Abstract
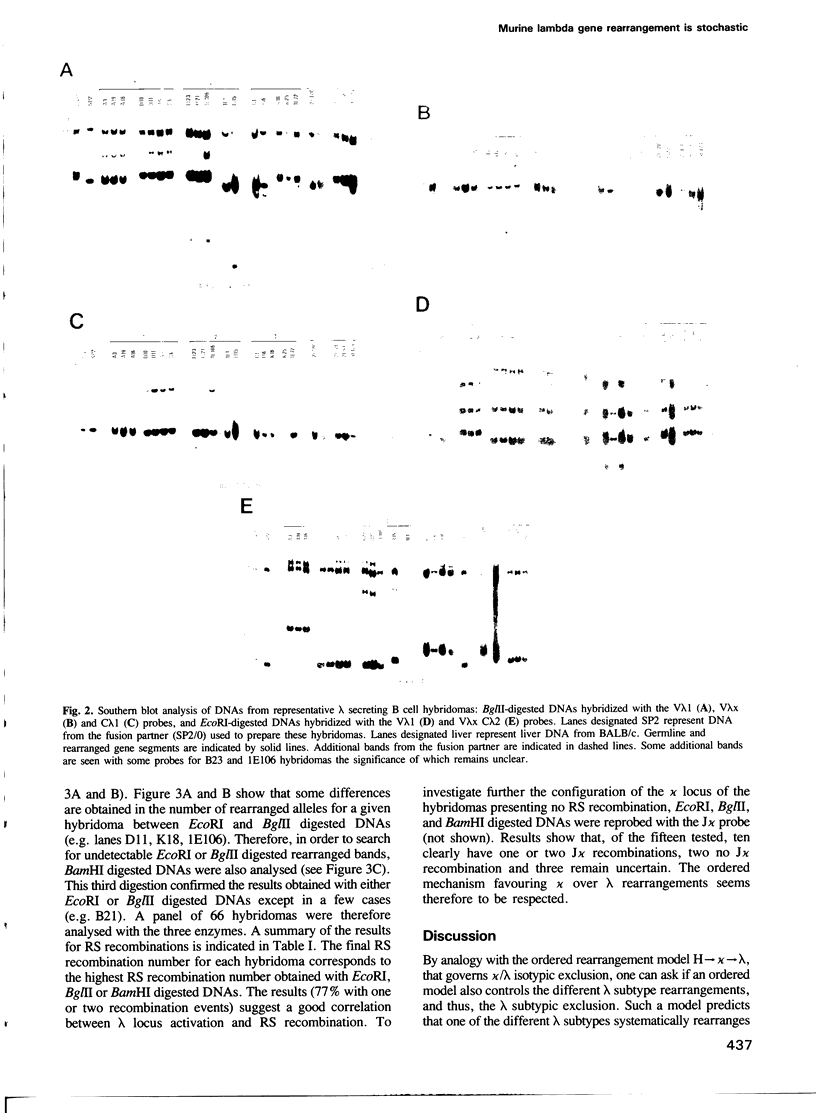
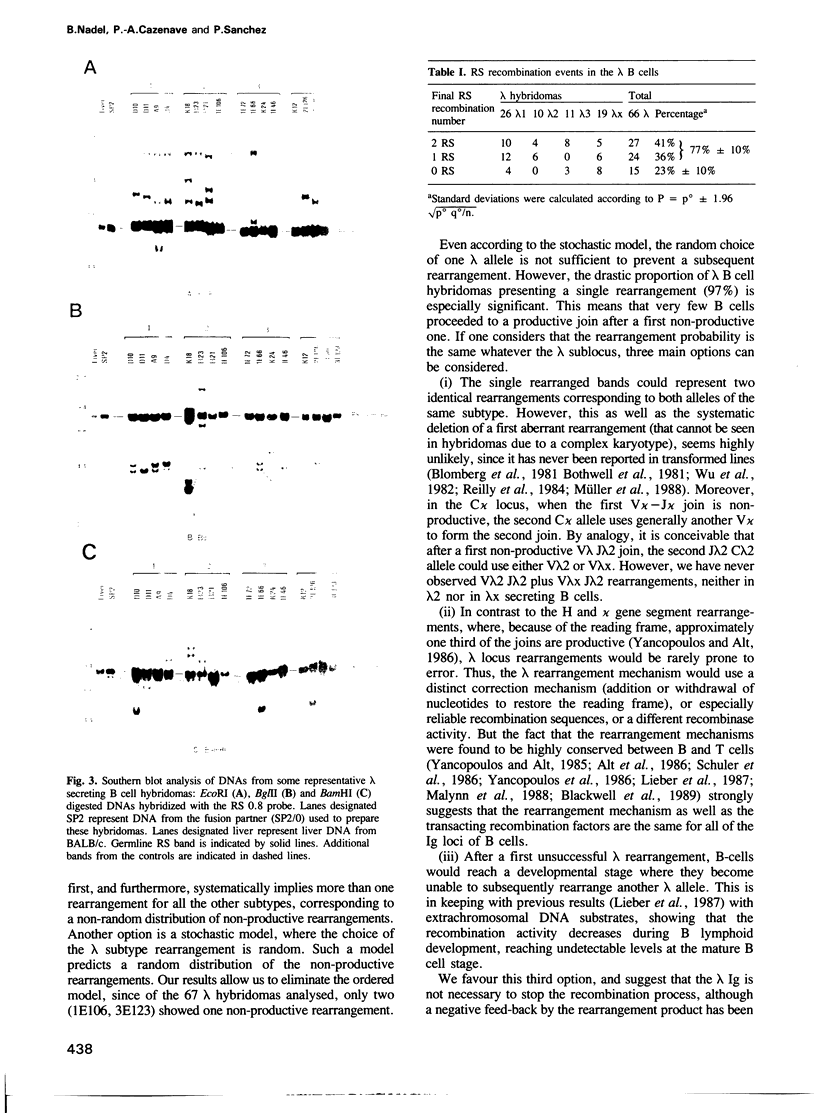
The ontogeny of the immunoglobulin (Ig) gene rearrangement in mammalian B cells seems to be ordered. Heavy chain gene segments rearrange first, followed by light chain gene segments, kappa before lambda. The genomic organization of murine lambda locus does not preclude the simultaneous expression of two subtypes from the same chromosome. In order to distinguish between an ordered and a stochastic model of rearrangement, a panel of 67 B cell hybridomas secreting either lambda 1, lambda 2, lambda 3 or lambda x (recently described) were analysed for V lambda J lambda rearrangements. The results show that in 97% of cases, a single rearrangement occurred, favouring the stochastic model over the ordered one. Strikingly, the possibility of having a productive rearrangement if the first try results in an aberrant one is rare. We propose therefore, that the lambda Ig is not necessarily required to ensure allelic and subtypic exclusion mechanisms. Moreover, in 97% of the cases, at least one kappa allele is rearranged. Furthermore, the RS recombination has been detected in 77% of the cases. This suggests that, although the stimulation of kappa precedes that of lambda locus, the RS recombination acts as a transacting albeit dispensable lambda activator.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp B., McMullen M. D., Storb U. Sequences of immunoglobulin lambda 1 genes in a lambda 1 defective mouse strain. Nature. 1982 Jul 8;298(5870):184–187. doi: 10.1038/298184a0. [DOI] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Malynn B. A., Pollock R. R., Ferrier P., Covey L. R., Fulop G. M., Phillips R. A., Yancopoulos G. D., Alt F. W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989 Mar;8(3):735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Tonegawa S. DNA sequences of the joining regions of mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):530–533. doi: 10.1073/pnas.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Traunecker A., Eisen H., Tonegawa S. Organization of four mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3765–3769. doi: 10.1073/pnas.78.6.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Carson S., Wu G. E. A linkage map of the mouse immunoglobulin lambda light chain locus. Immunogenetics. 1989;29(3):173–179. doi: 10.1007/BF00373642. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Colberg J. E., Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C. Chance, necessity and antibody gene dynamics. Nature. 1983 May 5;303(5912):23–26. doi: 10.1038/303023a0. [DOI] [PubMed] [Google Scholar]
- Durdik J., Moore M. W., Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984 Feb 23;307(5953):749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- Elliott B. W., Jr, Eisen H. N., Steiner L. A. Unusual association of V, J and C regions in a mouse immunoglobulin lambda chain. Nature. 1982 Oct 7;299(5883):559–561. doi: 10.1038/299559a0. [DOI] [PubMed] [Google Scholar]
- Hagman J., Lo D., Doglio L. T., Hackett J., Jr, Rudin C. M., Haasch D., Brinster R., Storb U. Inhibition of immunoglobulin gene rearrangement by the expression of a lambda 2 transgene. J Exp Med. 1989 Jun 1;169(6):1911–1929. doi: 10.1084/jem.169.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Lamers M., Köhler G. Expression of immunoglobulin delta chain causes allelic exclusion in transgenic mice. Nature. 1987 Dec 3;330(6147):482–484. doi: 10.1038/330482a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane mu, but not by secreted mu heavy chains. J Exp Med. 1988 Oct 1;168(4):1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Barth C. F., Carlson L. M., Petryniak B., Humphries E. H., Thompson C. B. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Genes Dev. 1989 Jun;3(6):838–847. doi: 10.1101/gad.3.6.838. [DOI] [PubMed] [Google Scholar]
- Müller B., Reth M. Ordered activation of the Ig lambda locus in Abelson B cell lines. J Exp Med. 1988 Dec 1;168(6):2131–2137. doi: 10.1084/jem.168.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Caskey H. M., Pettersson S., Williams G. T., Surani M. A. Isotype exclusion and transgene down-regulation in immunoglobulin-lambda transgenic mice. Nature. 1989 Mar 23;338(6213):350–352. doi: 10.1038/338350a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persiani D. M., Durdik J., Selsing E. Active lambda and kappa antibody gene rearrangement in Abelson murine leukemia virus-transformed pre-B cell lines. J Exp Med. 1987 Jun 1;165(6):1655–1674. doi: 10.1084/jem.165.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E. B., Blomberg B., Imanishi-Kari T., Tonegawa S., Eisen H. N. Restricted association of V and J-C gene segments for mouse lambda chains. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2484–2488. doi: 10.1073/pnas.81.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Reth M., Petrac E., Wiese P., Lobel L., Alt F. W. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987 Nov;6(11):3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Sanchez P., Cazenave P. A. A new variable region in mouse immunoglobulin lambda light chains. J Exp Med. 1987 Jul 1;166(1):265–270. doi: 10.1084/jem.166.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P., Marche P. N., Le Guern C., Cazenave P. A. Structure of a third murine immunoglobulin lambda light chain variable region that is expressed in laboratory mice. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9185–9188. doi: 10.1073/pnas.84.24.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Selsing E., Miller J., Wilson R., Storb U. Evolution of mouse immunoglobulin lambda genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4681–4685. doi: 10.1073/pnas.79.15.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblott M. J., Litman G. W. Complete nucleotide sequence of primitive vertebrate immunoglobulin light chain genes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4684–4688. doi: 10.1073/pnas.86.12.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch K. A., Bakhshi A., Goldman P., Korsmeyer S. J. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 1985 Jul 18;316(6025):260–262. doi: 10.1038/316260a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storb U., Haasch D., Arp B., Sanchez P., Cazenave P. A., Miller J. Physical linkage of mouse lambda genes by pulsed-field gel electrophoresis suggests that the rearrangement process favors proximate target sequences. Mol Cell Biol. 1989 Feb;9(2):711–718. doi: 10.1128/mcb.9.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Neiman P. E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987 Feb 13;48(3):369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Weiss S., Meyer J., Wabl M. R. V lambda 2 rearranges with all functional J lambda segments in the mouse. Eur J Immunol. 1985 Aug;15(8):765–768. doi: 10.1002/eji.1830150805. [DOI] [PubMed] [Google Scholar]
- Wu G. E., Govindji N., Hozumi N., Murialdo H. Nucleotide sequence of a chromosomal rearranged lambda 2 immunoglobulin gene of mouse. Nucleic Acids Res. 1982 Jul 10;10(13):3831–3843. doi: 10.1093/nar/10.13.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]




