Abstract
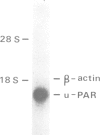
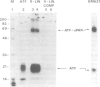
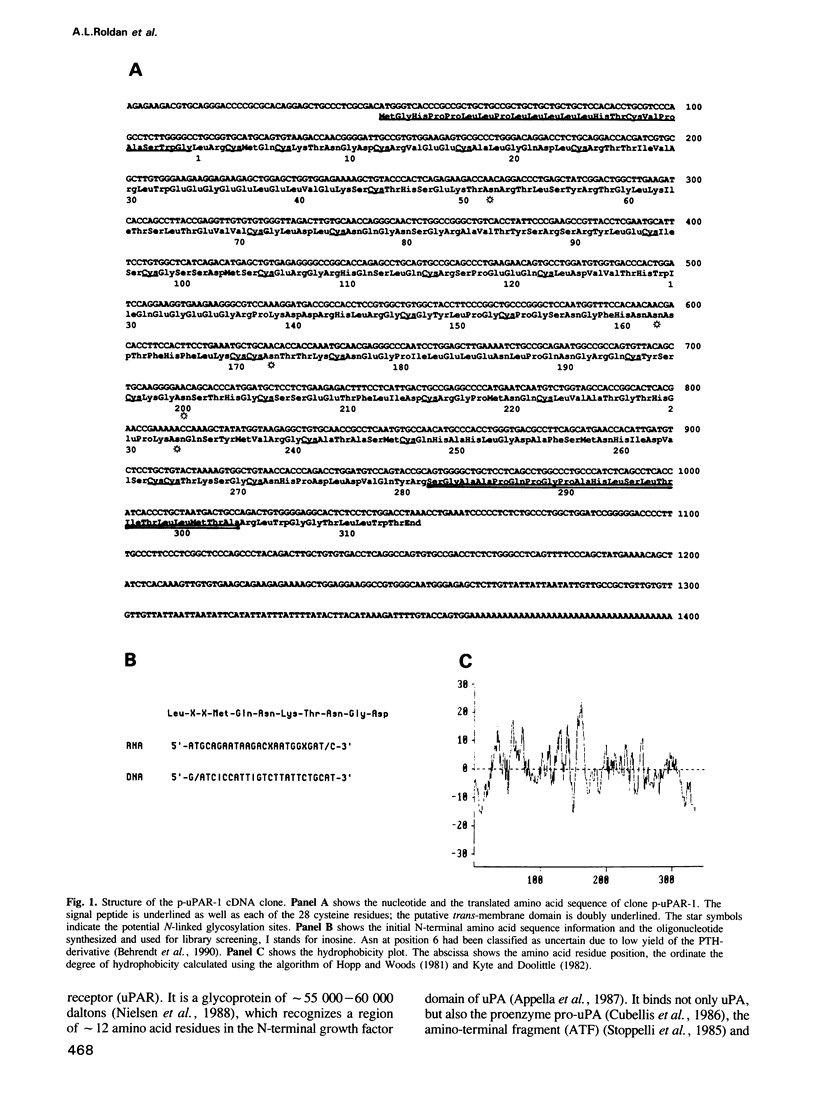
The surface receptor for urokinase plasminogen activator (uPAR) has been recognized in recent years as a key molecule in regulating plasminogen mediated extracellular proteolysis. Surface plasminogen activation controls the connections between cells, basement membrane and extracellular matrix, and therefore the capacity of cells to migrate and invade neighboring tissues. We have isolated a 1.4 kb cDNA clone coding for the entire human uPAR. An oligonucleotide synthesized on the basis of the N-terminal sequence of the purified protein was used to screen a cDNA library made from SV40 transformed human fibroblasts [Okayama and Berg (1983) Mol. Cell Biol., 3, 280-289]. The cDNA encodes a protein of 313 amino acids, preceded by a 21 residue signal peptide. A hydrophobicity plot suggests the presence of a membrane spanning domain close to the C-terminus. The cDNA hybridizes to a 1.4 kb mRNA from human cells, a size very close to that of the cloned cDNA. Expression of the uPAR cDNA in mouse cells confirms that the clone is complete and expresses a functional uPA binding protein, located on the cell surface and with properties similar to the human uPAR. Caseinolytic plaque assay, immunofluorescence analysis, direct binding studies and cross-linking experiments show that the transfected mouse LB6 cells specifically bind human uPA, which in turn activates plasminogen. The Mr of the mature human receptor expressed in mouse cells is approximately 55,000, in accordance with the naturally occurring, highly glycosylated human uPAR. The Mr calculated on the basis of the cDNA sequence, approximately 35,000, agrees well with that of the deglycosylated receptor.
Full text
PDF

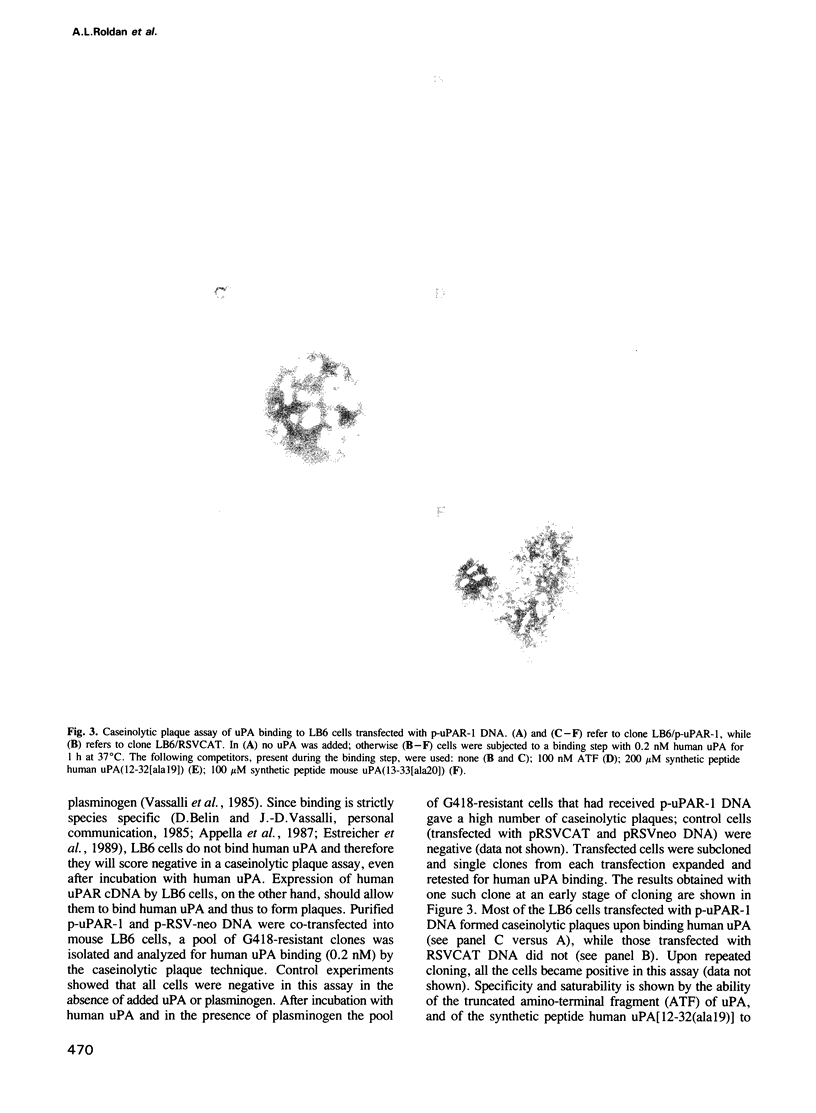
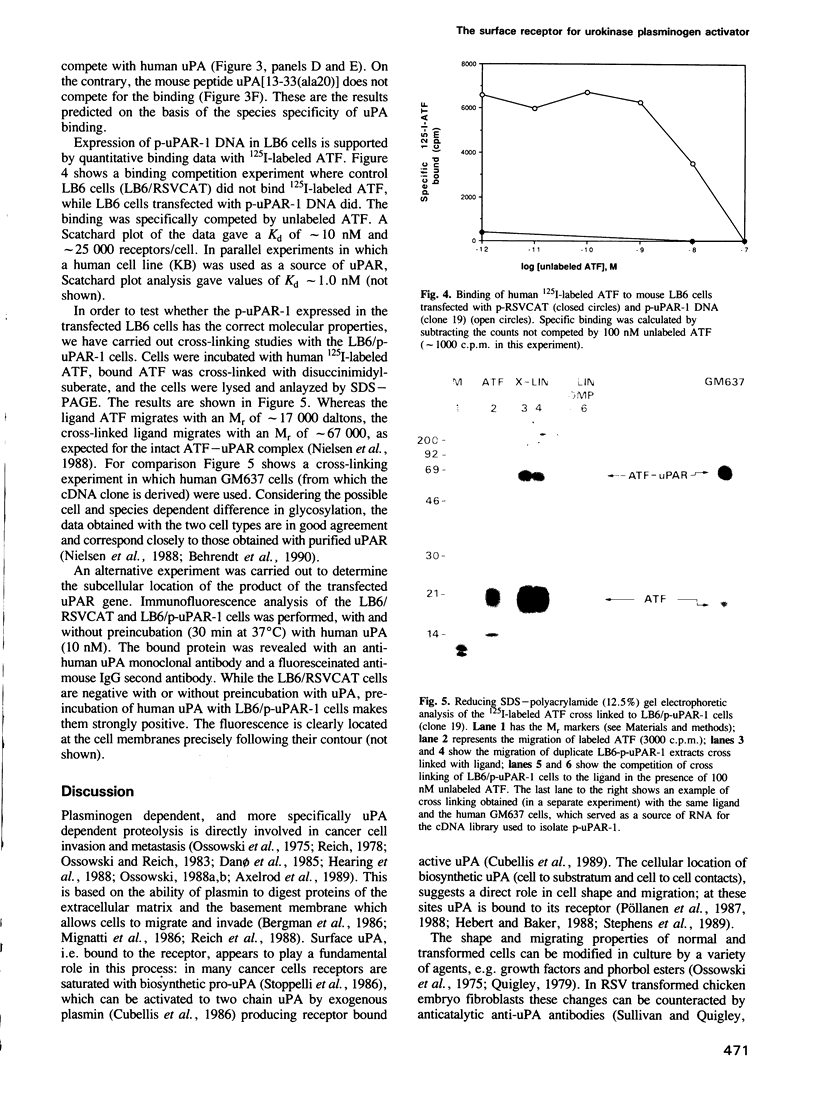



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Appella E., Weber I. T., Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988 Apr 11;231(1):1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- Axelrod J. H., Reich R., Miskin R. Expression of human recombinant plasminogen activators enhances invasion and experimental metastasis of H-ras-transformed NIH 3T3 cells. Mol Cell Biol. 1989 May;9(5):2133–2141. doi: 10.1128/mcb.9.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai A., Baker J. B. Urokinase binding sites on human foreskin cells. Evidence for occupancy with endogenous urokinase. Biochem Biophys Res Commun. 1985 Dec 31;133(3):994–1000. doi: 10.1016/0006-291x(85)91234-3. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B. L., Scott R. W., Bajpai A., Watts S., Baker J. B. Inhibition of tumor-cell-mediated extracellular matrix destruction by a fibroblast proteinase inhibitor, protease nexin I. Proc Natl Acad Sci U S A. 1986 Feb;83(4):996–1000. doi: 10.1073/pnas.83.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Andreasen P., Ragno P., Mayer M., Danø K., Blasi F. Accessibility of receptor-bound urokinase to type-1 plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4828–4832. doi: 10.1073/pnas.86.13.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubellis M. V., Nolli M. L., Cassani G., Blasi F. Binding of single-chain prourokinase to the urokinase receptor of human U937 cells. J Biol Chem. 1986 Dec 5;261(34):15819–15822. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem. 1989 Feb 5;264(4):2185–2188. [PubMed] [Google Scholar]
- Estreicher A., Wohlwend A., Belin D., Schleuning W. D., Vassalli J. D. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J Biol Chem. 1989 Jan 15;264(2):1180–1189. [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert C. A., Baker J. B. Linkage of extracellular plasminogen activator to the fibroblast cytoskeleton: colocalization of cell surface urokinase with vinculin. J Cell Biol. 1988 Apr;106(4):1241–1247. doi: 10.1083/jcb.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund L. R., Riccio A., Andreasen P. A., Nielsen L. S., Kristensen P., Laiho M., Saksela O., Blasi F., Danø K. Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987 May;6(5):1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Richards R. I., Crawford R. J., Hamilton J. A. Suppression of urokinase-type plasminogen activator mRNA levels in human fibrosarcoma cells and synovial fibroblasts by anti-inflammatory glucocorticoids. EMBO J. 1986 Sep;5(9):2217–2222. doi: 10.1002/j.1460-2075.1986.tb04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Fakhrai H., Edgington T. S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987 Jul 3;50(1):129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Andreasen P. A., Grøndahl-Hansen J., Huang J. Y., Kristensen P., Danø K. Monoclonal antibodies to human 54,000 molecular weight plasminogen activator inhibitor from fibrosarcoma cells--inhibitor neutralization and one-step affinity purification. Thromb Haemost. 1986 Apr 30;55(2):206–212. [PubMed] [Google Scholar]
- Nielsen L. S., Kellerman G. M., Behrendt N., Picone R., Danø K., Blasi F. A 55,000-60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J Biol Chem. 1988 Feb 15;263(5):2358–2363. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L. In vivo invasion of modified chorioallantoic membrane by tumor cells: the role of cell surface-bound urokinase. J Cell Biol. 1988 Dec;107(6 Pt 1):2437–2445. doi: 10.1083/jcb.107.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L. Plasminogen activator dependent pathways in the dissemination of human tumor cells in the chick embryo. Cell. 1988 Feb 12;52(3):321–328. doi: 10.1016/s0092-8674(88)80025-4. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Picone R., Kajtaniak E. L., Nielsen L. S., Behrendt N., Mastronicola M. R., Cubellis M. V., Stoppelli M. P., Pedersen S., Danø K., Blasi F. Regulation of urokinase receptors in monocytelike U937 cells by phorbol ester phorbol myristate acetate. J Cell Biol. 1989 Feb;108(2):693–702. doi: 10.1083/jcb.108.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzatti R., Muschel R., Williams J., Padmanabhan R., Howard B., Liotta L., Khoury G. Primary rat embryo cells transformed by one or two oncogenes show different metastatic potentials. Science. 1986 Apr 11;232(4747):223–227. doi: 10.1126/science.3456644. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R. K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Tacchetti C., Cubellis M. V., Corti A., Hearing V. J., Cassani G., Appella E., Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986 Jun 6;45(5):675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: induction by concanavalin A and phorbol myristate acetate. Cell. 1977 Jul;11(3):695–705. doi: 10.1016/0092-8674(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]