Abstract
It has been suggested that epidermal growth factor-like (EGF-like) domains, containing conserved carboxylate residues, are responsible for the high-affinity calcium binding exhibited by a number of vitamin K-dependent plasma proteins involved in the control of the blood coagulation cascade. These include the procoagulant factors IX and X, and the anticoagulants protein C and protein S. To test this hypothesis we have expressed the first EGF-like domain from human factor IX (residues 46-84) using a yeast secretion system, and examined calcium binding to the domain. Using 1H-NMR to measure a calcium-dependent shift assigned to Tyr69 we have detected a high-affinity calcium binding site (Kd = 200-300 microM). We suggest that other EGF-like domains of this type may have similar calcium binding properties. In addition, we have completely assigned the aromatic region of the NMR spectrum by NOESY and COSY analysis, and have used these data to discuss the effect of calcium and pH on the conformation of the domain with reference to a model based on the structure of human EGF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Borowski M., Furie B. C., Bauminger S., Furie B. Prothrombin requires two sequential metal-dependent conformational transitions to bind phospholipid. Conformation-specific antibodies directed against the phospholipid-binding site on prothrombin. J Biol Chem. 1986 Nov 15;261(32):14969–14975. [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. M., Wilkinson A. J., Baron M., Pastore A., Tappin M. J., Campbell I. D., Gregory H., Sheard B. The solution structure of human epidermal growth factor. 1987 May 28-Jun 3Nature. 327(6120):339–341. doi: 10.1038/327339a0. [DOI] [PubMed] [Google Scholar]
- Davis C. G., Goldstein J. L., Südhof T. C., Anderson R. G., Russell D. W., Brown M. S. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 1987 Apr 23;326(6115):760–765. doi: 10.1038/326760a0. [DOI] [PubMed] [Google Scholar]
- Davis L. M., McGraw R. A., Ware J. L., Roberts H. R., Stafford D. W. Factor IXAlabama: a point mutation in a clotting protein results in hemophilia B. Blood. 1987 Jan;69(1):140–143. [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S. Computer-based characterization of epidermal growth factor precursor. Nature. 1984 Feb 9;307(5951):558–560. doi: 10.1038/307558a0. [DOI] [PubMed] [Google Scholar]
- Esmon N. L., DeBault L. E., Esmon C. T. Proteolytic formation and properties of gamma-carboxyglutamic acid-domainless protein C. J Biol Chem. 1983 May 10;258(9):5548–5553. [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Beta-hydroxyaspartic acid in vitamin K-dependent proteins. J Biol Chem. 1983 Oct 25;258(20):12509–12512. [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Green P. M., Bentley D. R., Mibashan R. S., Nilsson I. M., Giannelli F. Molecular pathology of haemophilia B. EMBO J. 1989 Apr;8(4):1067–1072. doi: 10.1002/j.1460-2075.1989.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Ke X. H., Sweeney W., Tam J. P. Calcium binding and putative activity of the epidermal growth factor domain of blood coagulation factor IX. Biochem Biophys Res Commun. 1989 Apr 14;160(1):133–139. doi: 10.1016/0006-291x(89)91631-8. [DOI] [PubMed] [Google Scholar]
- Højrup P., Magnusson S. Disulphide bridges of bovine factor X. Biochem J. 1987 Aug 1;245(3):887–891. doi: 10.1042/bj2450887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman H. A., Furie B. C., Furie B. The factor IX phospholipid-binding site is required for calcium-dependent activation of factor IX by factor XIa. J Biol Chem. 1987 Jun 5;262(16):7605–7612. [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Morita T., Isaacs B. S., Esmon C. T., Johnson A. E. Derivatives of blood coagulation factor IX contain a high affinity Ca2+-binding site that lacks gamma-carboxyglutamic acid. J Biol Chem. 1984 May 10;259(9):5698–5704. [PubMed] [Google Scholar]
- Morita T., Kisiel W. Calcium binding to a human factor IXa derivative lacking gamma-carboxyglutamic acid: evidence for two high-affinity sites that do not involve beta-hydroxyaspartic acid. Biochem Biophys Res Commun. 1985 Jul 31;130(2):841–847. doi: 10.1016/0006-291x(85)90493-0. [DOI] [PubMed] [Google Scholar]
- Ohlin A. K., Linse S., Stenflo J. Calcium binding to the epidermal growth factor homology region of bovine protein C. J Biol Chem. 1988 May 25;263(15):7411–7417. [PubMed] [Google Scholar]
- Ohlin A. K., Stenflo J. Calcium-dependent interaction between the epidermal growth factor precursor-like region of human protein C and a monoclonal antibody. J Biol Chem. 1987 Oct 5;262(28):13798–13804. [PubMed] [Google Scholar]
- Persson E., Selander M., Linse S., Drakenberg T., Ohlin A. K., Stenflo J. Calcium binding to the isolated beta-hydroxyaspartic acid-containing epidermal growth factor-like domain of bovine factor X. J Biol Chem. 1989 Oct 5;264(28):16897–16904. [PubMed] [Google Scholar]
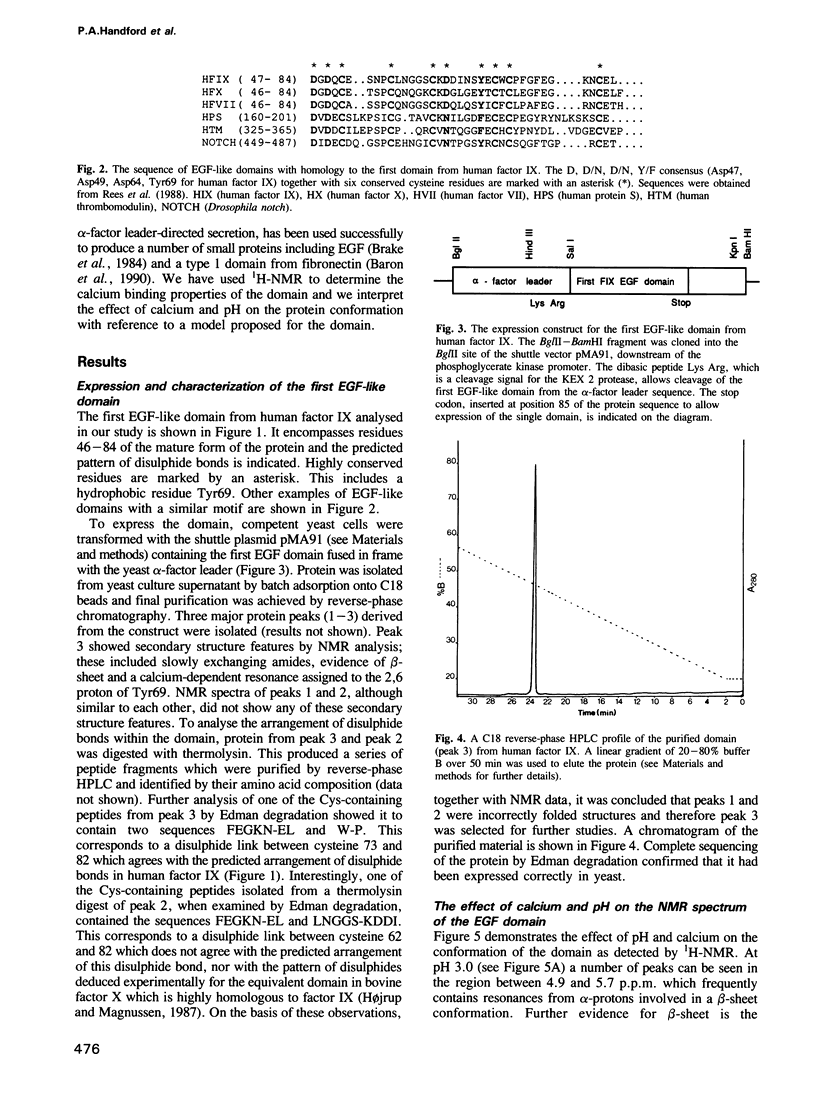
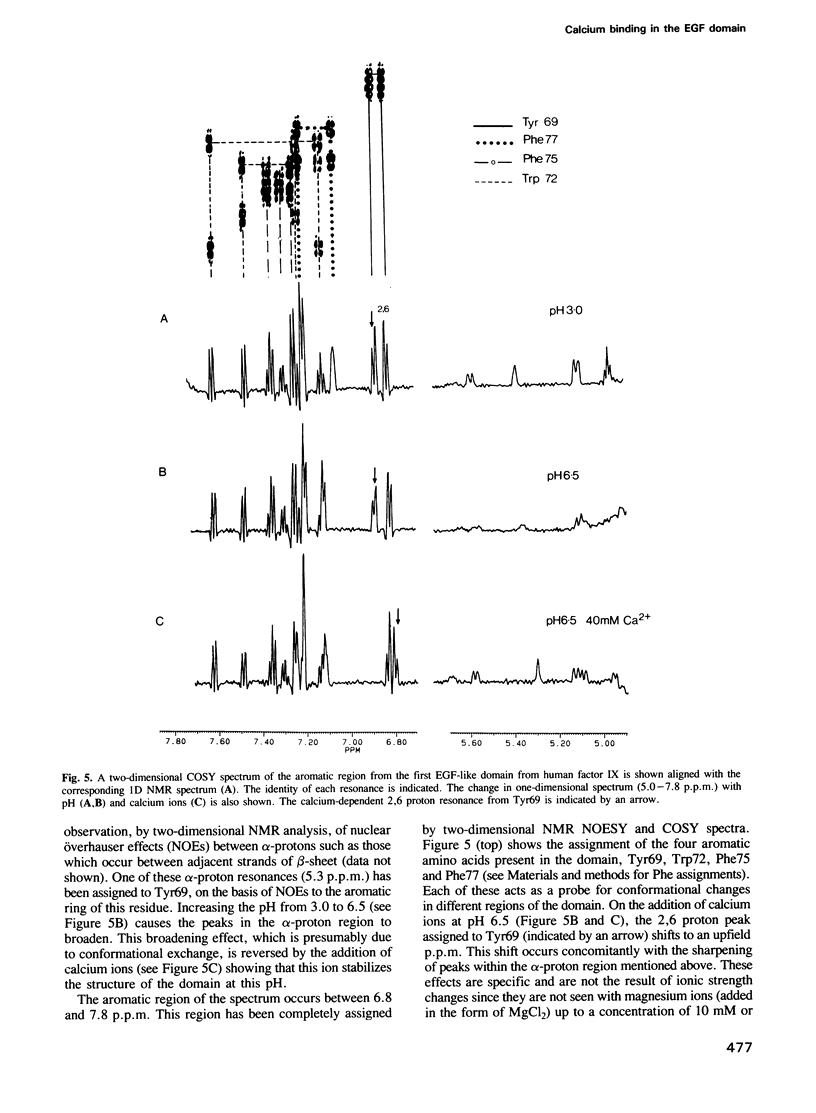
- Rees D. J., Jones I. M., Handford P. A., Walter S. J., Esnouf M. P., Smith K. J., Brownlee G. G. The role of beta-hydroxyaspartate and adjacent carboxylate residues in the first EGF domain of human factor IX. EMBO J. 1988 Jul;7(7):2053–2061. doi: 10.1002/j.1460-2075.1988.tb03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Urdea M., Quiroga M., Sanchez-Pescador R., Fong N., Selby M., Rutter W. J., Bell G. I. Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science. 1983 Jul 15;221(4607):236–240. doi: 10.1126/science.6602382. [DOI] [PubMed] [Google Scholar]
- Sugo T., Björk I., Holmgren A., Stenflo J. Calcium-binding properties of bovine factor X lacking the gamma-carboxyglutamic acid-containing region. J Biol Chem. 1984 May 10;259(9):5705–5710. [PubMed] [Google Scholar]
- Sugo T., Dahlbäck B., Holmgren A., Stenflo J. Calcium binding of bovine protein S. Effect of thrombin cleavage and removal of the gamma-carboxyglutamic acid-containing region. J Biol Chem. 1986 Apr 15;261(11):5116–5120. [PubMed] [Google Scholar]


