Abstract
Purpose
To prospectively evaluate cardiotoxicity risk with sunitinib in metastatic renal cell carcinoma (mRCC) routine clinical practice using comprehensive echocardiography and biomarker phenotyping.
Patients and Methods
In a multi-center prospective study of 90 mRCC patients, echocardiography and biomarkers of cardiovascular injury and stress were quantified at baseline, 3.5, 15, and 33 weeks following sunitinib initiation. These “on-drug” visits corresponded to cycles 1, 3, and 6, respectively. LV dysfunction was defined as an absolute decline in LV ejection fraction (LVEF) by ≥10% to a value of <50%. Conditional survival analyses predicted the risk of LV dysfunction. Linear mixed effects models estimated changes in LVEF, high-sensitivity Troponin I (hsTnI), and B-type natriuretic peptide (BNP) over time.
Results
The predicted risk of LV dysfunction by cycle 6 was 9.7% (95% CI 3,17%). The majority of events occurred in the first treatment cycle. This risk diminished to 5% and 2% in patients who had not experienced dysfunction by the completion of cycles 1 and 3, respectively. All evaluable patients who experienced LV dysfunction had subsequent improvement in LVEF with careful management. Six patients (6.7%) developed hsTnI elevations >21.5 pg/ml, and eleven additional patients (12.2%) developed BNP elevations >100 pg/ml. These elevations similarly tended to occur early and resolved over time.
Conclusion
On average, mRCC patients receiving sunitinib exhibit modest declines in LVEF and nonsignificant changes in hsTnI and BNP. However, approximately 9.7% to 18.9% of patients develop more substantive abnormalities. These changes occur early and are largely recoverable with careful management.
Keywords: cardiotoxicity, vascular endothelial growth factor receptor, tyrosine kinase inhibitor, renal cell carcinoma, echocardiography, biomarkers
1. Introduction
Numerous therapies targeting the vascular endothelial growth factor (VEGF) molecular pathway have been approved for the treatment of metastatic renal cell carcinoma (mRCC).(1–5) The availability of such therapies has resulted in a doubling of the median overall survival to approximately two years, and VEGF-directed therapies remain the current standard of care for frontline mRCC management. Sunitinib is a multi-targeted VEGF receptor tyrosine kinase inhibitor (TKI) and is a standard first-line treatment option for mRCC.(6) Indeed, a recent review of treatment practices at U.S. community oncology practices indicated that sunitinib was the preferred initial therapeutic option for mRCC management.(7)
Although sunitinib and other VEGF-directed therapies have significantly improved clinical outcomes for mRCC patients, they have also been associated with several cardiovascular toxicities, including hypertension, left ventricular (LV) dysfunction, and heart failure.(8–10) While the exact mechanisms for cardiotoxicity remain unclear, VEGF signaling is known to play an important role in maintaining cardiac function and homeostasis in both ischemic and non-ischemic cardiomyopathy.(11–13) In addition, as a multi-targeted kinase inhibitor, sunitinib has effects on the AMP-activated protein kinase (AMPK) and platelet derived growth factor receptor (PDGFR), which are critical for cardiomyocyte function and survival.(11, 12) Finally, an increase in systemic arterial load may result from a reduction in vasodilatory nitric oxide production and from vascular rarefaction, and may further adversely affect LV systolic function.(14, 15)
However, our understanding of sunitinib-related cardiotoxicity and its clinical significance in mRCC patients remains limited and is largely defined by retrospective analyses in clinical trial populations. In a meta-analysis of mostly phase II and III clinical trials in a variety of advanced solid tumors, the overall incidence of heart failure in sunitinib-treated patients was estimated to be 4.1%.(16) Several retrospective studies performed in a variety of treatment settings have reported that the incidence of sunitinib-induced cardiotoxicity specifically in mRCC patients ranges from approximately 3% to 30%.(17–20) The clinical interpretation of these varied findings has been further limited by the use of non-standardized cardiac monitoring protocols, varying definitions of cardiotoxicity, including composites of LV ejection fraction (LVEF) decline and non-specific heart failure symptoms, and a lack of quantitative or core laboratory assessment of these measures. As a result, there is little consensus regarding recommended cardiac toxicity monitoring strategies in the setting of mRCC and sunitinib therapy.(6) This has the potential to be of significant impact given the prospect for expanding indications for sunitinib use, particularly in the adjuvant treatment setting.
Given the therapeutic importance of VEGF receptor TKIs, it is critical to better understand the treatment-related cardiovascular risk in the general mRCC patient population in order to guide effective cardioprotective monitoring. As such, we performed a multicenter prospective cohort study to precisely define the longitudinal changes in cardiac function that occur in a real-world cohort of mRCC patients newly initiated on sunitinib therapy. In particular, we aimed to define the risk of sunitinib-induced subclinical cardiac injury through detailed quantitative assessment of both cardiac function by echocardiography, and myocardial injury and stress by cardiac biomarkers, including high-sensitivity Troponin I (hsTnI) and B-type natriuretic peptide (BNP).
2. Patients and Methods
2.1 Study Design
This was a multicenter prospective cohort study performed at five academic medical centers, including the University of Pennsylvania, the Vanderbilt University Medical Center, the University of Wisconsin, University Hospitals Case Medical Center, and the University of Utah. Eligible participants were accrued between December 2011 and December 2015 and included patients with mRCC who were planned to initiate sunitinib therapy. Sunitinib starting dose, schedule, and dose adjustments were determined at the discretion of the treating medical oncologist. All participants provided written informed consent, and the study protocol was approved by the Institutional Review Board at each individual participating site.
Prior to the initiation of sunitinib, enrolled participants underwent a detailed review of their medical history, including prior cardiac events, cardiovascular risk factors, and current medications, with all findings verified by the oncology provider. Cardiac symptoms were assessed using the MD Anderson Symptom Inventory – Heart Failure (MDASI-HF) survey, which records heart failure symptoms on a scale of 0–10.(21) In addition, all participants underwent a baseline transthoracic echocardiogram, blood pressure assessment, and blood sample collection. Study follow-up was designed to detect both the peak and late incidences of LV dysfunction as a result of sunitinib exposure.(18) As such, follow-up echocardiograms, plasma biomarkers, and clinical assessments (including cardiac history, symptom assessment, blood pressure measurement, and cardiac medication use) were performed during follow-up visits at 3.5 weeks (+/− 1 week), 15 weeks (+/− 2 weeks), and 33 weeks (+/− 2 weeks) after the initiation of sunitinib (Figure 1). These follow-up visits were timed to coincide with sunitinib exposure during cycles 1, 3 and 6 of therapy, respectively, and were scheduled at a time when the participant would be actively taking sunitinib (i.e. ‘on drug’). The decision to initiate anti-hypertensive or other cardiovascular medications was at the discretion of the treating medical provider. In the event of sunitinib discontinuation due to disease progression or intolerable toxicity, follow-up cardiac assessment was obtained per protocol.
Figure 1. Study Schema.
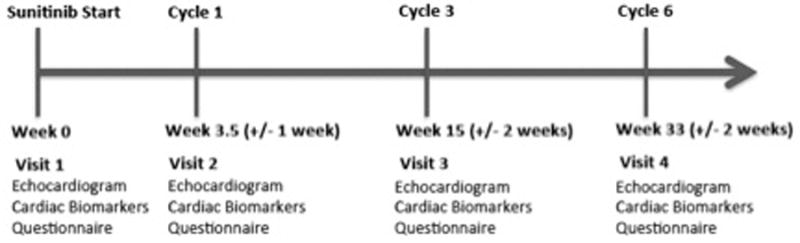
Protocol-specified cardiac assessments timed to coincide with sunitinib exposure during cycles 1, 3 and 6 of therapy.
2.2 Echocardiography Quantitation and Definition of LV Dysfunction
Transthoracic echocardiograms were performed at the participating sites according to a standardized protocol. Two-dimensional images were acquired using Philips IE33 machines (Andover, MA). Echocardiography quantitation was independently performed in the imaging core laboratory at the Hospital of the University of Pennsylvania by trained sonographers blinded to all patient characteristics. Quantitation of end-systolic and end-diastolic LV volumes was performed using TomTec Image Arena (TomTec Imaging Systems, Unterschleissheim, Germany). LVEF was derived from the stroke volume (defined as the difference between end-diastolic and end-systolic volumes), divided by the end-diastolic volume.(22) LV dysfunction was defined as an absolute decline in the LVEF by ≥ 10% to a resultant value of < 50%.(23–26)
2.3 Biomarker Analyses
Plasma samples were collected in EDTA tubes, processed at 3353 RPM for 20 minutes at room temperature, aliquoted, and stored at −80°C until the time of assay. High sensitivity TnI and BNP were quantitatively measured using the Singulex Single Molecule Counting laboratory assay (Alameda, CA).(27) Elevations in hsTnI > 99th percentile for a population with, or at risk for, comorbid cardiovascular disease (> 21.5 pg/ml), or elevations in BNP to > 100 pg/ml were considered abnormal.(27) High sensitivity TnI or BNP was missing for 13 of the 281 (4.6%) protocol-defined biomarker assessments.
2.4 Statistical Analysis
Participants were eligible for analysis if they received sunitinib therapy and underwent at least a baseline echocardiogram and clinical assessment. Descriptive statistics for key demographic and clinical variables were performed. The Kaplan-Meier method was used to analyze the time-to-event endpoints of mRCC-specific survival, time to discontinuation of sunitinib, and time to incident LV dysfunction. Participants who did not experience an event of interest were censored at the date of last follow-up. Individual linear mixed effects models with a random intercept to account for intra-participant correlation of repeated measures were used to estimate LVEF, hsTnI, or BNP changes over time. Linear regression models adjusted for baseline LVEF were also used to estimate the associations between individual baseline factors or biomarkers (hsTnI or BNP) and changes in LVEF. Baseline clinical characteristics selected a priori for univariable analyses included age, sex, body mass index (BMI), cardiac medication use, sunitinib starting dose, systolic blood pressure, pulse pressure, and history of hypertension or hyperlipidemia. Similarly, univariate linear models were used to evaluate the association between changes in cardiac symptoms and changes in LVEF. Specific cardiac symptoms included the change in dyspnea severity and change in the MDASI-HF score (average response for 8 heart failure symptoms: severity of ankle edema, abdominal bloating, sudden weight gain, lack of energy, orthopnea, paroxysmal nocturnal dyspnea, nocturnal cough, and palpitations).(21) Finally, in order to determine the predicted risk of LV dysfunction over time, unadjusted conditional survival analyses were used to estimate the risk of LV dysfunction prior to 33 weeks of sunitinib therapy (cycle 6). Here, the predicted risk was derived conditional on ‘surviving’ without LV dysfunction at either 6 weeks or 18 weeks of sunitinib therapy (completion of cycles 1 or 3, respectively). Hypothesis tests were two-sided with a type I error rate of 0.05. All analyses were performed with R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1 Study Population
A total of 90 participants were eligible for analysis and contributed 281 echocardiograms and 272 biomarker measures over the course of study follow-up. Participant demographic and clinical characteristics are summarized in Table 1. The median age was 63 years (IQR 55, 68). The majority of participants had clear cell mRCC with a history of prior nephrectomy (78%) and received initial systemic therapy with sunitinib (87% with no prior systemic RCC therapy). Cardiovascular risk factors were highly prevalent at baseline, including hypertension (54%), coronary artery disease (9%), and current/former tobacco use (57%). The median baseline LVEF was 49.8% (IQR 44.6, 54.1), and the median baseline values for hsTnI and BNP were 1.7 pg/ml (IQR 1.0 – 3.6) and 32.8 pg/ml (IQR 16.7, 65.4), respectively.
Table 1.
Baseline Patient Characteristics (N = 90)
| Variable | N (%) |
|---|---|
| Age (years) | |
| Median (IQR) | 63 (55, 68) |
| Male Sex | 59 (66) |
| Tumor Histology | |
| Clear Cell | 74 (82) |
| Papillary | 7 (8) |
| Chromophobe | 2 (2) |
| Other | 7 (8) |
| Site of Metastases | |
| Lung | 28 (31) |
| Liver | 7 (8) |
| Bone | 10 (11) |
| Brain | 1 (1) |
| Other | 14 (16) |
| Unknown | 30 (33) |
| Prior Nephrectomy | 70 (78) |
| Prior Systemic RCC Therapy | |
| None | 78 (87) |
| IL-2 | 7 (8) |
| Other Targeted Agent | 5 (6) |
| Sunitinib Starting Dose | |
| 50 mg | 55 (61) |
| 37.5 mg | 4 (4) |
| 25 mg | 6 (7) |
| Other (Escalating Dose) | 25 (28) |
| Baseline LVEF (%) | |
| Median (IQR) | 49.8 (44.6, 54.1) |
| Baseline Cardiac Troponin I (pg/ml) | |
| Median (IQR) | 1.7 (1.0 – 3.6) |
| Baseline BNP (pg/ml) | |
| Median (IQR) | 32.8 (16.7 – 65.4) |
| Baseline SBP (mmHg) | |
| Median (IQR) | 135 (123, 147) |
| Baseline Pulse Pressure (mmHg) | |
| Median (IQR) | 58 (47, 70) |
| Baseline Dyspnea Severitya | 0 (0, 2) |
| Baseline MDASI-HF Scorea | 0.6 (0.2, 1.5) |
|
Cardiovascular Co-morbidities/Risk Factors |
|
| Hypertension | 49 (54) |
| Coronary Disease | 8 (9) |
| Heart Failure | 4 (4) |
| BMI (median, IQR) | 27.0 (23.7, 32.9) |
| Hyperlipidemia | 47 (52) |
| Diabetes Mellitus | 22 (24) |
| Tobacco Use | 51 (57) |
| Cardiac Medication Use | |
| Aspirin | 23 (26) |
| ACEi or ARB | 24 (27) |
| Beta Blocker | 20 (22) |
| Calcium Channel Blocker | 19 (21) |
| Statin | 28 (31) |
Abbreviations: RCC, renal cell carcinoma; IQR, interquartile range; IL-2, interleukin-2; LVEF, left ventricular ejection fraction; BNP, B-type natriuretic peptide; SBP, systolic blood pressure; BMI, body mass index; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; Statin, HMG CoA reductase inhibitor
Symptoms assessed by the MD Anderson Symptom Inventory – Heart Failure and scored on a scale of 0–10. Heart Failure Symptom Score is derived from the average response for 8 heart failure symptoms, including severity of ankle edema, abdominal bloating, sudden weight gain, lack of energy, orthopnea, paroxysmal nocturnal dyspnea, nocturnal cough, and palpitations.
The median study follow-up time was 30.9 weeks (IQR 6.3, 35.0 weeks). Among the five study sites, 49 participants (54%) completed the full protocol-specified follow-up including echocardiograms and cardiac assessments through approximately 33 weeks of study follow-up time. Twenty-one participants (23%) withdrew from the study prior to 33 weeks, and 3 others (3%) were lost to routine oncologic follow-up. Reasons for early participant withdrawal included disease progression (N=16) and patient preference (N=5). Seventeen participants (19%) died from mRCC during the course of study follow-up, and there were no deaths from other causes (Figure 2A). Eighty-three participants (92%) and 62 participants (69%) completed follow-up through 3.5 weeks (cycle 1) and 15 weeks (cycle 3), respectively. Participants completing study follow-up had similar baseline characteristics as those participants who did not (Supplemental Table S1). At the end of protocol follow-up (33 weeks, cycle 6), the majority (87%) of evaluable participants active in the study remained on sunitinib therapy. The Kaplan-Meier analysis for time to discontinuation of sunitinib is displayed in Figure 2B.
Figure 2.
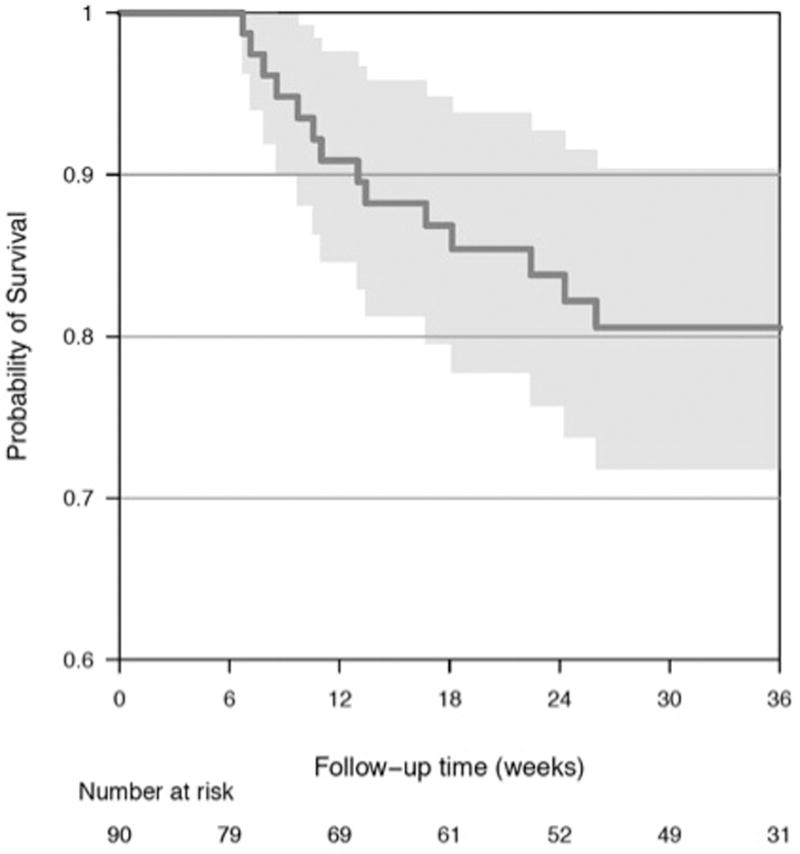
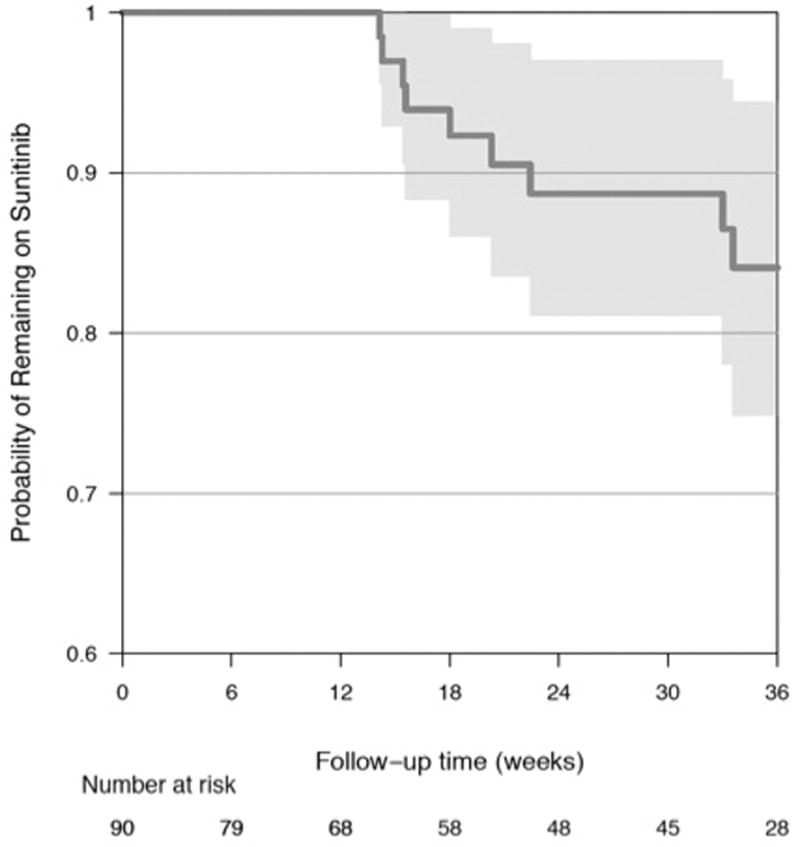
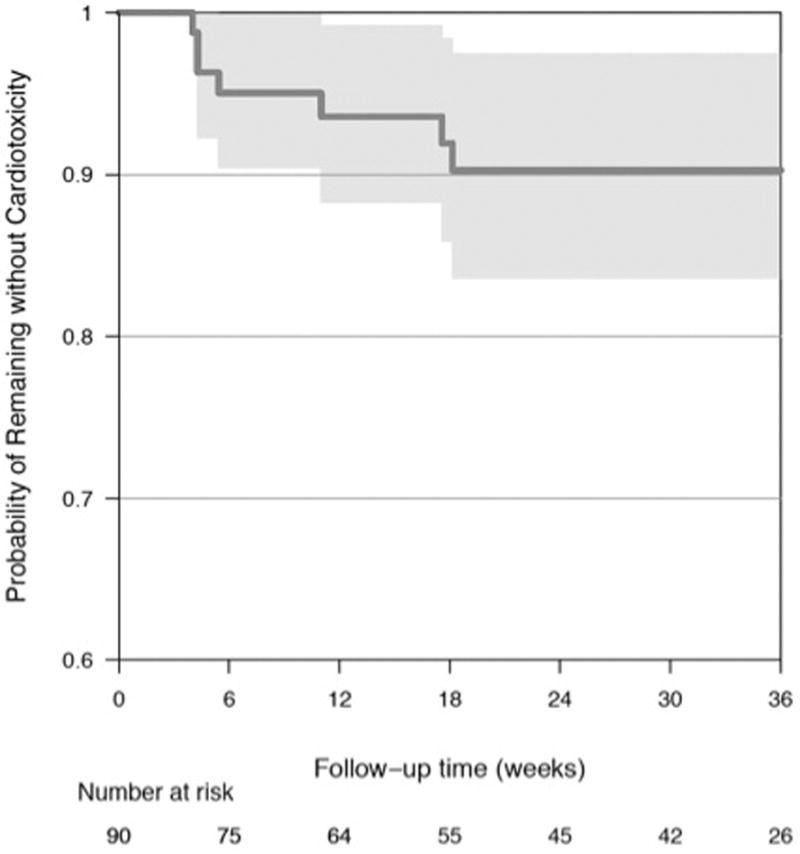
Kaplan-Meier Analyses for (A) Time to Death from mRCC (B) Time to Sunitinib Discontinuation (C) Time to LV Dysfunction
3.2 Incidence of LV Dysfunction and Change in Incidence Over Time
Overall, there was a very modest, but statistically significant decline in LVEF of 1.9% (95% CI −3.2, −0.5) at 3.5 weeks (cycle 1) when compared to baseline (p = 0.007) (Table 2, Figure 3). At subsequent visits, there were no significant differences in mean LVEF from baseline (Table 2). Patient and treatment characteristics including hypertension, systolic blood pressure, pulse pressure, and sunitinib starting dose or schedule were not associated with early LVEF changes (Supplemental Table S2). Similarly, early changes in dyspnea severity or the MDASI-HF score were not associated with early LVEF declines (Supplemental Table S2). At baseline, the overall predicted risk of developing LV dysfunction by 33 weeks (cycle 6) following the initiation of sunitinib was 9.7% (95% CI 3, 17%). The majority of these events occurred by 3.5 weeks of the first treatment cycle, with a substantial decrease in risk over time (Figure 2C). The estimated risk of LV dysfunction diminished to 5% (95% CI 0, 11%) and 2% (95% CI 0, 5%) in patients who had not experienced LV dysfunction by the completion of cycles 1 and 3, respectively.
Table 2.
Change in LVEF and Cardiac Biomarkers from Baseline
| Study Timepoint | Change in LVEF (%)a | p-value | Change in hsTnI (pg/ml)a | p-value | Change in BNP (pg/ml)a | p-value | |
|---|---|---|---|---|---|---|---|
| Visit 2 | 3.5 weeks (+/− 1 week) |
−1.9 (−3.2, −0.5) |
0.007 | −8.1 (−18.3, 2.1) |
0.123 | 21.2 (−23.8, 66.2) |
0.361 |
| Visit 3 | 15 weeks (+/− 2 weeks) |
−0.3 (−1.8, 1.2) |
0.714 | −3.7 (−14.6, 7.2) |
0.510 | −8.4 (−57.3, 40.4) |
0.737 |
| Visit 4 | 33 weeks (+/− 2 weeks) |
0.1 (−1.6, 1.8) |
0.933 | −6.3 (−18.3, 5.7) |
0.308 | 6.9 (−48.0, 61.7) |
0.807 |
Mean absolute change from baseline, with 95% CI; Analyses performed with linear mixed effects model with random intercept
Figure 3. Change in LVEF from Baseline with Sunitinib Therapy.
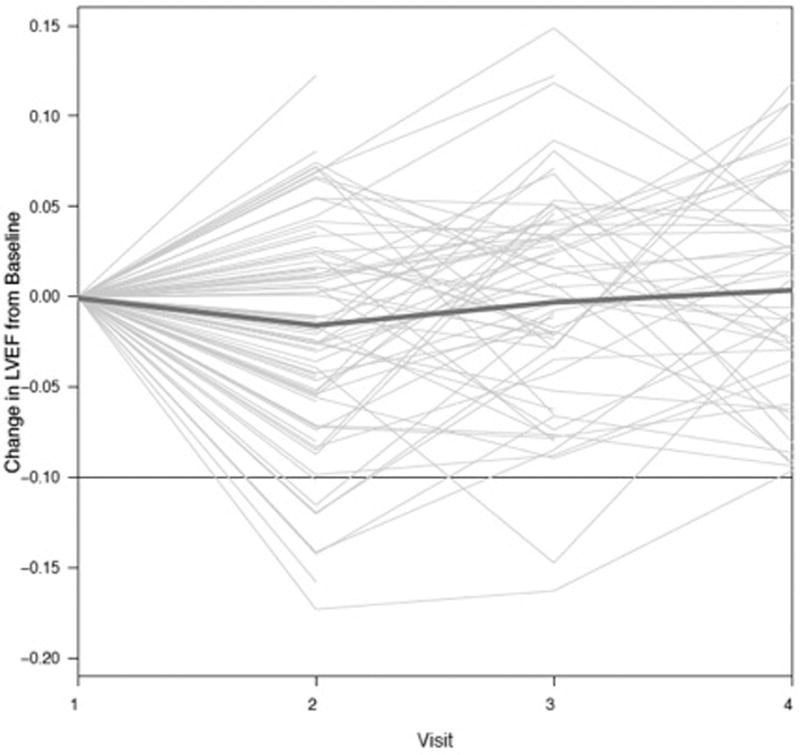
Trajectory of LVEF plotted for each individual patient. Dark blue line indicates a summary spline curve of mean change in LVEF. Threshold at −0.10 denotes the development of LV dysfunction, as defined by an absolute LVEF decline by ≥ 10% to a value of < 50%.
3.3 Changes in Cardiac Biomarkers Over Time
There were no significant changes in either hsTnI or BNP over time across the study cohort (Table 2). However, at baseline, 7 participants (7.8%) had an abnormal hsTnI (>21.5 pg/ml), and 15 participants (16.7%) had an abnormal BNP (>100 pg/ml). Only one participant had baseline elevations in both biomarkers. A total of 17 participants (18.9%) developed subsequent cardiac biomarker increases exceeding abnormal thresholds. Six participants (6.7%) developed an increase in hsTnI exceeding the 99th percentile (>21.5 pg/ml) following the initiation of sunitinib therapy. High sensitivity TnI elevations occurred in the setting of recent intra-abdominal surgery (N=1) and a recent hospitalization for dehydration and electrolyte derangements (N=1). All hsTnI elevations occurred by week 15 (cycle 3), with the exception of 2 participants who developed hsTnI elevation at week 33 (cycle 6). Similarly, 11 additional participants (12.2%) developed increases in BNP to >100 pg/ml (N=1 with incident brain metastases, N=1 with malignant ascites, N=1 with end-stage renal disease requiring hemodialysis, and N=1 with hemorrhagic hepatic metastases). Five of these participants had abnormal BNP values (>100 pg/ml) at baseline. All BNP increases occurred by week 15 (cycle 3), with the exception of 1 participant who developed an increase at week 33. While there was no significant association between hsTnI and LVEF (0.1% LVEF decline per 10 unit increase in hsTnI, p = 0.407), BNP was modestly associated with LVEF change (0.4% decrease in LVEF for 100 unit increase in BNP, p = 0.007).
3.4 Characteristics and Clinical Course of Patients who Developed LV Dysfunction
Of the nine participants who experienced incident LV dysfunction, as defined by an absolute LVEF decline by ≥ 10% to an absolute LVEF value of < 50%, 8 had a sunitinib starting dose of 50 mg daily, and all participants were initially treated on a 4 weeks on/2 weeks off dosing schedule (Table 3). The median quantitated LVEF at baseline was 53.1%, with 3 participants having an LVEF < 50% (47.1% to 49.9%). There was no significant difference in the median baseline LVEF between those with and without the development of subsequent LV dysfunction (53.1% vs 49.2%, p = 0.105). Eight of these 9 participants developed LV dysfunction by the 3.5-week study visit (cycle 1), and the remaining one patient developed LV dysfunction by the 15-week visit (cycle 3). The median absolute decline in LVEF among these patients was 12.5% (IQR 12.0, 14.7). The majority of participants had non-specific symptoms, most commonly generalized fatigue. Only 2 participants with a decline in LVEF also experienced an elevation in hsTnI or BNP. The median changes from baseline for hsTnI and BNP among patients with sunitinib-induced LV dysfunction were 0.6 pg/ml (IQR −1.9, 6.5) and 4.6 pg/ml (IQR −2.9, 19.6), respectively (Table 3).
Table 3.
Clinical Characteristics of Individual Patients Experiencing Left Ventricular Dysfunction While on Sunitinib
| Patient | Age (yrs) | Gender | Baseline Cardiac Medications | Sunitinib Starting Dose (mg) | Baseline LVEF (%) | Maximum LVEF Decline (%)a | Maximum Change in Biomarker (pg/ml)b | HF Symptoms | New Cardiac Medications | Sunitinib Continued | LVEF Recovery | Recovery LVEF (%) | Timing of LVEF Recovery | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | BB, Statin, Diuretic | 50 | 49.9 | 10.1 | TnI: −4.1 BNP: −504 |
Fatigue, Dyspnea | None | Yes | N/A | N/A | N/A | Expired from RCC prior to subsequent ECHO |
| 2 | 43 | M | Statin | 50 | 53.4 | 17.2 | TnI: +227.5 BNP: +3.9 |
Fatigue | BB, CCB | Yes | Yes | 43.8 | Wk 33 | Changed RCC therapy wk 30 secondary to disease progression |
| 3 | 56 | F | None | 50 | 53.1 | 14.1 | Unk | Fatigue, Dyspnea, PND | None | Yes | Yes | 67.6 | Initial recovery Wk 15; Full recovery by Wk 52 | SU dose reduction and changed to 2/1 schedule wk 15 |
| 4 | 41 | F | None | 50 | 51.7 | 14.7 | TnI: +0.5 BNP: +5.2 |
Fatigue, Ankle Swelling | None | Yes | Yes | 50.9 | Wk 33 | SU dose reduction and changed to 2/1 schedule wk 15 |
| 5 | 61 | M | ASA, ARB, CCB, Diuretic | 50 | 47.1 | 12.0 | TnI: +3.8 BNP: +3.8 |
Fatigue | Clonidine BB |
Yes | Yes | 57.9 | Wk 33 | |
| 6 | 66 | M | CCB | 50 | 55.0 | 12.0 | TnI: +0.7 BNP: +23.6 |
Fatigue, Ankle Swelling | ARB, diuretic BB |
No | Yes | 52.0 | Wk 33 | Changed RCC therapy wk 12 secondary to disease progression |
| 7 | 46 | M | None | 37.5 | 53.5 | 11.5 | TnI: −22.4 BNP: +15.6 |
Fatigue, Dyspnea | ACEi | Yes | Yes | 52.6 | Wk 15 | |
| 8 | 45 | M | ASA | 50 | 49.7 | 12.5 | TnI: +0.3 BNP: −9.5 |
Fatigue, | None | Yes | Yes | 46.2 | Wk 33 | Changed to 2/1 schedule wk 15 |
| 9 | 56 | M | BB, CCB, Other | 50 | 62.1 | 15.8 | TnI: +9.2 BNP: +1726 |
None | None | Yes | N/A | N/A | N/A | Expired from RCC prior to subsequent ECHO |
For all patients with the exception of patient 4, LV dysfunction was detected at week 3.5. For patient 4, this was detected at 15 weeks.
Maximum change in biomarker from baseline visit (i.e. biomarker value at follow-up visit – biomarker value at baseline visit)
Abbreviations: LVEF, left ventricular ejection fraction; LV, left ventricular; BB, beta-blocker; ASA, aspirin; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; ACEi, angiotensin converting enzyme inhibitor; Statin, HMG CoA reductase inhibitor; TnI, high sensitivity cardiac troponin I; BNP, B-type natriuretic peptide; HF, heart failure; RCC, renal cell carcinoma; ECHO, echocardiography; SU, sunitinib; N/A, not applicable
Sunitinib therapy was continued in 8 participants and discontinued in 1 participant. Two participants were not evaluable for improvement in LVEF (both died from mRCC prior to subsequent echocardiography). All evaluable participants had a subsequent improvement in LVEF by 15 to 33 weeks, including the 6 participants who remained on sunitinib. Four participants had recovery of LVEF to at least within 3% of their respective baseline LVEF. Following the detection of LV dysfunction, 4 participants initiated new anti-hypertensive medications, and 2 had a dose-reduction of sunitinib and were changed to a 2 weeks on/1 week off dosing schedule (Table 3). All 8 participants who continued sunitinib therapy remained on drug without pause in treatment.
4. Discussion
Although LV dysfunction is a known cardiovascular toxicity of the VEGF receptor TKI sunitinib, the clinical significance of this toxicity remains poorly defined. In an effort to improve our understanding of the epidemiology and natural history of LV dysfunction as a result of sunitinib exposure, our prospective study comprehensively evaluated changes in LVEF, derived by quantitative echocardiography, in a “real-world” mRCC cohort. Furthermore, we evaluated circulating cardiac biomarkers as additional surrogate measures of subclinical myocardial injury and stress. Our study revealed several key findings. First, on the population level, mRCC patients receiving sunitinib demonstrate very modest declines in LVEF and minimal change in plasma cardiac biomarkers. Second, 9.7% of patients do experience a more substantial decline in LV function; however, these patients largely demonstrate recovery of LVEF to near baseline values despite the continuation of sunitinib therapy, but in the setting of careful cardiovascular management or sunitinib dose-reduction. Third, LV dysfunction occurs early in the treatment course — often within the first treatment cycle — and the subsequent risk of significant subclinical LV dysfunction following 3 cycles of therapy is low. Fourth, a similar proportion of patients also develop early abnormalities in hsTnI and BNP, which may be more sensitive markers of cardiac injury and stress. We believe these findings have important implications for the cardiovascular and oncologic management of patients on sunitinib therapy, particularly with regard to the timing of cardiovascular assessment and the continuation of therapy.
To our knowledge, this is the first prospective evaluation of sunitinib-induced cardiotoxicity using precise assessment of cardiac function obtained at standardized intervals and quantified in an echocardiography core laboratory. Prior reports of sunitinib-related cardiovascular toxicity have mostly been retrospective in nature, with various composite endpoint definitions, cardiac monitoring procedures, and study populations.(9, 17, 19, 20) Overall, retrospective analyses suggest that LVEF declines occur in 18.9% of patients, while a larger percentage (33.8%) develop an elevation in cardiac enzymes, symptomatic arrhythmia, or new LV dysfunction.(8, 20) However, quantitative echocardiography was not performed in these reports. Many studies also demonstrate some degree of LVEF recovery following sunitinib-induced LV dysfunction. (26, 28) Our study definitively validates these findings through its prospective design, standardized data collection, and inclusion of a population that is generalizable to every day practice.
As such, these findings provide important evidence to guide screening and toxicity management with sunitinib in routine clinical practice. Although cardiovascular risk on VEGF receptor TKIs is widely recognized, current consensus statements do not provide guidance for the monitoring of cardiac function.(6) As a result, institutional algorithms for the cardiac monitoring of patients treated with VEGF receptor TKIs have been developed and recommend varying strategies, including monthly or every 3-month LVEF assessment.(19, 20) In contrast, our data suggest that while cardiovascular toxicity may occur early during therapy (9.7% with LVEF declines of ≥ 10% to a value of < 50%), routine cardiac monitoring in asymptomatic individuals is unlikely to be of widespread clinical benefit, specifically beyond cycle 3 of therapy when the observed rates of cardiac dysfunction were very low (approximately 2% by 33 weeks).
Furthermore, the standard management of mRCC is to exploit the addiction of mRCC to VEGF-signaling by utilizing serial VEGF-targeted agents despite disease progression on an initial VEGF receptor inhibitor.(3, 29) Thus, early cessation of therapy is not favorable, and novel multi-targeted VEGFR inhibitors are achieving unprecedented survival benefit in pre-treated mRCC patients.(30) In our study, the majority of patients who experienced a significant decline in LVEF were able to recover LV function to within 3 percentage points of their baseline with careful cardiovascular management. Of note, many patients had initiation of anti-hypertensive medications and/or dose-reduction of sunitinib. However, recovery of cardiac function occurred primarily in the setting of sunitinib continuation/dose-reduction and without treatment delay. While there were no specific clinical risk factors that could be clearly identified, our findings suggest that LVEF declines are largely reversible and the discontinuation of sunitinib therapy in the setting of incident asymptomatic LV dysfunction is not universally mandated. Our findings suggest that therapy may be continued in these patients with careful cardiovascular and oncologic management in an effort to derive maximum benefit from VEGF-directed therapies with minimal “cost” to treatment intensity. Therefore, the modest and reversible nature of LV dysfunction in our study may provide reassurance regarding cardiotoxicity in this setting, as has been similarly demonstrated in a recent cardiac monitoring study of RCC patients treated adjuvantly with VEGF receptor TKIs.(26)
Elevations in plasma cardiac biomarkers have been prospectively identified as indicators of cardiac toxicity occurring in the absence of overt LV systolic dysfunction.(31–33) In retrospective studies of patients with mRCC, cardiac biomarker alterations have similarly been reported following sunitinib initiation, with resulting algorithms recommending routine serial BNP monitoring.(19) Although there were no significant mean changes in measures of hsTnI and BNP from baseline following the initiation of sunitinib in our study cohort, a total of 18.9% of patients developed marked increases in hsTnI or BNP. Of note, most of these patients did not have a corresponding detectable substantial decline in LVEF. Therefore, while these biomarker elevations may be indicative of early subclinical cardiac toxicity, the clinical utility of plasma cardiac biomarkers in an advanced oncologic population with multiple clinical confounders is unclear. Indeed, a nontrivial number of patients had baseline biomarker abnormalities, potentially reflecting the sequelae of the oncologic disease burden, common medical comorbidities in this population such as renal disease, and possibly even an increased risk of oncologic mortality.(34) Thus, our findings indicate a lack of clear clinical utility for hsTnI or BNP in this setting, and suggest the need to understand whether the test characteristics may differ according to the severity of oncologic disease.
In our study, no baseline patient or treatment factors, including sunitinib dose or schedule, were associated with an early LVEF decline. Interestingly, while hypertension is a risk factor for heart failure and cardiomyopathy and a widely recognized toxicity of sunitinib therapy, neither baseline systolic blood pressure nor pulse pressure was associated with change in LVEF. It is possible that measures such as blood pressure may not adequately quantify the changes in vascular function that occur with this therapy. Ongoing clinical studies, including detailed phenotyping with arterial tonometry, will seek to clarify the relationship between early changes in systemic vascular load with sunitinib, LV dysfunction, and cardiac recovery. In addition, our sample size may have limited our ability to detect a significant association between patient or treatment factors and early LVEF change.
Additional limitations of this study are noted. In a patient population with an advanced malignancy, disease-related death serves as a competing risk to treatment-related cardiovascular toxicity and may therefore bias the reported risk estimates. In addition, in our study, 23% of participants prematurely withdrew from study follow-up, primarily in the setting of oncologic disease progression. However, the results of the reported conditional survival analyses indicate that if a patient continues on sunitinib therapy without significant LV dysfunction through cycle 3, then the subsequent risk of LV dysfunction is low at 33 weeks. Furthermore, as the majority of active patients remained on sunitinib therapy at cycle 6, or 33 weeks of follow-up, it is unlikely that early cessation of sunitinib therapy significantly impacted the reported conditional survival. Finally, our sample size precluded us from determining the relationship between sunitinib-induced LV dysfunction and oncologic outcomes.
When compared to prior reports of sunitinib-induced cardiovascular toxicities, the strengths of this current study include its prospective nature, the detailed assessments performed at pre-defined intervals designed to coincide with the sunitinib cycle length and drug exposure, and detailed cardiovascular phenotyping with central review of echocardiograms in a core laboratory and cardiovascular biomarker data. In addition, the enrollment of a non-clinical trial patient population with medical comorbidities common to the mRCC population allows for external validity and generalizability of these findings to routine clinical practice.
In conclusion, we found that while the majority of mRCC patients experience modest declines in LVEF and cardiac biomarker changes with sunitinib, 9.7% of patients experience a substantial LVEF change and 18.9% develop cardiac biomarker elevations. LV dysfunction, as defined by LVEF declines, occurs early in the treatment course and is not directly reflected by cardiac symptoms or changes in hsTnI or BNP. Taken together with recently reported cardiac toxicity findings from the adjuvant use of sunitinib, these results indicate that LVEF declines are largely reversible in the setting of sunitinib continuation with careful cardiovascular management or sunitinib dose-reduction. While individual clinical discretion remains prudent, routine LVEF or cardiac biomarker monitoring in asymptomatic individuals with a low index of suspicion of cardiotoxicity may be of limited utility, especially beyond 3 cycles of therapy.
Supplementary Material
Statement of Translational Relevance.
This is the first multi-center, prospective study of sunitinib-induced cardiotoxicity in patients with metastatic renal cell carcinoma (mRCC) using precise and standardized cardiac imaging and biomarker assessments. Our results demonstrate that on average mRCC patients receiving sunitinib exhibit modest declines in left ventricular ejection fraction (LVEF) and nonsignificant changes in high-sensitivity Troponin I (hsTnI) and B-type natriuretic peptide (BNP) over time. Left ventricular (LV) dysfunction, as defined by LVEF declines of ≥10% to <50%, occurred in approximately 10% of patients. Similarly, incident increases in hsTnI to >21.5 pg/ml or BNP to >100 pg/ml occurred in a total of 18.9% of patients (6.7% and 12.2% for hsTnI and BNP, respectively). LVEF declines and biomarker increases occurred early and were not sustained. These findings provide prospective evidence to guide strategies for cardiotoxicity monitoring with sunitinib therapy.
Acknowledgments
None.
Financial Support: This study was funded in part by an Investigator Initiated Research Award to BK from Pfizer, Inc. Biomarker assays were performed in collaboration with Singulex, Inc. through Investigator Initiated Research support (BK). Sponsors were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: The authors declare no potential conflicts of interest.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, Michaelson D, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 6.NCCN Guidelines: Kidney Cancer. National Comprehensive Cancer Network;Version I.2016 [Google Scholar]
- 7.Jonasch E, Signorovitch JE, Lin PL, Liu Z, Culver K, Pal SK, et al. Treatment patterns in metastatic renal cell carcinoma: A retrospective review of medical records from US community oncology practices. Curr Med Res Opin. 2014;30(10):2041–50. doi: 10.1185/03007995.2014.938730. [DOI] [PubMed] [Google Scholar]
- 8.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–12. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 9.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi W, He A, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: A systematic review and meta-analysis. Br J Clin Pharmacol. 2013;76(3):348–57. doi: 10.1111/bcp.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rainer PP, Doleschal B, Kirk JA, Sivakumaran V, Saad Z, Groschner K, et al. Sunitinib causes dose-dependent negative functional effects on myocardium and cardiomyocytes. BJU Int. 2012;110(10):1455–62. doi: 10.1111/j.1464-410X.2012.11134.x. [DOI] [PubMed] [Google Scholar]
- 12.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 13.Ky B, French B, Ruparel K, Sweitzer NK, Fang JC, Levy WC, et al. The vascular marker soluble fms-like tyrosine kinase 1 is associated with disease severity and adverse outcomes in chronic heart failure. J Am Coll Cardiol. 2011;58(4):386–94. doi: 10.1016/j.jacc.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamnvik O-R, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121(2):311–9. doi: 10.1002/cncr.28972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20(5):807–15. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 16.Richards CJ, Je Y, Schutz FAB, Heng DYC, Dallabrida SM, Moslehi JJ, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450–6. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 17.Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, et al. Heart failure associated with sunitinib malate: A multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112(11):2500–8. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 18.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19(9):1613–8. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 19.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72–8. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Di Lorenzo G, Autorino R, Bruni G, Cartentì G, Ricevuto E, Tudini M, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: A multicenter analysis. Ann Oncol. 2009;20(9):1535–42. doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 21.Fadol A, Mendoza T, Gning I, Kernicki J, Symes L, Cleeland CS, et al. Psychometric Testing of the MDASI-HF: A Symptom Assessment Instrument for Patients With Cancer and Concurrent Heart Failure. J Card Fail. 2008;14(6):497–507. doi: 10.1016/j.cardfail.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Badano LP, Victor M, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–81. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 24.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 25.Narayan HK, French B, Khan AM, Plappert T, Hyman D, Bajulaiye A, et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics-Related Cardiac Dysfunction. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas NB, Manola J, Ky B, Flaherty KT, Uzzo RG, Kane CJ, et al. Effects of adjuvant sorafenib and sunitinib on cardiac function in renal cell carcinoma patients without overt metastases: Results from ASSURE, ECOG 2805. Clin Cancer Res. 2015;21(18):4048–54. doi: 10.1158/1078-0432.CCR-15-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu A, Estis J, Heseltine P, Bui K, Todd J, Kavsak P. High-sensitivity Cardiac Troponin I in a Large Community-Based Population at Risk for Cardiovascular Disease. Clinical Chemistry. 2015;61(10, Supplement) [Google Scholar]
- 28.Ewer MS, Suter TM, Lenihan DJ, Niculescu L, Breazna A, Demetri GD, et al. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: A comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur J Cancer. 2014;50(12):2162–70. doi: 10.1016/j.ejca.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32(8):760–7. doi: 10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choueiri T, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1510016. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen SA, Hasbak P, Mortensen J, Sørensen JB. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J Clin Oncol. 2010;28(36):5280–6. doi: 10.1200/JCO.2009.27.3953. [DOI] [PubMed] [Google Scholar]
- 32.Kuittinen T, Jantunen E, Vanninen E, Mussalo H, Vuolteenaho O, Ala-Kopsala M, et al. Cardiac effects within 3 months of BEAC high-dose therapy in non-Hodgkin’s lymphoma patients undergoing autologous stem cell transplantation. Eur J Haematol. 2006;77(2):120–7. doi: 10.1111/j.1600-0609.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 33.Ky B, Putt M, Sawaya H, French B, Januzzi JL, Jr, Sebag IA, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63(8):809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101(23):1874–80. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


