and densitometric data show that high Pi induces rapid phosphorylation of Akt and delayed phosphorylation of PKD. b Human AVICs were treated with high Pi in the presence or absence of PKD inhibitor (CID755673, 40 µM) for 72 h. Representative immunoblots and densitometric data show that inhibition of PKD reduces the levels of Runx2 and ALP in human AVICs exposed to high Pi. c Human AVICs were treated with high Pi (3 mmol/l) in the presence or absence of PKD inhibitor (CID755673, 40 µmol/l) for 14 days in the conditioning medium. Representative images (×10 objective for original magnification in the higher power images) of alizarin red staining and spectrophotometric data show that inhibition of PKD attenuates calcium deposit formation induced by high Pi. d Inhibition of Akt by MK2206 (5 µmol/l) reduces the levels of Runx2 and ALP in cells exposed to high Pi. e Inhibition of Akt by MK2206 (5 µmol/l) attenuates calcium deposit formation (×10 objective for original magnification in the higher power images) in human AVICs induced by high Pi in the conditioning medium. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P<0.05 vs. untreated control; #P<0.05 vs. Pi alone or Pi + DMSO
High Pi upregulates Sox9 through PKD and Akt
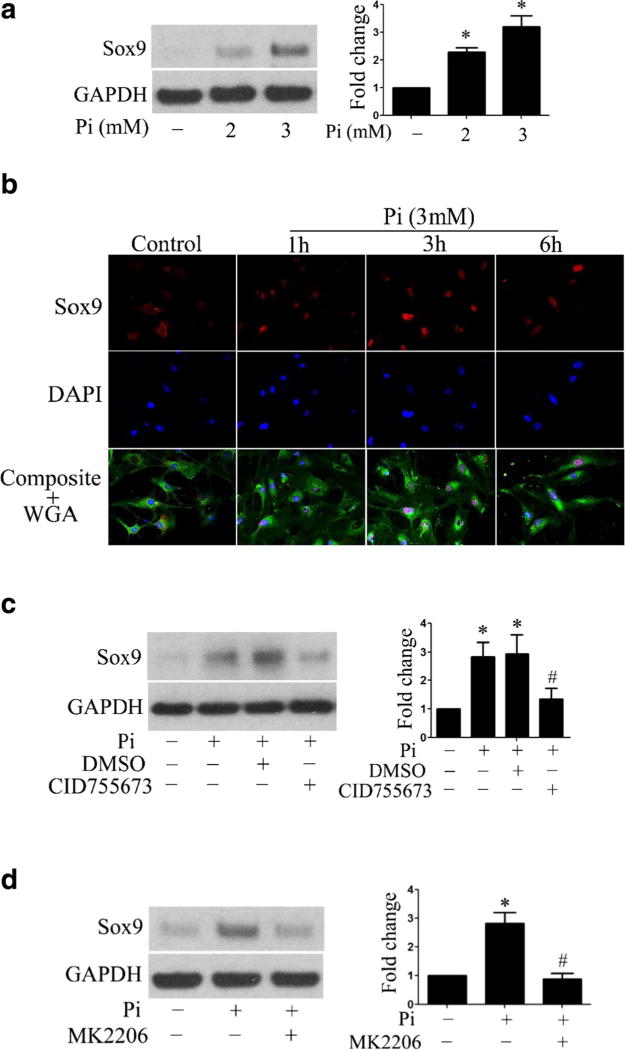
Sox9, a key transcriptional factor of chondrogenesis, has been implicated in valvular calcification [25]. A previous study shows that high Pi upregulates the expression of Sox9 and increases ALP activity in human aortic smooth muscle cells [26]. We found that the levels of Sox9 protein were increased in a dose-dependent manner in human AVICs exposed to high Pi (Fig. 3a). Furthermore, high Pi induced Sox9 translocation from the cytoplasm to the nucleus (Fig. 3b). To determine whether PKD and Akt pathways are involved in high Pi-induced Sox9 upregulation, we pretreated human AVICs with PKD inhibitor or Akt inhibitor prior to high Pi stimulation. As shown in Fig. 3c, inhibition of PKD decreased Sox9 levels in human AVICs exposed to high Pi, and inhibition of Akt had a similar effect (Fig. 3d). Thus, high Pi induces Sox9 upregulation via the PKD and Akt pathways.
Fig. 3. High Pi upregulates Sox9 through PKD and Akt.
a Human AVICs were treated with high Pi (2 or 3 mmol/l) for 72 h. High Pi upregulates the levels of Sox9 protein in a dose-dependent manner. b Representative immunofluorescence images (original magnification, ×40 objective) show that high Pi induces Sox9 (red) intranuclear translocation. Alexa 488-tagged wheat germ agglutinin (WGA, green) was used to outline plasma membrane. 4′,6-Diamidino-2-phenylindole (DAPI, blue) was used for nuclear counterstaining. c Inhibition of PKD (CID755673, 40 µmol/l) reduces the levels of Sox9 in cells exposed to high Pi. d Inhibition of Akt (MK2206, 5 µmol/l) suppresses high Pi-induced Sox9 expression. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. untreated control; #P < 0.05 vs. Pi alone or Pi + DMSO
In exploration of the role of Pi transporters in high Pi-induced Sox9 upregulation, we found that high Pi increased the levels of Pit 2, but it had a minimal effect on Pit 1 levels (Supplemental Fig. 3A). Knockdown of Pit 2 with shRNA moderately attenuated the upregulation of Sox9 induced by high Pi (Supplemental Fig. 3B). It appears that Pi transporter Pit 2 is also involved in the upregulation of Sox9 expression by high Pi although its role is less important in comparison to that of PKD and Akt.
Sox9 mediates high Pi-induced osteogenic responses in human AVICs
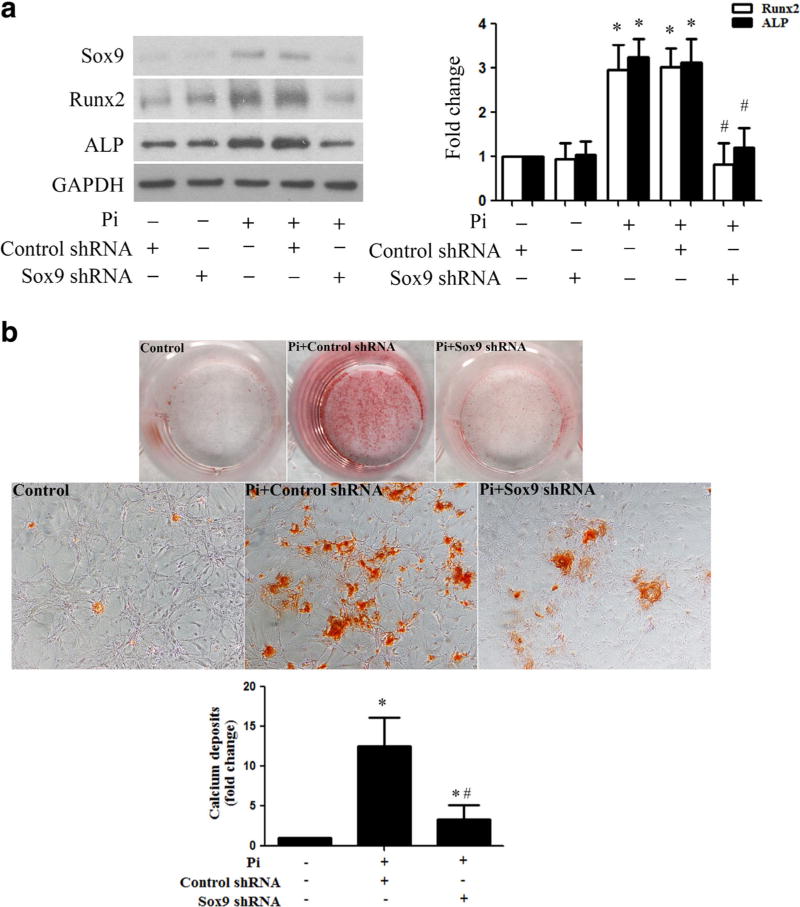
To determine the role of Sox9 in AVIC osteogenic responses to high Pi, we treated cells for 72 h with lentivirus expressing Sox9 shRNA and then exposed them to high Pi. As shown in Fig. 4a, lentiviral Sox9 shRNA markedly reduced the levels of Sox9. Inhibition of Sox9 by shRNA suppressed high Pi-induced upregulation of Runx2 and ALP. Moreover, Sox9 knockdown attenuated AVIC calcium deposition induced by prolonged stimulation with high Pi (Fig. 4b). These data demonstrate that Sox9 plays a critical role in mediating AVIC osteogenic responses to high Pi.
Fig. 4. Sox9 mediates high Pi-induced osteogenic responses in human AVICs.
a Human AVICs were pretreated with lentivirus expressing Sox9 shRNA (100 nmol/l) for 72 h and then treated with high Pi (3 mmol/l) for 72 h. Representative immunoblots show that knockdown of Sox9 with shRNA abolishes Sox9 upregulation and reduces the levels of Runx2 and ALP in cells exposed to high Pi. b Representative images (×10 objective for original magnification in the higher power images) of alizarin red staining show that knockdown of Sox9 attenuates high Pi-induced calcium deposit formation in human AVICs. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. control shRNA; #P < 0.05 vs. Pi alone or Pi + control shRNA
We also stimulated human AVICs with LPS that has been shown to elevate AVIC pro-osteogenic activity in our previous studies [19, 27]. As shown in Supplemental Fig. 4, LPS failed to upregulate Sox9, whereas it upregulated the expression of BMP-2 and ALP in human AVICs. Conversely, it moderately reduced Sox9 levels. It seems that the role of Sox9 in AVIC osteogenic responses is stimulus-dependent. It is likely that the upregulation of Sox9 in human AVICs observed in the present study is specific for high Pi.
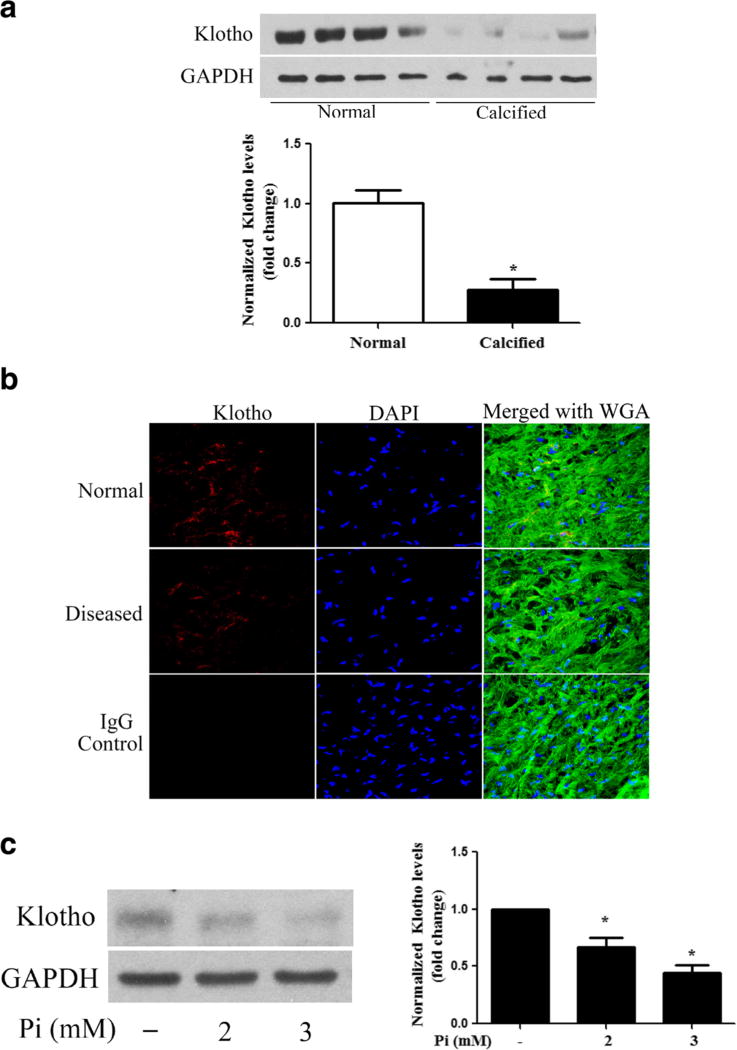
Calcified human aortic valves have reduced levels of Klotho
Patients with CKD have lower levels of Klotho in the kidney and blood [28]. However, it remains unclear whether human aortic valves express Klotho and whether high Pi and calcification have effects on valvular Klotho. In this study, we observed that Klotho protein was detectable in human aortic valve tissue, and calcified human aortic valves had lower levels of Klotho compared to normal aortic valves (Fig. 5a, b). In addition, we observed that high Pi decreased Klotho protein levels in AVICs from normal human aortic valves (Fig. 5c).
Fig. 5. High Pi downregulates Klotho levels in human AVICs.
a Calcified aortic valve tissues (from donors of 56 to 77 years old) have lower levels of Klotho compared to normal aortic valve tissues (from donors of 28 to 56 years old). Data are mean ± SE. n = 4 per group; *P < 0.05 vs. normal. b Representative images (×40 objective for original magnification) of immunofluorescence staining show that Klotho (red) is present in the interstitial cells and interstitial spaces of aortic valve tissue, and diseased aortic valves have lower levels of Klotho. 4′,6-Diamidino-2-phenylindole (DAPI, blue) was used for nuclear counterstaining. Alexa 488-tagged wheat germ agglutinin (WGA, green) was used to outline plasma membrane. c AV I C s o f normal valves were treated with high Pi (2 or 3 mmol/l) for 72 h. Representative immunoblots and densitometric data show that high Pi reduces Klotho protein levels in human AVICs. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. untreated control
Klotho suppresses high Pi-induced osteogenic responses in human AVICs through suppressing Sox9 upregulation
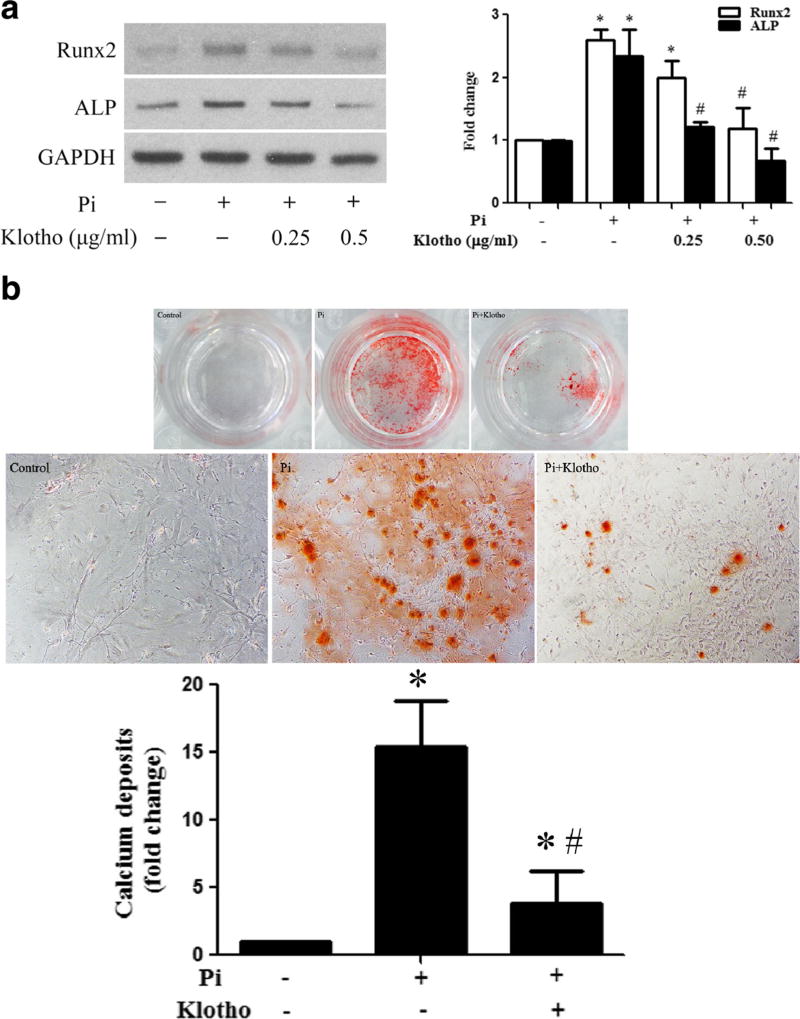
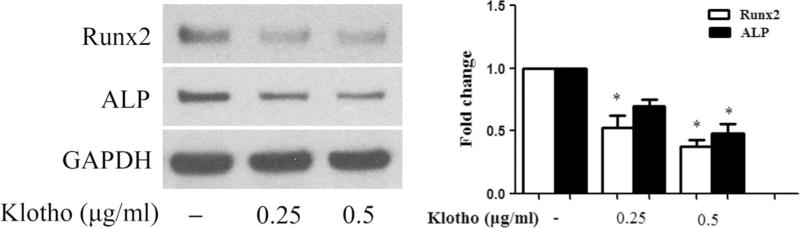
To determine whether recombinant Klotho inhibits high Pi-induced osteogenic responses in human AVICs, we pretreated cells with different concentrations of recombinant Klotho (0.25 and 0.5 µg/ml) 1 h prior to high Pi treatment for 72 h. Klotho reduced the protein levels of Runx2 and ALP in cells exposed to high Pi (Fig. 6a). Additionally, calcium deposition in normal AVICs induced by a prolonged exposure to high Pi was attenuated by recombinant Klotho (Fig. 6b). Furthermore, Klotho reduced the basal levels of Runx2 and ALP in AVICs isolated from calcified valves (Fig. 7). Thus, Klotho suppresses AVIC osteogenic responses to high Pi.
Fig. 6. Klotho suppresses high Pi-induced osteogenic responses in human AVICs.
a AV I C s o f normal human valves were pretreated with different concentrations of recombinant Klotho (0.25 or 0.5 µg/ml) 1 h prior to high Pi treatment for 72 h. Representative immunoblots and densitometric data show that Klotho suppresses high Pi-induced Runx2 and ALP upregulation in human AVICs. b AVICs of normal human valves were treated with high Pi (3 mmol/l), in the presence or absence of Klotho (0.5 µg/ml) for 14 days in the conditioning medium. Representative images (×10 objective for original magnification in the higher power images) of alizarin red staining and spectrophotometric data show that recombinant Klotho suppresses high Pi-induced calcium deposit formation. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. untreated control; #P < 0.05 vs. Pi alone
Fig. 7.
Klotho reduces Runx2 and ALP levels in AVICs of calcified valves. AVICs of calcified human aortic valves were treated with different concentrations of recombinant Klotho (0.25 or 0.5 µg/ml) for 72 h. Representative immunoblots and densitometric data show that recombinant Klotho reduces the levels of Runx2 and ALP in diseased AVICs in the absence of high Pi stimulation. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. untreated control
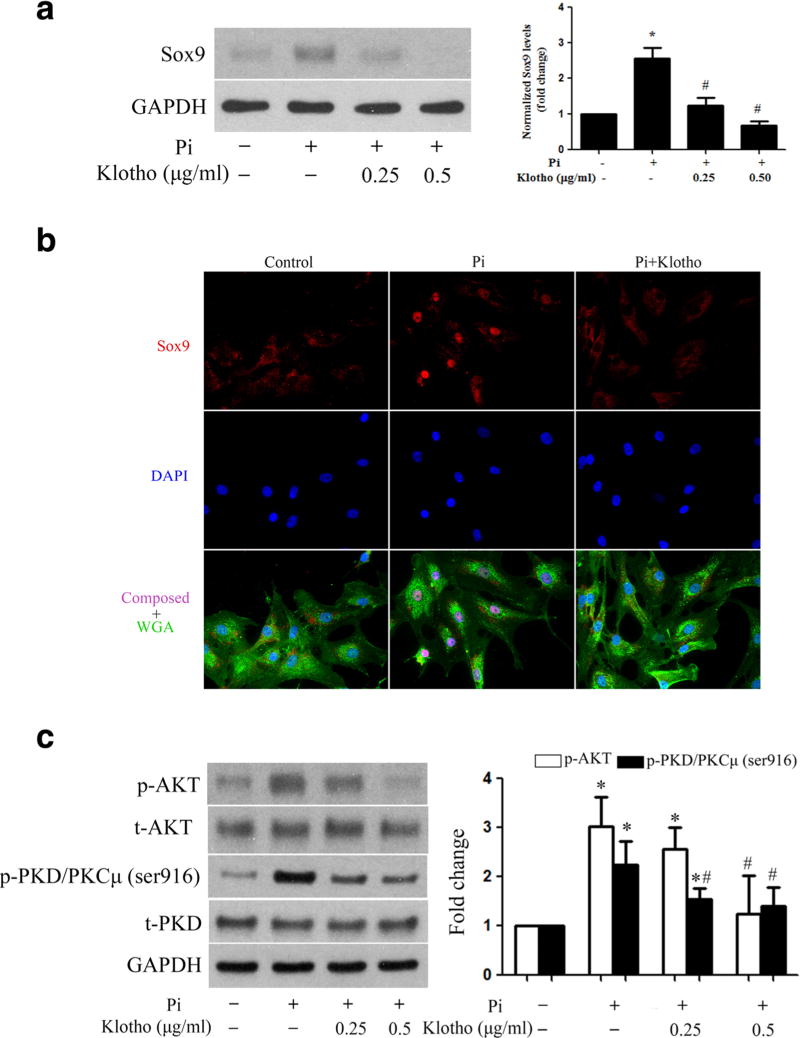
As Sox9 mediates high Pi-induced osteogenic responses and Klotho suppresses the response to high Pi, we conducted further experiment to examine whether and how Klotho inhibits Sox9 upregulation by high Pi. We treated normal AVICs with Klotho 1 h before the stimulation with high Pi. As shown in Fig. 8a, b, recombinant Klotho suppressed the upregulation of Sox9 by high Pi and prevented high Pi-induced Sox9 intranuclear translocation. Furthermore, Klotho inhibited the activation of PKD and Akt (Fig. 8c), two signaling molecules involved in the upregulation of Sox9 by high Pi. Together, these data reveal that Klotho suppresses the osteogenic responses in human AVICs through inhibition of PKD and Akt, which results in attenuated Sox9 upregulation.
Fig. 8. Klotho inhibits Akt and PKD phosphorylation and suppresses Sox9 upregulation induced by high Pi.
a AVICs were pretreated with different concentrations of recombinant Klotho (0.25 or 0.5 µg/ml) 1 h prior to high Pi treatment for 72 h. Immunoblots and densitometric data show that recombinant Klotho suppresses high Pi-induced Sox9 upregulation. b AVICs were pretreated with recombinant Klotho (0.5 µg/ml) 1 h prior to high Pi (3 mmol/l) stimulation for 3 h. Representative immunofluorescence images (×40 objective for original magnification) show that Klotho inhibits Sox9 (red) intranuclear translocation induced by high Pi. Alexa 488-tagged wheat germ agglutinin (WGA, green) was used to outline plasma membrane. 4′,6-Diamidino-2-phenylindole (DAPI, blue) was used for nuclear counterstaining. c AVICs were pretreated with different concentrations of recombinant Klotho (0.25 or 0.5 µg/ml) 1 h prior to high Pi treatment for 2 h. Immunoblots and densitometric data show that recombinant Klotho inhibits high Pi-induced phosphorylation of Akt and PKD. Data are mean ± SE. n = 4 experiments using distinct cell isolates; *P < 0.05 vs. untreated control; #P < 0.05 vs. Pi alone
Discussion
Numerous studies have confirmed that aortic valve calcification is an active process involving multiple mechanisms, including abnormal calcium or phosphate metabolism, valvular inflammation, and osteogenic reprogramming of AVICs. Elevated Pi levels in the circulation play an important role in aortic valve calcification associated with CKD. Currently, the precise mechanism underlying aortic valve calcification in CKD patients remains unclear. The present study demonstrates that high Pi elevates human AVIC pro-osteogenic activity, as evidenced by the upregulation of osteogenic biomarkers Runx2 and ALP, as well as calcium deposition, primarily through PKD-dependent and Akt-dependent Sox9 upregulation. In addition, our data show that human AVICs express Klotho, and Klotho levels in AVICs are negatively regulated by high Pi and calcification. Importantly, Klotho inhibits Sox9 upregulation to suppress the osteogenic responses of human AVICs to high Pi. These findings provide insights into the molecular mechanism underlying aortic valve calcification associated with CKD and indicate that Klotho may have therapeutic potential in attenuating aortic valve calcification.
The PKD and Akt pathways mediate high Pi-induced osteogenic responses
Several population-based studies demonstrate an increased prevalence of aortic valve calcification in patients with CKD [29, 30]. Studies on animal models of CKD also show that declined renal function and increased circulating levels of Pi are accompanied by the appearance of ectopic calcification in vascular and valvular tissues [31, 32]. Using a model of adenine-induced renal failure, Shuvy and colleagues demonstrated that rats fed with a high Pi diet develop aortic valve calcification [33]. Further, AVICs display greater mineral deposition in response to high Pi stimulation [5, 34]. However, the signaling mechanism remains incompletely understood. The results of the present study show that high Pi induces the phosphorylation of PKD and Akt, and inhibition of one of these two signaling pathways suppresses high Pi-induced upregulation of Runx2 and ALP and calcium deposit formation in human AVICs. Akt signaling has been implicated in AVIC osteoblast-like differentiation [35]. The results of the present study suggest that both Akt and PKD pathways mediate high Pi-induced osteogenic responses in human AVICs. It appears that these two signaling pathways work in concert to mediate the upregulation of Runx2 and ALP, and blocking either of these two pathways would markedly attenuate the responses to high Pi.
The next question is how the Akt and PKD pathways upregulate the expression of Runx2 and ALP. The osteogenic transcription factor Runx2 is known to upregulate ALP level and activity [36]. The upregulation of Runx2 by high Pi may be responsible for the elevated ALP levels in human AVICs exposed to high Pi. A factor modulated by PKD and Akt may upregulate the expression of Runx2, and possibly ALP as well.
Sox9 plays a critical role in mediating high Pi-induced osteogenic responses
Sox9 is a master regulator of chondrogenesis and is required for chondrocyte proliferation, differentiation, and maturation [37]. It has been reported that Sox9 is overexpressed in stenotic human aortic valves [38], indicating a role of this factor in aortic valve calcification. However, Sox9 appears to have an anti-osteogenic effect in animal aortic valves. Mice with Sox9 deficiency have been found to develop heart valve calcification, and knockdown of Sox9 in murine heart valves in vitro results in increased Runx2 expression and calcium deposition [10]. In porcine AVICs, calcium nodule formation is associated with translocation of Sox9 from the nucleus to the cytoplasm [39]. Interestingly, the results of the present study show that high Pi upregulates Sox9 in human AVICs in a PKD-dependent and Akt-dependent manner and promotes Sox9 intranuclear translocation. Importantly, knockdown of Sox9 abolishes high Pi-induced expression of Runx2 and ALP, as well as the greater calcium deposition in human AVICs. Thus, Sox9 plays a pro-osteogenic role in human AVICs and mediates the osteogenic responses to high Pi. The discrepancy between our finding and the findings from the animal study [10] may be due to the difference between human and animal. In addition, heart valve calcification involves a complicated pathobiology. Other altered factors may be responsible for heart valve calcification observed in the Sox9-deficient mice.
LPS does not upregulate Sox9 levels in human AVICs although it induces the osteogenic responses. It appears that different stimuli utilize different mechanisms to mediate the osteogenic responses in human AVICs, and Sox9 upregulation appears to be specific to high Pi stimulation. It is likely that high Pi is responsible for the upregulation of Sox9 observed in this study. In this regard, high Pi has been reported to upregulate the expression of Sox9 in human endothelial cells and smooth muscle cells [26, 40]. The findings of the present study highlight a novel role of Sox9 in mediating the osteogenic responses of human AVICs to high Pi.
It should be noted that high Pi also elevates Pit 2 levels in human AVICs and that knockdown of Pit 2 suppresses the upregulation of Sox9 by high Pi. It appears that Pit 2 also plays a role in mediating high Pi-induced Sox9 upregulation. Future studies are needed to determine the relative role of Pit 2 in mediating the osteogenic responses to high Pi in human AVICs.
Klotho suppresses human AVIC osteogenic responses to high Pi
The anti-aging protein Klotho has attracted great attention because of its implication in diverse biological processes related to human longevity. Interestingly, aortic valve calcification score of patients is negatively correlated with circulating Klotho levels [41]. The Klotho−/− mouse model, which exhibits premature aging associated with hyperphosphatemia and low vitamin D, has been used to study age-related diseases, including CKD and vascular calcification. Klotho−/− mice exhibit aortic valve calcification at the hinge region of the fibrosa side [16], a pathological change similar to human CAVD. Reduced expression of this protein has been observed in patients with CKD, and this may be one of the factors underlying the aortic valve calcification in CKD. However, the impact of high Pi on Klotho has not been examined, and the role of Klotho in AVIC osteogenic responses to high Pi is unknown.
We observed that Klotho protein is present in human aortic valves, and calcified aortic valve tissue and AVICs from such tissue have lower levels of Klotho. The reduction of Klotho in calcified aortic valves may be due to both disease and aging since patients with aortic valve calcification are usually older. Interestingly, high Pi decreases the levels of Klotho in AVICs isolated from normal human valves, and recombinant Klotho suppresses the upregulation of Runx2 and ALP and attenuates calcium deposition in normal human AVICs exposed to high Pi. More importantly, Klotho reduces the basal levels of Runx2 and ALP in AVICs isolated from calcified valves. Therefore, Klotho negatively modulates AVIC pro-osteogenic activity, and downregulation of Klotho in AVICs by high Pi may contribute to the elevated valvular pro-osteogenic activity associated with CKD and CAVD.
The anti-osteogenic effect of Klotho on human AVIC appears due to its suppression of Sox9 upregulation by high Pi since recombinant Klotho markedly reduces the upregulation of Sox9 that is critical in mediating high Pi-induced osteogenic responses in human AVICs. Our finding is consistent with a previous report showing that Klotho null mice have increased expression of Sox9 in aortic valve tissue [42]. Currently, the existence of a specific receptor for Klotho is a matter of debate. Nevertheless, our data show that Klotho inhibits PKD and Akt, two signaling molecules involved in the upregulation of Sox9 by high Pi. Together, the data of the present study reveal that Klotho suppresses the osteogenic responses to high Pi in human AVICs through attenuation of Sox9 upregulation by inhibition of PKD and Akt.
This study has several limitations. Although we observed that Sox9 plays a critical role in mediating high Pi-induced osteogenic responses in human AVICs and that Klotho attenuates Sox9 upregulation to suppress AVIC osteogenic responses to high Pi, it is important to confirm these in vitro findings in future studies using animal models. In addition, it remains unclear from this study what receptor mediates the effect of Klotho on human AVIC osteogenic responses to high Pi. Further studies are warranted to identify the receptor.
Conclusions
The present study demonstrates that high Pi induces osteogenic responses in human AVICs via PKD-dependent and Akt-dependent Sox9 upregulation. Klotho is preset in human aortic valves and AVICs, and high Pi reduces Klotho levels in human AVICs. Furthermore, Klotho suppresses the pro-osteogenic effect of high Pi through inhibition of Sox9 upregulation. These findings suggest that modulation of Klotho levels and/or function may have therapeutic potential for mitigation of aortic valve calcification associated with CKD.
Supplementary Material
Key messages.
CAVD associated with chronic kidney disease is a significant clinical problem.
High phosphate upregulates Sox9 through AKT and PKD in human AVICs.
Calcified human aortic valves have lower levels of Klotho.
Klotho suppresses Sox9 upregulation and intranuclear translocation.
Klotho inhibits high phosphate-induced osteogenic activity in human AVICs.
Acknowledgments
This study was supported by National Institutes of Heart, Lung, and Blood Grants HL106582 and HL121776.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00109-017-1527-3) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, et al. A prospective survey of patients with valvular heart disease in Europe: the euro heart survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Rattazzi M, Bertacco E, Del Vecchio A, Puato M, Faggin E, Pauletto P. Aortic valve calcification in chronic kidney disease. Nephrol Dial Transplant. 2013;28:2968–2976. doi: 10.1093/ndt/gft310. [DOI] [PubMed] [Google Scholar]
- 4.Lau WL, Pai A, Moe SM, Giachelli CM. Direct effects of phosphate on vascular cell function. Adv Chronic Kidney Dis. 2011;18:105–112. doi: 10.1053/j.ackd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seya K, Furukawa K, Chiyoya M, Yu Z, Kikuchi H, Daitoku K, Motomura S, Murakami M, Oshima Y, Fukuda I. 1-Methyl-2-undecyl-4(1H)-quinolone, a derivative of quinolone alkaloid evocarpine, attenuates high phosphate-induced calcification of human aortic valve interstitial cells by inhibiting phosphate cotransporter PiT-1. J Pharmacol Sci. 2016;131:51–57. doi: 10.1016/j.jphs.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Towler DA. Molecular and cellular aspects of calcific aortic valve disease. Circ Res. 2013;113:198–208. doi: 10.1161/CIRCRESAHA.113.300155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neven E, Persy V, Dauwe S, De Schutter T, De Broe ME, D’Haese PC. Chondrocyte rather than osteoblast conversion of vascular cells underlies medial calcification in uremic rats. Arterioscler Thromb Vasc Biol. 2010;30:1741–1750. doi: 10.1161/ATVBAHA.110.204834. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Ji G, Shen J, Wang X, Zhou J, Li L. SOX9 and myocardin counteract each other in regulating vascular smooth muscle cell differentiation. Biochem Biophys Res Commun. 2012;422:285–290. doi: 10.1016/j.bbrc.2012.04.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao J, Hu N, Zhou N, Lin L, Zhao C, Yi S, Fan T, Bao W, Liang X, Chen H, et al. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One. 2014;9:e89025. doi: 10.1371/journal.pone.0089025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 13.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol. 2013;9:650–660. doi: 10.1038/nrneph.2013.111. [DOI] [PubMed] [Google Scholar]
- 15.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Stallons MV, Wirrig-Schwendeman EE, Hassel KR, Conway SJ, Yutzey KE. Bone morphogenetic protein signaling is required for aortic valve calcification. Arterioscler Thromb Vasc Biol. 2016;36:1398–1405. doi: 10.1161/ATVBAHA.116.307526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Lin Y, Sun Z. Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKalpha-mediated activation of RUNX2. Aging Cell. 2016 doi: 10.1111/acel.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Cai Z, Chen F, Shi X, Zhang Q, Chen S, Shi J, Wang DW, Dong N. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res Cardiol. 2012;107:306. doi: 10.1007/s00395-012-0306-0. [DOI] [PubMed] [Google Scholar]
- 19.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC, Jr, Fullerton DA. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294:C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Q, Jin C, Ao L, Cleveland JC, Jr, Song R, Xu D, Fullerton DA, Meng X. Cross-talk between the Toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation. 2012;126:S222–S230. doi: 10.1161/CIRCULATIONAHA.111.083675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song R, Fullerton DA, Ao L, Zheng D, Zhao KS, Meng X. BMP-2 and TGF-beta1 mediate biglycan-induced pro-osteogenic reprogramming in aortic valve interstitial cells. J Mol Med (Berl) 2015;93:403–412. doi: 10.1007/s00109-014-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song R, Zeng Q, Ao L, Yu JA, Cleveland JC, Zhao KS, Fullerton DA, Meng X. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via Toll-like receptor-2. Arterioscler Thromb Vasc Biol. 2012;32:2711–2720. doi: 10.1161/ATVBAHA.112.300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Shan J, Yang W, Zheng H, Xue S. High mobility group box 1 (HMGB1) mediates high-glucose-induced calcification in vascular smooth muscle cells of saphenous veins. Inflammation. 2013;36:1592–1604. doi: 10.1007/s10753-013-9704-1. [DOI] [PubMed] [Google Scholar]
- 24.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 25.Kauffenstein G, Pizard A, Le Corre Y, Vessieres E, Grimaud L, Toutain B, Labat C, Mauras Y, Gorgels TG, Bergen AA, et al. Disseminated arterial calcification and enhanced myogenic response are associated with abcc6 deficiency in a mouse model of pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol. 2014;34:1045–1056. doi: 10.1161/ATVBAHA.113.302943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alesutan I, Musculus K, Castor T, Alzoubi K, Voelkl J, Lang F. Inhibition of phosphate-induced vascular smooth muscle cell osteo-/chondrogenic signaling and calcification by bafilomycin A1 and methylamine. Kidney Blood Press Res. 2015;40:490–499. doi: 10.1159/000368524. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Q, Song R, Ao L, Weyant MJ, Lee J, Xu D, Fullerton DA, Meng X. Notch1 promotes the pro-osteogenic response of human aortic valve interstitial cells via modulation of ERK1/2 and nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2013;33:1580–1590. doi: 10.1161/ATVBAHA.112.300912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotondi S, Pasquali M, Tartaglione L, Muci ML, Mandanici G, Leonangeli C, Sales S, Farcomeni A, Mazzaferro S. Soluble alpha-Klotho serum levels in chronic kidney disease. Int J Endocrinol. 2015;2015:872193. doi: 10.1155/2015/872193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 30.Guerraty MA, Chai B, Hsu JY, Ojo AO, Gao Y, Yang W, Keane MG, Budoff MJ, Mohler ER, 3rd, Investigators CS. Relation of aortic valve calcium to chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study) Am J Cardiol. 2015;115:1281–1286. doi: 10.1016/j.amjcard.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 33.Shuvy M, Abedat S, Beeri R, Danenberg HD, Planer D, Ben-Dov IZ, Meir K, Sosna J, Lotan C. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res. 2008;79:492–499. doi: 10.1093/cvr/cvn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rattazzi M, Iop L, Faggin E, Bertacco E, Zoppellaro G, Baesso I, Puato M, Torregrossa G, Fadini GP, Agostini C, et al. Clones of interstitial cells from bovine aortic valve exhibit different calcifying potential when exposed to endotoxin and phosphate. Arterioscler Thromb Vasc Biol. 2008;28:2165–2172. doi: 10.1161/ATVBAHA.108.174342. [DOI] [PubMed] [Google Scholar]
- 35.Yao Q, Song R, Ao L, Zhan Q, Cleveland JC, Jr, Yu X, Fullerton DA, Meng X. Over-expression of neurotrophin 3 in human aortic valves affected by calcific disease induces the osteogenic responses via the Trk-Akt pathway. Biochim Biophys Acta. 2015;1852:1940–1949. doi: 10.1016/j.bbadis.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 37.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 38.Alexopoulos A, Bravou V, Peroukides S, Kaklamanis L, Varakis J, Alexopoulos D, Papadaki H. Bone regulatory factors NFATc1 and Osterix in human calcific aortic valves. Int J Cardiol. 2010;139:142–149. doi: 10.1016/j.ijcard.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Huk DJ, Austin BF, Horne TE, Hinton RB, Ray WC, Heistad DD, Lincoln J. Valve endothelial cell-derived Tgfbeta1 signaling promotes nuclear localization of Sox9 in interstitial cells associated with attenuated calcification. Arterioscler Thromb Vasc Biol. 2016;36:328–338. doi: 10.1161/ATVBAHA.115.306091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balogh E, Toth A, Tolnai E, Bodo T, Banyai E, Szabo DJ, Petrovski G, Jeney V. Osteogenic differentiation of human lens epithelial cells might contribute to lens calcification. Biochim Biophys Acta. 2016;1862:1724–1731. doi: 10.1016/j.bbadis.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Di Lullo L, Gorini A, Bellasi A, Morrone LF, Rivera R, Russo L, Santoboni A, Russo D. Fibroblast growth factor 23 and parathyroid hormone predict extent of aortic valve calcifications in patients with mild to moderate chronic kidney disease. Clin Kidney J. 2015;8:732–736. doi: 10.1093/ckj/sfv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J Mol Cell Cardiol. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.