Abstract
Higher-order aberrations (HOAs) and intraocular scatter lead to the degradation of image quality on the retina, and consequently deteriorate subjective visual performance. In this article, we modified an adaptive optics double-pass system to combine objective and subjective visual testing capabilities. Employing the modified DP system, we investigated the effects of HOAs and intraocular scatter on contrast sensitivity. Contrast sensitivity measurements were performed with HOAs either retained or corrected by adaptive optics, and with scatter either remaining at the natural eye-induced level or further enhanced by a set of three different scatter filters. Contrast sensitivity was found to be worse when HOAs were uncorrected or scatter increased. Quantitative analysis indicated that the joint effect of HOAs and scatter on contrast sensitivity was not a simple summation of each contributing factor, suggesting a potential compensatory mechanism between HOAs and intraocular scatter on contrast sensitivity.
OCIS codes: (170.4460) Ophthalmic optics and devices, (330.4595) Optical effects on vision
1. Introduction
Higher-order aberrations (HOAs) and intraocular scatter lead to the degradation of image quality on the retina, and consequently deteriorate subjective visual performance. Contrast sensitivity (CS) proved to be clinically useful for assessing the subjective visual performance [1, 2]. It is valuable to assess the effect of HOAs and intraocular scatter on CS to aid in better understanding of the visual performance.
In 1997, Liang et al. built the world’s first adaptive optics (AO) system for the eye and obtained supernormal vision through HOAs correction [3]. With the introduction of AO into vision researches, we were then well equipped to explore vision beyond traditional domains. A number of investigations have explored the effect of HOAs on CS [3–8]. Most of them discovered a marked improvement in CS with HOAs correction. Our recent study [8] also showed that AO correction of HOAs improved CS at all spatial frequencies for all subjects. Therefore, it’s reasonable to speculate a negative effect of HOAs on CS.
Intraocular scatter is another important optical factor affecting CS. There have been several studies [9-10] assessing the effect of intraocular scatter on CS, and inconsistent conclusions were made from the experimental data. Using the statistical analysis method, Lee et al. [9] compared parameters of the double-pass (DP) system with the CS. They discovered that the objective scatter index (OSI) was not correlated with CS at any spatial frequencies. In a more recent study, Bueno et al. [10] measured CS with different scatter levels induced by pre-defined filters. The results indicated that CS decreased with increasing scatter for all spatial frequencies.
Efforts have been made with respect to the combined impact of HOAs and intraocular scatter on CS. In their research on the role of HOAs and intraocular scatter in the visual deterioration of eyes with cortical or nuclear cataract, Fujikado et al. [4] presented that loss of CS was predominantly due to backward intraocular scatter and HOAs in eyes with nuclear cataract, and to forward intraocular scatter and HOAs in eyes with cortical cataract. Using multivariate regression analysis method, Kamiya et al. [11] investigated the clinical factors that influenced CS in a population of myopic and otherwise healthy subjects. They found CS was significantly correlated with intraocular scatter, but not with HOAs, suggesting that the intraocular scatter played a more essential role in CS among myopic subjects. In fact, most of previous studies focused on which one of the two factors played a more important role on CS. As HOAs and intraocular scatter both affect CS, it’s questionable whether the joint impact of the two factors is a simple summation operation. A quantitative relation between the effect introduced by the simultaneous presence of the two factors and the effects introduced by the respective presence of the two factors is more valuable to understand the combined effect of the two factors on visual performance. However, no relevant results have been reported to date and it’s of interest to make a research on this issue.
In this article, we modified an adaptive optics double-pass system to combine objective and subjective visual testing. Employing the modified DP system, we investigated the effect of HOAs and intraocular scatter on CS. CS was measured with HOAs either retained or corrected, and with scatter either remaining at the natural eye-induced level or further enhanced by one of a set of three different scatter filters, and then compared across different optical conditions. In order to quantitatively assess the relationship of the effect of HOAs and intraocular scatter on CS, three impact metrics were introduced and calculated for three different optical conditions, and their relations were analyzed.
2. Methods
2.1 Subjects
Three well-trained subjects (LB, HSY and CH) aged between 25 and 28 participated in this study. For each subject, the data were measured with the left eye. Clinical refraction for each subject was implemented for complete correction of their low order aberrations. Paralysis of accommodation and dilation of pupil was achieved through administering 1% cyclopentolate. Informed consent was obtained from all subjects and the experiment procedures conformed to the tenets of the Declaration of Helsinki.
2.2 System
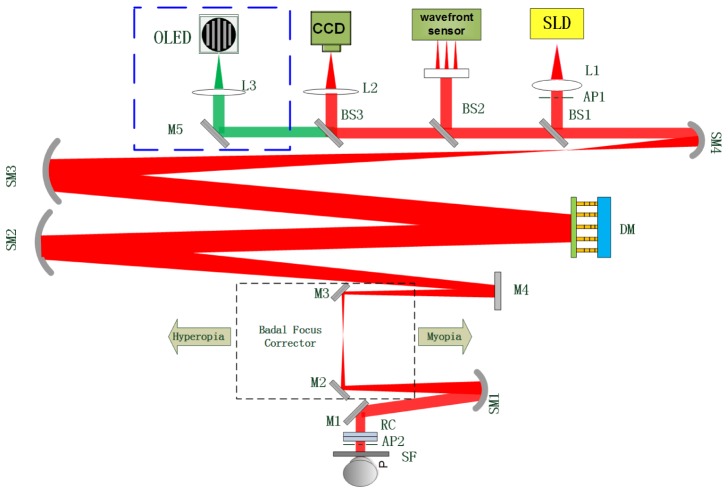
An adaptive optics double-pass PSF measurement system was designed for accurate intraocular scatter estimation, and it was described in detail elsewhere [12]. This system has been modified by incorporating an additional channel for visual testing. The visual testing channel is highlighted by the blue dotted rectangle box of Fig. 1. The visual stimuli for visual testing were generated by Matlab 2011b and Psychtoolbox extensions on another computer, and projected by an OLED display (EMA-100110, eMagin Corporation). The OLED display emitted at 540 nm (green light) with a bandwidth of 70 nm. It had a resolution of 800 × 600 pixels and a maximum refresh rate of 75 Hz. An interference filter (550 nm ± 5 nm) was placed in front of the OLED display. The OLED display and interference filter were fixed on a stepping motor for adjusting the chromatic focus shift between the HS infrared light source and the green stimulus. A gamma correction setting was used to calibrate the OLED display for its luminance nonlinearity. An objective lens L3 and a reflecting mirror M5 were used to form an image of the stimulus on the retina. Considering that BS2, BS3, M5 and L 3 might slightly deteriorate the contrast level of the display, the static aberrations introduced by them were calibrated and pre-corrected.
Fig. 1.
Schematic diagram of the modified adaptive optics double-pass PSF measurement. SLD, super-luminescent diode; SM, spherical mirror, BS, beam splitter; M, mirror; DM, 145-element PZT deformable mirror; L, lens; AP1, artificial entrance pupil; AP2, artificial exit pupil; RC, rotating cylinders; SF, scatter filter.
2.3 Experiment procedures
Tests involving OSI and contrast sensitivity function (CSF) measurements were performed under eight experimental conditions for all subjects. The same tests were repeated for 4 scatter situations and 2 aberration conditions of the eyes as follows: (1) basic correction of defocus and astigmatism; (2) AO correction of HOAs beyond the basic correction. The defocus and astigmatism were corrected by means of a pair of rotating cylinders (RC) in combination with a Badal focus corrector. For each aberration condition, the measurements were repeated at 4 scatter levels. The eyes were tested without scatter filters in the control situation, while three different diffusers (scatter filters) including S0.5 (Tiffen, Pro Mist 1/2), S1 (Tiffen, Pro Mist 1) and S2 (Tiffen, Pro Mist 2) were used in front of the eyes in the rest situations. A higher grade indicates an increasing scattering effect of the filter. The eight experimental conditions were randomized with the subjects.
The OSI values were obtained from each DP image as the ratio of the amount of light within an annular area of 12 and 20 arcmin to that recorded within a circular area with a one arcmin radius centered on the central peak of the acquired DP PSF image [13]. Five DP PSF images were taken to calculate the OSI values for each condition. These five OSI values were averaged as the final value of the OSI.
The CSF was measured at spatial frequencies of 2, 4, 8, 16 and 32 cycles/degree (cpd). In CSF measurements, the stimuli were vertical sinusoidal grating with a background luminance of 5.2 cd/m2 in the pupil plane, which was measured with an IL17000 Radiometer (International Light Technologies Inc., Peabody, MA). A two-alternative forced choice procedure was adopted in which only one of two consecutive presentations of a trial contained the gratings (the other was a blank scene with background luminance). Each presentation lasted for 100 ms. The contrast of the stimuli was controlled through an adaptive staircase procedure. Three successive correct responses led to a contrast decrease by 10% of the previous value; one incorrect response brought a 10% increase. The psychometric data were obtained from 8 staircases of 88 trials each. Contrast threshold for each spatial frequency was calculated as the average of the last 5 turnarounds, and its reciprocal was CS. More details about the procedures can be found in reference [14].
In order to quantitatively assess the relationship of the effect of HOAs and intraocular scatter on CS, three impact metrics were defined as follows:
| (1) |
| (2) |
| (3) |
With CS the contrast sensitivity measured without HOAs correction and CSHC the contrast sensitivity measured with HOAs correction, CSHC + F the contrast sensitivity measured with scatter filters after HOAs correction, CSF the contrast sensitivity measured with scatter filters before HOAs correction. and quantify the effect of HOAs and scatter on CS, respectively. quantify the joint effect of HOAs and scatter on CS. A null value of the metrics means no effect on CS, a value close to 1 means a large decrease in CS.
3. Results
3.1 Real-time measurement and correction of HOAs
Figure 2 shows the time course of total RMS aberration for all the subjects. Data during eye blinks have already been removed. The average native RMS was 0.20 μm, 0.39 and 0.35 μm for Subject LB, HSY and CH, respectively. After AO correction was initiated (T = 10s), the residual RMS of all subjects converged rapidly to a small value of about 0.06 μm and leveled off, demonstrating a good performance of the AO system.
Fig. 2.
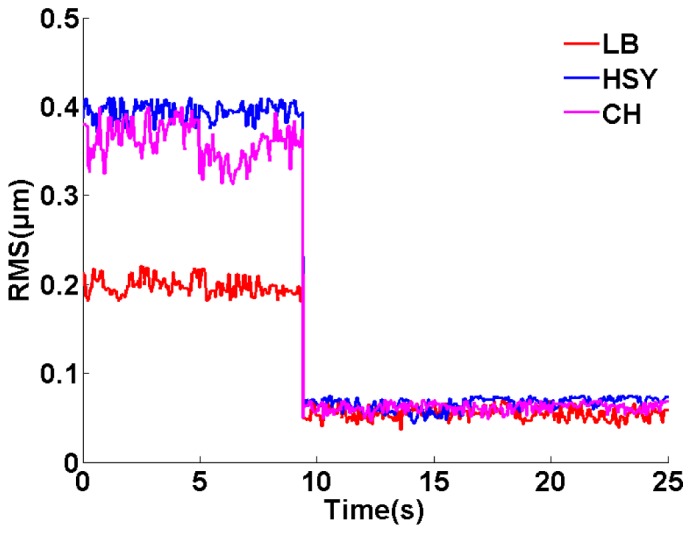
The time-course of total RMS aberrations upon AO correction for the three subjects. The red, blue and magenta lines represent RMS aberration for Subject LB, HSY and CH, respectively. AO is initiated at T = 10s.
3.2 Contrast sensitivity before and after HOAs correction
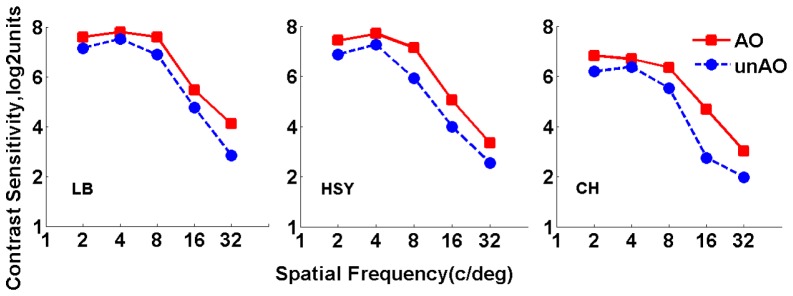
Figure 3 shows the results of CSF measurements for the three subjects, in log 2 scale. Different columns display data for different subjects, respectively. Blue circles stand for results obtained without AO correction (defocus and astigmatism are corrected); red squares stand for results obtained with AO correction of HOAs. It’s evident that AO correction of HOAs improved CS for all spatial frequencies in all eyes.
Fig. 3.
Contrast sensitivity function measured with and without HOAs correction. Red squares stand for results obtained with AO correction; blue circles stand for results obtained without AO correction.
The average improvement across different eyes was by a factor of 1.47, 1.27, 1.91, 2.50 and 2.06 at 2, 4, 8, 16, and 32 cpd, respectively. The average improvements across different spatial frequencies were 1.65, 2.08, and 1.80 for LB, HSY, and CH, respectively. Associated with the results in Table 1, it seemed that a greater amount of HOAs brought larger improvement in CS. The data were referred to a paired t test for statistical analysis. Results showed that the improvement in CS was significant for all spatial frequencies (p<0.05), indicating the validity of our optical correction. For a better understanding of the effect of HOAs on visual performance, parameter was computed for each spatial frequency. Figure 4 shows the corresponding results.
Fig. 4.
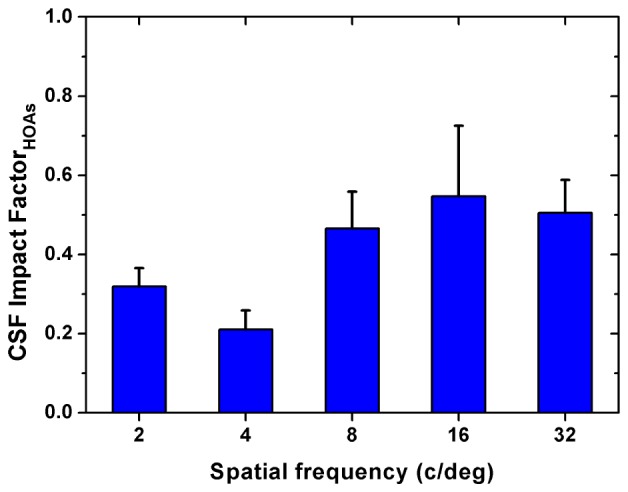
Average impact factor of HOAs on contrast sensitivity. Error bars represent the standard deviations across subjects.
3.3 Contrast sensitivity at different scatter levels after HOAs correction
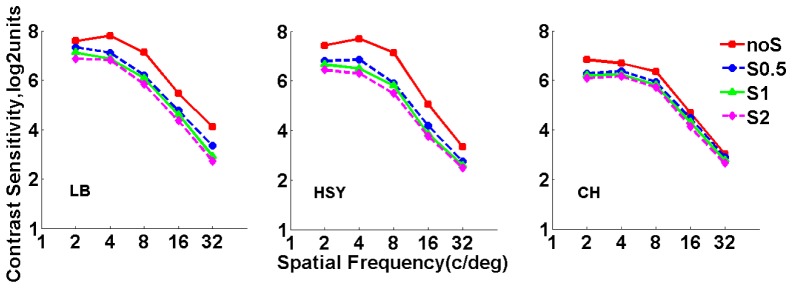
For each subject, DP images were recorded and CSF were measured at different scatter levels with HOAs corrected. The OSI values were obtained from the DP images. Figure 5 shows the OSI values for all subjects at 4 scatter levels. As expected OSI values increased with higher scatter level. When no scatter filter was applied, the OSI value was similar in all subjects (~0.9); when the filter S2 was applied, OSI ranged between ~3.8 (Subject HSY) and ~5.5 (Subject LB). Figure 6 shows the results of CSF for all subjects measured with and without scatter filters. It can be observed that the CS decreased with increasing scatter for each spatial frequency, which was consistent with the previous results reported by Bueno et al. [10]. For a better understanding of the effect of scatter on visual performance, parameter was computed for each scatter filter and spatial frequency. The corresponding results are shown in Fig. 7.
Fig. 5.
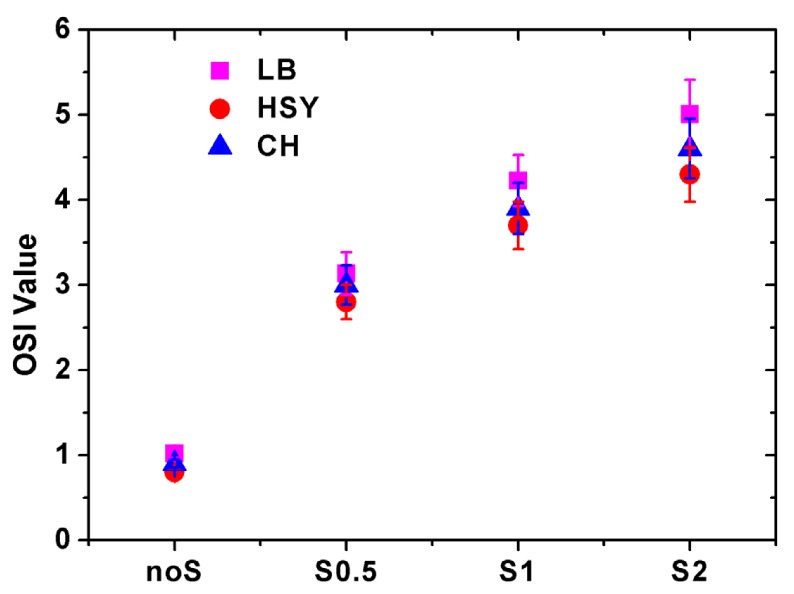
Average OSI values for all subjects obtained from DP images acquired with the eye of the subject alone and using the three scatter filters. Magenta squares stand for results of Subject LB; red circles stand for results of Subject HSY; blue triangles stand for results of Subject CH. Error bars correspond to the standard deviation computed from five DP images.
Fig. 6.
Contrast sensitivity function measured with and without scatter filters. Red squares stand for results obtained without scatter filters; blue circles stand for results obtained with Filter S0.5; green triangles stand for results obtained with Filter S1; magenta diamonds stand for results obtained with Filter S2.
Fig. 7.
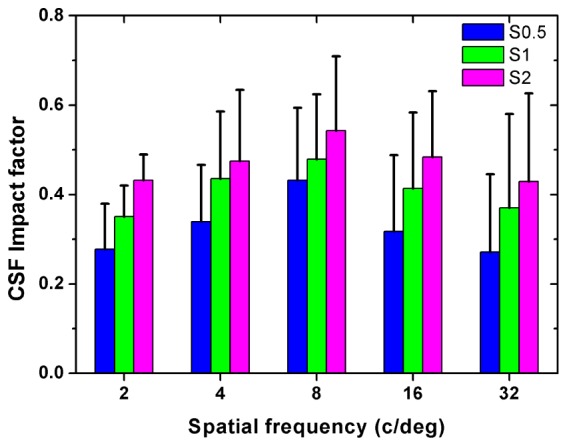
Average impact factor on contrast sensitivity when using the different scatter filters after HOAs correction. Error bars represent the standard deviations across subjects.
3.4 Contrast sensitivity at different scatter levels before HOAs correction
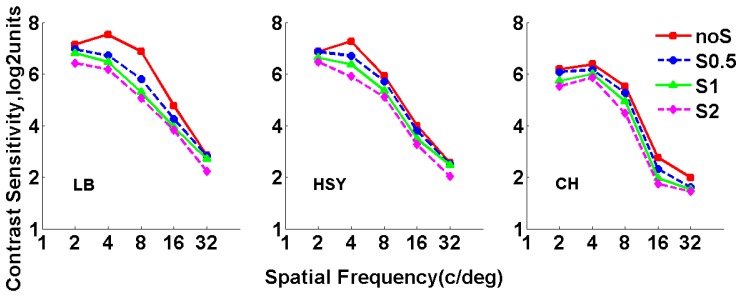
Figure 8 shows the results of CSF for all subjects measured at different scatter levels without HOAs correction. In this case, CS decreased with increasing scatter, and was smaller than the corresponding results shown in Fig. 6 at the same scatter level with HOAs corrected, for all subjects and spatial frequencies. These indicated that both scatter and HOAs had an effect on the CSF. For a better understanding of the combined effect of scatter and HOAs on visual performance, parameter was computed for each spatial frequency. Figure 9 shows the corresponding results.
Fig. 8.
Contrast sensitivity function measured with and without scatter filters before HOAs correction. Red squares stand for results obtained without scatter filters; blue circles stand for results obtained with Filter S0.5; green triangles stand for results obtained with Filter S1; magenta diamonds stand for results obtained with Filter S2.
Fig. 9.
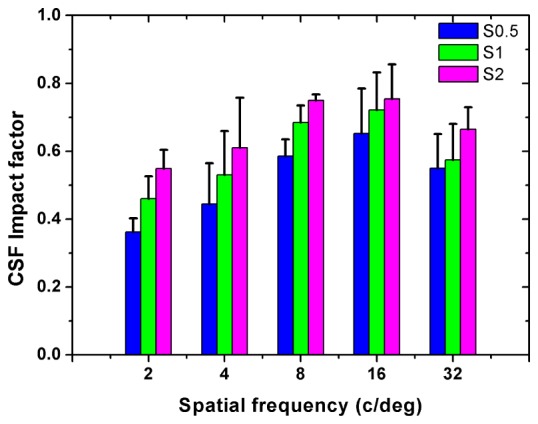
Average impact factor on the CS when using the different scatter filters before HOAs correction. Error bars represent the standard deviations across subjects.
The impact factor data including these shown in Fig. 4, Fig. 7 and Fig. 9 were referred to a two-way analysis of variance (ANOVA) with factors being HOAs and scatter condition. The results indicated significant interaction between scatter and HOAs on CSF (F = 3.921, p = 0.017). To further explore the combined effect of scatter and HOAs on CSF, we assessed the relationships of with the sum of and . Figure 10 shows the as a function of the sum of and . The increased with the sum of two impact factors, following the relation: . This relationship indicated that it was not a simple summation effect on CS for HOAs and intraocular scatter, suggesting a compensatory mechanism between HOAs and intraocular scatter on CS.
Fig. 10.
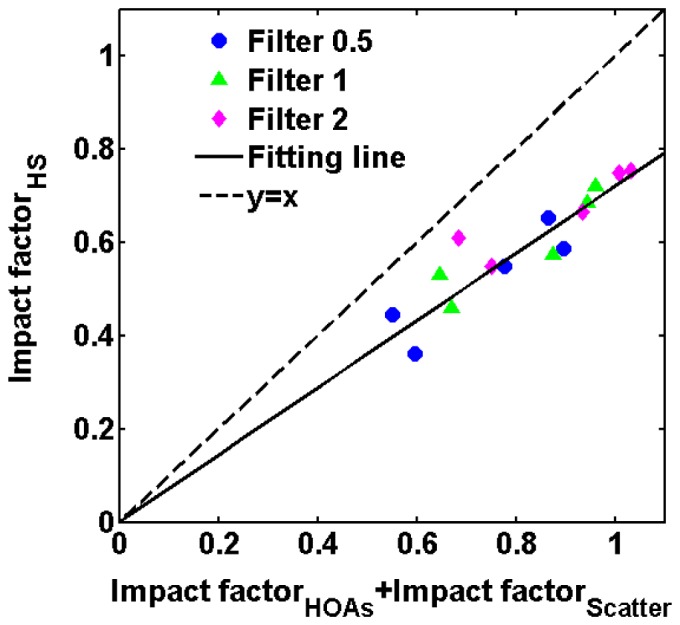
Linear fit of versus the sum of and . Each point represent an average value across subjects for a spatial frequency. Blue circles stand for results obtained with Filter S0.5; green triangles stand for results obtained with Filter S1; magenta diamonds stand for results obtained with Filter S2. The solid line represent the fitting line; the dotted line stand for the line y = x.
4. Discussion
We modified an adaptive optics double-pass PSF measurement to combine objective and subjective visual testing. Although a visual testing channel was added to the original configuration, the original DP channel was kept intact. The biggest advantage of this DP system is the combination of aberrations correction, scatter measurement and visual testing in a unique platform. The effect on visual performance of both intraocular scatter and HOAs can be assessed with this system. In clinical practice, they are carried out using different instruments and it is complex to establish reliable relationships between the intraocular scatter and HOAs on visual performance. The combination reported here overcomes these limitations and enables not only a direct comparison between the individual impact of HOAs and scatter on visual performance, but also a more in-depth analysis of their interaction.
Results showed that CS decreased with increasing scatter and AO correction of HOAs improved CS in all spatial frequencies for each subject, suggesting that both HOAs and intraocular scatter played an essential role in the CSF. To date, there have been several studies [8, 10] assessing the respective effect of HOAs and intraocular scatter on CSF, and similar results were reported. Kang et al. [8] measured the CS with and without HOAs correction, and their results showed that AO correction of HOAs improved CS for all spatial frequencies in all eyes. Bueno et al. [10] measured CS with different scatter levels induced by pre-defined filters, they found that CS decreased with increasing scatter for each spatial frequency.
This study concentrated on the combined effect of HOAs and intraocular scatter on the CS. The quantitative relation between the combined effect of the two factors and the sum of the effect introduced individually by the two factors was achieved. To the best of our knowledge, this is the first report on the quantitative estimation of the combined effect of HOAs and intraocular scatter on CS. The relation indicated that it was not a simple summation effect on CS for HOAs and intraocular scatter, suggesting a compensatory mechanism between HOAs and intraocular scatter on CS. A similar compensatory mechanism between spherical aberration and intraocular scatter on CS was found by Pe’rez et al. [5]. Previous studies have demonstrated an increase in optical aberrations of the eye with age [15–18], and a progressive increase of intraocular scatter with age has been clearly demonstrated by using both subjective [19] and objective techniques [20]. Since both of them increased with age, it could be argued that there was a balance between HOAs and intraocular scatter. Moreover, for the eyes with cataract, studies showed that both of HOAs and intraocular scatter were greater than those in eyes without cataract [21, 13].
There are some limitations in the current study and future work is needed for further verification. The intraocular scatter mentioned in this study refers to forward scatter only and the effect of the backward scatter on CS is not included. In addition, the accuracy of the quantitative relation between the combined impact factor and the sum of the two respective impact factors limited to the number of subject, scatter levels and the measured spatial frequencies.
5. Conclusion
In this article, we first modified an adaptive optics double-pass PSF measurement system to combine objective and subjective visual testing. Then we investigated the effect of HOAs and intraocular scatter on CS with the modified DP system. CS was measured with HOAs either retained or corrected, and with scatter either remaining at the natural eye-induced level or further enhanced by one of a set of three different scatter filters, and then compared across different optical conditions. Contrast sensitivity was found to be worse when HOAs were uncorrected or scatter was increased. Finally, three impact metrics were introduced and calculated for three different optical conditions, and their relations were analyzed. The results showed that the joint effect of HOAs and scatter on contrast sensitivity was not a simple summation of each contributing factor, suggesting a potential compensatory mechanism between HOAs and intraocular scatter on contrast sensitivity.
Acknowledgments
The authors would like to thank Professor Wenhan Jiang and Dr. Jian Kang for their critical comments and thoughtful suggestions. The authors would like to thank the Vision Research Lab of the University of Science and Technology of China for providing the Matlab routines for visual stimulus display. We also thank all subjects for their positive supports in this work.
Funding
National Natural Science Foundation of China (61378064); National High Technology Research and Development Program of China (2015AA020510).
References and links
- 1.Jindra L. F., Zemon V., “Contrast sensitivity testing: a more complete assessment of vision,” J. Cataract Refract. Surg. 15(2), 141–148 (1989). 10.1016/S0886-3350(89)80002-1 [DOI] [PubMed] [Google Scholar]
- 2.Adamsons I., Rubin G. S., Vitale S., Taylor H. R., Stark W. J., “The effect of early cataracts on glare and contrast sensitivity. A pilot study,” Arch. Ophthalmol. 110(8), 1081–1086 (1992). 10.1001/archopht.1992.01080200061025 [DOI] [PubMed] [Google Scholar]
- 3.Liang J., Williams D. R., Miller D. T., “Supernormal vision and high-resolution retinal imaging through adaptive optics,” J. Opt. Soc. Am. A 14(11), 2884–2892 (1997). 10.1364/JOSAA.14.002884 [DOI] [PubMed] [Google Scholar]
- 4.Fujikado T., Kuroda T., Maeda N., Ninomiya S., Goto H., Tano Y., Oshika T., Hirohara Y., Mihashi T., “Light scattering and optical aberrations as objective parameters to predict visual deterioration in eyes with cataracts,” J. Cataract Refract. Surg. 30(6), 1198–1208 (2004). 10.1016/j.jcrs.2003.12.023 [DOI] [PubMed] [Google Scholar]
- 5.Pérez G. M., Manzanera S., Artal P., “Impact of scattering and spherical aberration in contrast sensitivity,” J. Vis. 9(3), 19 (2009). 10.1167/9.3.19 [DOI] [PubMed] [Google Scholar]
- 6.Oshika T., Okamoto C., Samejima T., Tokunaga T., Miyata K., “Contrast sensitivity function and ocular higher-order wavefront aberrations in normal human eyes,” Ophthalmology 113(10), 1807–1812 (2006). 10.1016/j.ophtha.2006.03.061 [DOI] [PubMed] [Google Scholar]
- 7.Feizi S., Karimian F., “Effect of higher order aberrations on contrast sensitivity function in myopic eyes,” Jpn. J. Ophthalmol. 53(4), 414–419 (2009). 10.1007/s10384-009-0677-4 [DOI] [PubMed] [Google Scholar]
- 8.Kang J., Xiao F., Zhao J., Zhao H., Hu Y., Tang G., Dai Y., Zhang Y., “Effects of higher-order aberration correction on stereopsis at different viewing durations,” J. Biomed. Opt. 20(7), 075005 (2015). 10.1117/1.JBO.20.7.075005 [DOI] [PubMed] [Google Scholar]
- 9.Lee H., Lee K., Ahn J. M., Kim E. K., Sgrignoli B., Kim T. I., “Double-pass system assessing the optical quality of pseudophakic eyes,” Optom. Vis. Sci. 91(4), 437–443 (2014). 10.1097/OPX.0000000000000190 [DOI] [PubMed] [Google Scholar]
- 10.Bueno J. M., Pérez G., Benito A., Artal P., “Impact of scatter on double-pass image quality and contrast sensitivity measured with a single instrument,” Biomed. Opt. Express 6(12), 4841–4849 (2015). 10.1364/BOE.6.004841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamiya K., Shimizu K., Iijima A., Kobashi H., “Factors Influencing Contrast Sensitivity Function in Myopic Eyes,” PLoS One 9(11), e113562 (2014). 10.1371/journal.pone.0113562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J., Xiao F., Kang J., Zhao H., Dai Y., Zhang Y., “Quantifying intraocular scatter with near diffraction-limited double-pass point spread function,” Biomed. Opt. Express 7(11), 4595–4604 (2016). 10.1364/BOE.7.004595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artal P., Benito A., Pérez G. M., Alcón E., De Casas A., Pujol J., Marín J. M., “An objective scatter index based on double-pass retinal images of a point source to classify cataracts,” PLoS One 6(2), e16823 (2011). 10.1371/journal.pone.0016823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y., Huang C., Xu P., Tao L., Qiu Z., Li X., Lu Z.-L., “Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia,” Vision Res. 46(5), 739–750 (2006). 10.1016/j.visres.2005.07.031 [DOI] [PubMed] [Google Scholar]
- 15.Calver R. I., Cox M. J., Elliott D. B., “Effect of aging on the monochromatic aberrations of the human eye,” J. Opt. Soc. Am. A 16(9), 2069–2078 (1999). 10.1364/JOSAA.16.002069 [DOI] [PubMed] [Google Scholar]
- 16.McLellan J. S., Marcos S., Burns S. A., “Age-related changes in monochromatic wave aberrations of the human eye,” Invest. Ophthalmol. Vis. Sci. 42(6), 1390–1395 (2001). [PubMed] [Google Scholar]
- 17.Brunette I., Bueno J. M., Parent M., Hamam H., Simonet P., “Monochromatic aberrations as a function of age, from childhood to advanced age,” Invest. Ophthalmol. Vis. Sci. 44(12), 5438–5446 (2003). 10.1167/iovs.02-1042 [DOI] [PubMed] [Google Scholar]
- 18.Artal P., Berrio E., Guirao A., Piers P., “Contribution of the cornea and internal surfaces to the change of ocular aberrations with age,” J. Opt. Soc. Am. A 19(1), 137–143 (2002). 10.1364/JOSAA.19.000137 [DOI] [PubMed] [Google Scholar]
- 19.IJspeert J. K., de Waard P. W. T., van den Berg T. J. T. P., de Jong P. T. V. M., “The intraocular straylight function in 129 healthy volunteers: dependence on angle, age and pigmentation,” Vision Res. 30(5), 699–707 (1990). 10.1016/0042-6989(90)90096-4 [DOI] [PubMed] [Google Scholar]
- 20.Westheimer G., Liang J., “Evaluating diffusion of light in the eye by objective means,” Invest. Ophthalmol. Vis. Sci. 35(5), 2652–2657 (1994). [PubMed] [Google Scholar]
- 21.Kuroda T., Fujikado T., Maeda N., Oshika T., Hirohara Y., Mihashi T., “Wavefront analysis in eyes with nuclear or cortical cataract,” Am. J. Ophthalmol. 134(1), 1–9 (2002). 10.1016/S0002-9394(02)01402-2 [DOI] [PubMed] [Google Scholar]