Abstract
Prostate cancer is one of the leading causes of cancer death in US men. There is an unmet need to identify modifiable risk factors for prostate cancer survival. Experimental studies have suggested that nonsteroidal anti-inflammatory drugs (NSAIDs) may improve prostate cancer survival through anti-thrombotic and anti-inflammation mechanisms. Results from previous observational studies have been equivocal, and few have assessed whether an etiologically relevant time window of exposure exists. We sampled prostate cancer cases from two large US prospective cohorts—NIH-AARP Diet and Health Study and PLCO Cancer Screening Trial—to investigate whether pre- and post-diagnostic aspirin and non-aspirin NSAID use were associated with prostate cancer-specific and all-cause mortality. Cox proportional hazards regression models estimated hazard ratios (HRs) and 95% confidence intervals (CIs). Study-specific results were meta-analyzed using fixed-effects models. Pre- and post-diagnostic aspirin or non-aspirin NSAID use were not statistically significantly associated with prostate cancer-specific mortality. However, occasional (less than daily) and daily aspirin users five years or more before prostate cancer diagnosis had 18% (HR=0.82; 95%CI=0.75 to 0.90) and 15% (HR=0.85; 95%CI=0.77 to 0.94) reduced all-cause mortality versus nonusers. Similarly, post-diagnostic occasional and daily aspirin use were associated with 17% (HR=0.83; 95%CI=0.72 to 0.95) and 25% (HR=0.75; 95%CI=0.66 to 0.86) reduced all-cause mortality, independent of pre-diagnostic aspirin use. This study suggests that aspirin or non-aspirin NSAIDs are not associated with prostate cancer survival. However, aspirin use both before and after prostate cancer diagnosis was associated with longer overall survival, highlighting the importance of comorbidity prevention among prostate cancer survivors.
Keywords: Nonsteroidal anti-inflammatory agents, Prostatic neoplasms, Survival, Cohort studies
Introduction
Despite a recent reduction in prostate cancer mortality, prostate cancer remains the third leading cause of cancer death in US men with an estimated 26,730 deaths in 2017 (1). Few modifiable risk factors have been established for prostate cancer progression and survival, despite putative evidence for body size, physical exercise, and smoking (2). Nonsteroidal anti-inflammatory drugs (NSAIDs) have been hypothesized to inhibit prostate cancer carcinogenesis and progression through anti-thrombotic and anti-inflammation mechanisms via blocking cyclooxygenase (COX)-1 and -2 isozymes, respectively (3,4).
Experimental studies support that platelets aid tumor metastasis by inducing angiogenesis, protecting tumor cells from immune surveillance, and promoting interactions between tumor cells and blood vessels (5). The anti-thrombotic effect of COX-1 inhibition in platelets can impair prostate cancer micrometastases (6,7). Meanwhile, COX-2 is highly expressed in human prostate cancer (8), and inhibition of COX-2 in mouse models down-modulates inflammation, suppresses angiogenesis, and retains anti-metastasis markers (9,10). Phase II trials of celecoxib found that the COX-2 inhibitor decreases prostate-specific antigen (PSA) velocity among biochemical recurrent prostate cancer cases after definitive treatments (11,12).
Despite biological plausibility, few observational studies have examined whether NSAIDs alter prostate cancer survival and whether an etiologically relevant time window of exposure exists. Four studies examined pre-diagnostic NSAID use (three for aspirin and two for non-aspirin NSAIDs) in relation to prostate cancer-specific mortality (PCSM), although the time between exposure and cancer diagnosis was often short, providing a limited time interval prior to cancer diagnosis for meaningful biological effects (13–16). Seven studies assessed post-diagnostic NSAID use (seven for aspirin and one for non-aspirin NSAIDs) and reported inconsistent results (14,16–21). Herein, we report associations of pre- and post-diagnostic aspirin and non-aspirin NSAID use with PCSM and all-cause mortality among prostate cancer cases in two large US prospective cohort studies.
Materials and Methods
Study Population
NIH-AARP Diet and Health Study
The study design has been previously described in detail (22). Briefly, this is a prospective cohort study of diet, health-related behaviors, and cancer. The cohort included 566,398 AARP members aged 50–71 years, who resided in one of six states or two metropolitan areas and completed a mailed Baseline Questionnaire (BQ) in 1995–1996. A Risk Factor Questionnaire (RFQ) was then mailed in 1996–1997 to participants without self-reported colon, breast or prostate cancers at baseline for additional epidemiologic information including frequency of NSAID use. A total of 334,905 participants completed RFQ. In 2004–2006, a Follow-Up Questionnaire (FUQ) including additional questions regarding NSAID use was sent, and a total of 221,189 participants responded. Because the baseline questionnaire did not collect NSAID information, we restricted our study population to men who completed RFQ.
Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
The trial design has been previously described in detail (23). Briefly, this is a multicenter, randomized, two-arm trial to evaluate the effect of screening on disease-specific mortality. The trial enrolled 76,685 men and 78,216 women aged 55–74 at ten screening centers in the US during 1993–2001. Men in the intervention arm received annual PSA tests for the first six years and annual digital rectal examinations for the first four years to screen for prostate cancer. Men in the control arm were encouraged to follow usual care. At randomization, participants in both arms were mailed a baseline questionnaire (BQM) to collect epidemiologic information including frequency of NSAID use. During 2006–2008, retained participants were mailed a supplemental questionnaire (SQX) including additional questions regarding NSAID use.
Both studies have been approved by institutional review boards at the National Cancer Institute.
Analytic Sample
Prostate cancers were ascertained by record linkage to state cancer registries through 2011 for AARP, and by annual questionnaires with subsequent medical record confirmation through 2009 for PLCO. The timeline of data collection in both studies is shown in Fig 1. To evaluate the critical time window of exposure associated with prostate cancer survival, we created two case-only cohorts within each study: the pre-diagnostic cohorts ascertained exposures 6 years (median) before prostate cancer diagnosis for AARP and 5 years for PLCO, and the post-diagnostic cohorts ascertained exposures 4 years (median) after prostate cancer diagnosis for AARP and 5 years for PLCO.
Figure 1. Timeline of data collection and survival follow-up in NIH-AARP and PLCO.
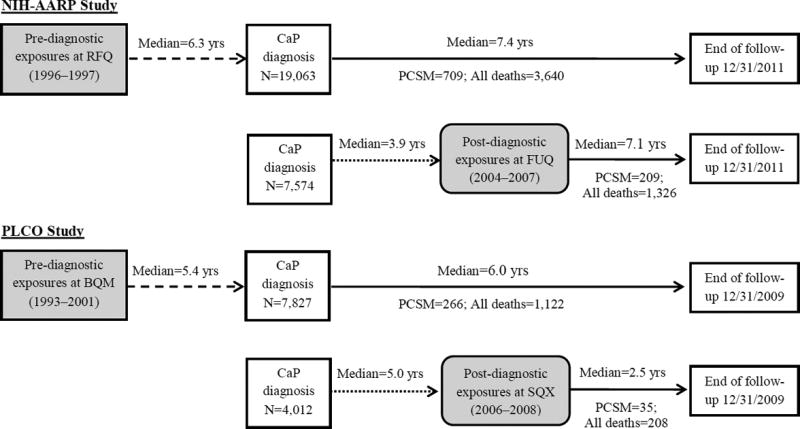
The dashed line indicates the time interval from exposure ascertainment to prostate cancer diagnosis in HIH-AARP and PLCO, the dotted line indicates the lag time and the solid line indicates the follow-up time in the model. The shaded area indicates exposures of interest. Therefore, for the pre-diagnostic analysis, follow-up started at the age of prostate cancer diagnosis and ended at the age of death due to the cause of interest or right censoring, whichever occurred earlier. For the post-diagnostic analysis, follow-up started at the age of SQX/FUQ and ended at the age of death due to the cause of interest or right-censoring, whichever occurred earlier. RFQ, Risk Factor Questionnaire; FUQ, Follow-up Questionnaire; BQM, Baseline Questionnaire for Men; SQX, Supplemental Questionnaire; CaP, prostate cancer; PCSM, prostate cancer-specific mortality.
Details of exclusion and inclusion of the AARP study population are shown in Supplementary Fig 1. In brief, of the 176,901 men considered at risk for prostate cancer at RFQ, 19,474 first primary prostate cancers occurred. The pre-diagnostic cohort included 19,063 invasive first primary prostate cancers, after excluding 24 cases diagnosed with carcinoma in situ, 38 without follow-up, and 349 lacking NSAID information on RFQ. The post-diagnostic cohort included 7,574 cases nested in the pre-diagnostic cohort, after additionally excluding 5,125 cases without FUQ, 5,583 diagnosed after FUQ, 769 lacking NSAID information on FUQ, and 12 without follow-up.
Details of exclusion and inclusion of the PLCO study population are shown in Supplementary Fig 1. In brief, of the 72,119 men considered at risk for prostate cancer at BQM, 7,916 first primary prostate cancers occurred. The pre-diagnostic cohort included 7,827 invasive first primary prostate cancers, after excluding 59 lacking NSAID information on BQM and 30 without follow-up. The post-diagnostic cohort included 4,012 cases nested in the pre-diagnostic cohort, after excluding 2,318 cases without SQX, 1,166 diagnosed after SQX, 322 lacking NSAID information on SQX, and 9 without follow-up.
Mortality and NSAID Ascertainment
Vital status and underlying cause of death were ascertained through linkage to the National Death Index for AARP, and by annual questionnaire with subsequent confirmation by death certificate supplemented with annual linkage to the National Death Index for PLCO.
Frequency of NSAID use in the past year was self-reported. Participants were specifically instructed not to include Tylenol or other pain relievers. AARP RFQ asked about frequency of aspirin and a list of 19 nonselective non-aspirin NSAID use (e.g., ibuprofen and naproxen) separately in eight categories (none, <2 times/month, 2–3/month, 1–2/week, 3–4/week, 5–6/week, 1/day, ≥2/day). PLCO BQM asked about frequency of aspirin and ibuprofen use in a similar fashion (no regular use, <2 pills/month, 2–3/month, 1/week, 2/week, 3–4/week, 1/day, ≥2/day). During follow-up, AARP again assessed frequency of aspirin and nonselective non-aspirin NSAID use in five categories (none, 1–3 times/month, 1–2/week, 3–6/week, ≥1/day) at FUQ. PLCO SQX assessed aspirin and nonselective non-aspirin NSAID use in similar categories (none or less than once/month, 1–3 times/month, 1–2/week, 3–6/week, ≥1/day). To avoid sparse numbers and to harmonize exposure categories, we combined frequency of exposure into three categories: no/no regular, occasional (<once/day), and daily use (≥once/day).
Statistical Methods
Study-specific hazard ratios (HRs) and 95% confidence intervals (95%CIs) for NSAID use in relation to PCSM and all-cause mortality were estimated using Cox proportional hazards regression models with age as the time metric. In pre-diagnostic cohorts, follow-up started at the age of prostate cancer diagnosis and ended at the age of death due to the cause of interest or right-censoring, whichever occurred earlier. In post-diagnostic cohorts, follow-up started at the age of FUQ for AARP or SQX for PLCO, and ended at the age of death due to the cause of interest or right-censoring, whichever occurred earlier. Censoring events included deaths due to causes other than the one of interest or end of study follow-up (12/31/2011 for AARP and 12/31/2009 for PLCO). The proportional hazards assumption was tested by including an interaction term of the exposure and log-transformed age and visual inspection of log(−log) survival plots.
To account for potential confounding, we fitted 1) a basic model, adjusted for Gleason score (prostatectomy or biopsy; <7 vs. ≥7), stage (pTNMs, cTNMs or SEER summary staging [AARP]; localized vs. regional/distant), primary cancer treatment, and intervention arm (PLCO); and 2) a full model, additionally adjusted for race, marital status, body mass index (BMI, kg/m2), smoking status, history of cardiovascular disease and diabetes, history of screening for prostate cancer (AARP), and self-perceived general health status (AARP). Potential confounding variables were decided a priori. For missing data, we conducted study-specific multiple imputation using a sequence of regression models (24,25) via IVEware (26). The multiple imputation model included Nelson–Aalen estimators, event indicators, and variables that had less than 30% missing data from the two questionnaires with one exception for primary cancer treatment in AARP (31%). Five imputed datasets were created and analyzed individually, and then HR estimates were combined using PROC MIANALYZE in SAS. Therefore, no analytical sample was excluded in the main analysis.
In sensitivity analyses, we 1) used four levels of frequency (no/no regular, 1–3 times/month, 1–6 times/week, and daily use) to explore whether there was a non-linear relationship; 2) restricted to exclusive users who only took aspirin or non-aspirin NSAIDs in the past year; 3) delayed cohort entry for one year by excluding the first year of observation following prostate cancer diagnosis to account for potential reverse causation resulting from increasing use of NSAIDs for palliative care; 4) excluded men who answered the questionnaire within one year before prostate cancer diagnosis to evaluate potential reverse causation resulting from latent-tumor symptoms; and 5) updated exposure and covariate information from the most recent pre-diagnostic questionnaire for cases diagnosed after the last study-specific questionnaire (n=5,205/19,063 for AARP and n=1,113/7,827 for PLCO). Subgroup analyses were conducted by stage, Gleason score, self-perceived general health (AARP), and trial arms (PLCO). Neither sensitivity (except for the first analysis using four-level exposure) nor subgroup analyses were conducted in post-diagnostic cohorts due to small sample sizes. We also assessed whether the time interval between pre-diagnostic aspirin use and prostate cancer diagnosis modified relationships between aspirin use and PCSM. This analysis was not performed for non-aspirin NSAIDs due to small sample sizes. Statistical analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, NC). Two-sided P < 0.05 were considered statistically significant.
Lastly, we meta-analyzed study-specific fully adjusted risk estimates using fixed-effects models in Stata version 14 (Stata Corp., College Station, TX, USA).
Results
In pre-diagnostic cohorts, 709 of 3,640 (19%) deaths were due to prostate cancer in AARP with a median follow-up of 7 years, and 266 of 1,122 (24%) deaths were due to prostate cancer in PLCO with a median follow-up of 6 years. The median age at prostate cancer diagnosis was 71 years old (IQR=67–74) in AARP and 70 (IQR=66–74) in PLCO. Distributions of study-specific pre-diagnostic characteristics of prostate cancer cases are shown in Supplementary Table 1. Compared with AARP, prostate cancer cases in PLCO were more likely to be diagnosed with high-grade (Glean score ≥7) and localized tumors, to receive radical prostatectomy as the primary treatment, and to have a history of cardiovascular disease. Approximately 29% and 8% of cases reported pre-diagnostic daily aspirin and non-aspirin NSAID use in the past year in both cohorts, whereas more cases in AARP versus PLCO reported occasional use of aspirin (50% vs. 25%) and non-aspirin NSAIDs (46% vs. 17%).
Table 1 shows characteristics by status of pre-diagnostic NSAID use in the past year. Study-specific aspirin users versus nonusers were more likely to have prostate cancer screening in the past 3 years (AARP), be non-Hispanic white, have a history of cardiovascular disease, and be overweight. Study-specific non-aspirin NSAID users were more likely to be younger at prostate cancer diagnosis, undergo prostatectomy, and be overweight/obese. Distributions of characteristics by status of post-diagnostic NSAID use in the past year (Supplementary Table 2) mirrored those in the pre-diagnostic cohorts.
Table 1.
Characteristics of prostate cancer cases by status of NSAID use in the past year in the pre-diagnostic NIH-AARP and PLCO cohorts
| Characteristics | Aspirin use
|
Non-aspirin NSAID use
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AARP (N=19,063)
|
PLCO total (N=7,827)
|
AARP (N=19,063)
|
PLCO total (N=7,827)
|
|||||||||
| No, n (%) | Yes, n (%) |
P- value |
No, n (%) | Yes, n (%) |
P- value |
No, n (%) | Yes, n (%) |
P- value |
No, n (%) | Yes, n (%) |
P- value |
|
| Age at diagnosis (years) | NS | * | *** | *** | ||||||||
| 55–65 | 680 (17.0) | 2,609 (17.3) | 1,032 (28.7) | 1,093 (25.9) | 1,287 (14.6) | 2,002 (19.6) | 1,515 (25.7) | 610 (31.6) | ||||
| 66–69 | 1,181 (29.5) | 4,353 (28.9) | 906 (25.2) | 1,073 (25.4) | 2,472 (28.0) | 3,062 (29.9) | 1,513 (25.7) | 466 (24.1) | ||||
| 70–74 | 1,325 (33.0) | 5,052 (33.6) | 1,020 (28.3) | 1,270 (30.1) | 3,126 (35.4) | 3,251 (31.8) | 1,762 (29.9) | 528 (27.3) | ||||
| ≥75 | 824 (20.5) | 3,039 (20.2) | 643 (17.9) | 790 (18.7) | 1,953 (22.1) | 1,910 (18.7) | 1,106 (18.8) | 327 (16.9) | ||||
|
| ||||||||||||
| Years from pre-diagnostic exposure ascertainment to prostate cancer diagnosis | NS | NS | ** | NS | ||||||||
| <2 | 569 (14.2) | 2,165 (14.4) | 742 (20.6) | 887 (21.0) | 1,313 (14.9) | 1,421 (13.9) | 1,253 (21.3) | 376 (19.5) | ||||
| 2–4 | 1,055 (26.3) | 3,695 (24.5) | 921 (25.6) | 1,068 (25.3) | 2,280 (25.8) | 2,470 (24.2) | 1,476 (25.0) | 513 (26.6) | ||||
| ≥5 | 2,386 (59.5) | 9,193 (61.1) | 1,938 (53.8) | 2,271 (53.7) | 5,245 (59.3) | 6,334 (61.9) | 3,167 (53.7) | 1,042 (54.0) | ||||
|
| ||||||||||||
| Trial arm | NA | NS | NA | NS | ||||||||
| Intervention | 1,917 (53.2) | 2,236 (52.9) | 3,136 (53.2) | 1,017 (52.7) | ||||||||
| Control | 1,684 (46.8) | 1,990 (47.1) | 2,760 (46.8) | 914 (47.3) | ||||||||
|
| ||||||||||||
| Prostate cancer screening in the past three years | *** | NA | *** | NA | ||||||||
| No | 405 (10.1) | 1,199 (8.0) | 911 (10.3) | 693 (6.8) | ||||||||
| Yes | 3,512 (87.6) | 13,510 (89.7) | 7,715 (87.3) | 9,307 (91.0) | ||||||||
|
| ||||||||||||
| Gleason score a | NS | NS | NS | NS | ||||||||
| <7 | 2,573 (64.2) | 9,574 (63.6) | 1,953 (54.2) | 2,323 (55.0) | 5,631 (63.7) | 6,516 (63.7) | 3,229 (54.8) | 1,047 (54.2) | ||||
| ≥7 | 1,222 (30.5) | 4,724 (31.4) | 1,600 (44.4) | 1,845 (43.7) | 2,739 (31.0) | 3,207 (31.4) | 2,584 (43.8) | 861 (44.6) | ||||
|
| ||||||||||||
| Tumor stage b | NS | NS | NS | NS | ||||||||
| Localized | 2,776 (69.2) | 10,435 (69.3) | 3,125 (86.8) | 3,689 (87.3) | 6,143 (69.5) | 7,068 (69.1) | 5,136 (87.1) | 1,678 (86.9) | ||||
| Regional/Distant | 335 (8.4) | 1,195 (7.9) | 475 (13.2) | 534 (12.6) | 700 (7.9) | 830 (8.1) | 759 (12.9) | 250 (12.9) | ||||
|
| ||||||||||||
| Primary cancer treatment | * | ** | *** | *** | ||||||||
| No/Other | 373 (9.3) | 1383 (9.2) | 472 (13.1) | 555 (13.1) | 851 (9.6) | 905 (8.9) | 814 (13.8) | 213 (11.0) | ||||
| Prostatectomy | 944 (23.5) | 3,486 (23.2) | 1,436 (39.9) | 1,546 (36.6) | 1,954 (22.1) | 2,476 (24.2) | 2,177 (36.9) | 805 (41.7) | ||||
| Radiation only | 739 (18.4) | 2,682 (17.8) | 768 (21.3) | 882 (20.9) | 1,557 (17.6) | 1,864 (18.2) | 1,247 (21.1) | 403 (20.9) | ||||
| Hormone only | 215 (5.4) | 621 (4.1) | 228 (6.3) | 327 (7.7) | 431 (4.9) | 405 (4.0) | 434 (7.4) | 121 (6.3) | ||||
| Radiation and hormone | 540 (13.5) | 2,121 (14.1) | 677 (18.8) | 891 (21.1) | 1,307 (14.8) | 1,354 (13.2) | 1,185 (20.1) | 383 (19.8) | ||||
|
| ||||||||||||
| Race/ethnicity | *** | *** | *** | NS | ||||||||
| White, non-Hispanic | 3,614 (90.1) | 14,062 (93.4) | 3,124 (86.8) | 3,815 (90.3) | 8,137 (92.1) | 9,539 (93.3) | 5,210 (88.4) | 1,729 (89.5) | ||||
| Other | 356 (8.9) | 847 (5.6) | 475 (13.2) | 410 (9.7) | 614 (6.9) | 589 (5.8) | 684 (11.6) | 201 (10.4) | ||||
|
| ||||||||||||
| Marital status | * | NS | *** | NS | ||||||||
| Married or cohabiting | 3,437 (85.7) | 13,114 (87.1) | 3,067 (85.2) | 3,621 (85.7) | 7,586 (85.8) | 8,965 (87.7) | 5,034 (85.4) | 1,654 (85.7) | ||||
| Other | 557 (13.9) | 1,870 (12.4) | 528 (14.7) | 597 (14.1) | 1,214 (13.7) | 1,213 (11.9) | 851 (14.4) | 274 (14.2) | ||||
|
| ||||||||||||
| Self-reported general health status | NS | NA | NS | NA | ||||||||
| Excellent/very good | 2,371 (59.1) | 8,935 (59.4) | 5,273 (59.7) | 6,033 (59.0) | ||||||||
| Good | 1,273 (31.7) | 4,853 (32.2) | 2,783 (31.5) | 3,343 (32.7) | ||||||||
| Fair/poor | 317 (7.9) | 1,103 (7.3) | 674 (7.6) | 746 (7.3) | ||||||||
|
| ||||||||||||
| History of cardiovascular disease(s) c | *** | *** | NS | NS | ||||||||
| Never | 1,945 (48.5) | 6,521 (43.3) | 2,465 (68.5) | 2,199 (52.0) | 3,904 (44.2) | 4,562 (44.6) | 3,499 (59.3) | 1,165 (60.3) | ||||
| Ever | 1,647 (41.1) | 7,119 (47.3) | 1,116 (31.0) | 1,990 (47.1) | 4,127 (46.7) | 4,639 (45.4) | 2,362 (40.1) | 744 (38.5) | ||||
|
| ||||||||||||
| History of diabetes | NS | ** | NS | NS | ||||||||
| Never | 3,757 (93.7) | 14,087 (93.6) | 3,380 (93.9) | 3,892 (92.1) | 8,256 (93.4) | 9,588 (93.8) | 5,485 (93.0) | 1,787 (92.5) | ||||
| Ever | 253 (6.3) | 966 (6.4) | 204 (5.7) | 308 (7.3) | 582 (6.6) | 637 (6.2) | 385 (6.5) | 127 (6.6) | ||||
|
| ||||||||||||
| Body mass index (BMI, kg/m2) | ** | *** | *** | *** | ||||||||
| <25.0 | 1,363 (34.0) | 4,730 (31.4) | 1,074 (29.8) | 1,072 (25.4) | 3,046 (34.5) | 3,047 (29.8) | 1,692 (28.7) | 454 (23.5) | ||||
| 25.0–29.9 | 1,881 (46.9) | 7,511 (49.9) | 1,774 (49.3) | 2,187 (51.8) | 4,326 (48.9) | 5,066 (49.5) | 2,963 (50.3) | 998 (51.7) | ||||
| ≥30.0 | 692 (17.3) | 2,582 (17.2) | 713 (19.8) | 876 (20.7) | 1,314 (14.9) | 1,960 (19.2) | 1,146 (19.4) | 443 (22.9) | ||||
|
| ||||||||||||
| Smoking status | NS | *** | * | ** | ||||||||
| Never | 1,383 (34.5) | 5,039 (33.5) | 1,597 (44.3) | 1,619 (38.3) | 3,057 (34.6) | 3,365 (32.9) | 2,472 (41.9) | 744 (38.5) | ||||
| Quit ≥10 years | 1,782 (44.4) | 6,833 (45.4) | 1,352 (37.5) | 1,816 (43.0) | 3,901 (44.1) | 4,714 (46.1) | 2,322 (39.4) | 846 (43.8) | ||||
| Quit <10 years | 359 (9.0) | 1,382 (9.2) | 225 (6.2) | 325 (7.7) | 811 (9.2) | 930 (9.1) | 425 (7.2) | 125 (6.5) | ||||
| Current/Quit<1 year | 359 (9.0) | 1,300 (8.6) | 394 (10.9) | 430 (10.2) | 771 (8.7) | 888 (8.7) | 628 (10.7) | 196 (10.2) | ||||
Note: Column percentage does not add up to 100% due to missing values. Study-specific P-values were calculated by Chi-square tests using non-missing data.
NA, not available; NS, not statistically significant at 0.05;
P-value<0.05;
P-value<0.01;
P-value<0.001.
Gleason score was derived from prostatectomy or biopsy, of which information from prostatectomy was preferentially used if available.
Tumor stage was derived from pathological/clinical TNMs or summary stage (only available in AARP), of which pathological information was preferentially used followed by clinical information and then summary stage.
Cardiovascular diseases include any of the following: heart attack, stroke and hypertension.
Table 2 shows associations between pre-diagnostic NSAID use and mortality. Aspirin use was not statistically significantly associated with PCSM in AARP or PLCO cohorts. In AARP, occasional non-aspirin NSAID use was associated with 16% (HR=0.84; 95%CI=0.72 to 0.98) reduced PCSM, and a similar non-significant inverse association was found for daily non-aspirin NSAID use. However, such associations were not observed in PLCO. When we meta-analyzed results from the two studies, neither aspirin nor non-aspirin NSAIDs were statistically significantly associated with PCSM. Occasional use of aspirin and non-aspirin NSAIDs were associated with reduced all-cause mortality in AARP, but not in PLCO. Pooled risk estimates mirrored what were observed in AARP. Sensitivity analyses using four-level exposure resulted in similar associations, and no obvious non-linear pattern was observed (Supplementary Table 3). The inverse association between occasional use of non-aspirin NSAIDs and PCSM in AARP became null in sensitivity analyses restricted to exclusive users of such in the past year (Supplemental Table 4). Other sensitivity analyses using three-level exposure, including delaying cohort entry for one year, excluding cases with exposure collected within one year before diagnosis, and using exposure and covariate information from the most recent pre-diagnostic questionnaire, yielded similar results (results not tabulated). The inverse associations of occasional aspirin and non-aspirin NSAID use with all-cause mortality were only restricted to localized (Supplementary Table 5) or less aggressive (Supplementary Table 6) prostate cancers. Subgroup analyses by self-perceived general health (AARP) and screen arm (PLCO) did not materially alter results (results not tabulated).
Table 2.
Frequency of pre-diagnostic NSAID use in relation to mortality in NIH-AARP and PLCO
| Aspirin
|
Non-aspirin NSAID
|
|||||
|---|---|---|---|---|---|---|
| None | Occasional | Daily | None | Occasional | Daily | |
| Prostate cancer-specific mortality | ||||||
|
| ||||||
| AARP | ||||||
| No. of deaths, n=709 | 161 | 332 | 216 | 374 | 286 | 49 |
| Model 1, HRs (95%CIs) a | ref | 0.94 (0.78, 1.14) | 1.05 (0.85, 1.29) | ref | 0.83 (0.71, 0.97) | 0.80 (0.59, 1.08) |
| Model 2, HRs (95%CIs) b | ref | 0.95 (0.78, 1.15) | 0.99 (0.80, 1.22) | ref | 0.84 (0.72, 0.98) | 0.77 (0.57, 1.05) |
| PLCO total | ||||||
| No. of deaths, n=266 | 122 | 68 | 76 | 202 | 40 | 24 |
| Model 1, HRs (95%CIs) c | ref | 1.15 (0.85, 1.55) | 0.99 (0.74, 1.32) | ref | 1.09 (0.77, 1.54) | 1.19 (0.78, 1.83) |
| Model 2, HRs (95%CIs) d | ref | 1.15 (0.85, 1.55) | 0.98 (0.72, 1.32) | ref | 1.02 (0.72, 1.44) | 1.07 (0.69, 1.65) |
|
| ||||||
| Pooled HRs (95%CIs) e | ref | 1.00 (0.85, 1.18) | 0.98 (0.83, 1.17) | ref | 0.87 (0.75, 1.00) | 0.86 (0.67, 1.10) |
|
| ||||||
| All-cause mortality | ||||||
|
| ||||||
| AARP | ||||||
| No. of deaths, n=3640 | 834 | 1534 | 1272 | 1850 | 1458 | 332 |
| Model 1, HRs (95%CIs) a | ref | 0.82 (0.76, 0.90) | 1.08 (0.99, 1.18) | ref | 0.88 (0.82, 0.95) | 1.11 (0.98, 1.24) |
| Model 2, HRs (95%CIs) b | ref | 0.87 (0.80, 0.95) | 0.97 (0.89, 1.07) | ref | 0.90 (0.84, 0.96) | 1.01 (0.90, 1.14) |
| PLCO total | ||||||
| No. of deaths, n=1122 | 476 | 259 | 387 | 856 | 169 | 97 |
| Model 1, HRs (95%CIs) c | ref | 1.06 (0.91, 1.23) | 1.22 (1.07, 1.40) | ref | 1.00 (0.84, 1.18) | 1.11 (0.90, 1.37) |
| Model 2, HRs (95%CIs) d | ref | 1.03 (0.89, 1.20) | 1.11 (0.97, 1.28) | ref | 1.00 (0.84, 1.18) | 1.07 (0.87, 1.33) |
|
| ||||||
| Pooled HRs (95%CIs) e | ref | 0.91 (0.84, 0.98) | 1.01 (0.94, 1.09) | ref | 0.91 (0.85, 0.97) | 1.02 (0.92, 1.14) |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference.
Model 1 used age as the time-metric, adjusted for Gleason score (<7 vs. ≥7), tumor stage (localized vs. regional/distant), and primary treatment (no curative treatment, prostatectomy, radiation only, hormone treatment only, radiation and hormone treatment, and other).
Model 2 additionally adjusted for race (non-Hispanic whites vs. other), marital status (married/cohabiting vs. other), history of cardiovascular disease (ever vs. never), history of diabetes (ever vs. never), BMI (<25, 25–29, and ≥30 kg/m2), smoking status (never, former & quit≥10 years, former & quit<10 years, and current & quit<1 year), history of prostate cancer screening (ever vs. never), and self-reported general health status (excellent/very good, good, and fair or poor).
Model 1 used age as the time-metric, adjusted for Gleason score (<7 vs. ≥7), tumor stage (localized vs. regional/distant), primary treatment (prostatectomy, radiation only, hormone treatment only, radiation and hormone treatment, and other), and trial arm (screening vs. standard of care).
Model 2 additionally adjusted for race (non-Hispanic whites vs. other), marital status (married/cohabiting vs. other), history of cardiovascular disease (ever vs. never), history of diabetes (ever vs. never), BMI (<25, 25–29, and ≥30 kg/m2), and smoking status (never, former & quit≥10 years, former & quit<10 years, and current & quit<1 year).
Pooled HRs and 95%CIs were computed using study-specific results from Model #2 in fixed-effects models.
Table 3 shows associations between pre-diagnostic aspirin use and mortality within strata of the time interval from exposure ascertainment to prostate cancer diagnosis. The study-specific multiplicative interactions between the time interval and exposure were statistically significant in AARP (PFisher's method<0.001) and PLCO (P Fisher's method<0.03). In AARP, occasional use within 2 years before diagnosis was positively associated with PCSM (HR=1.38; 95%CI=1.06 to 1.82), while occasional (HR=0.80; 95%CI=0.63 to 1.00) and daily (HR=0.91; 95%CI=0.70 to 1.19) aspirin use ≥5 years before diagnosis appeared inversely associated. Similarly, daily users diagnosed ≥5 years after exposure ascertainment tended to have reduced PCSM (HR=0.62; 95%CI=0.39 to 1.01) in PLCO, although such association was not observed among occasional users. For all-cause mortality, consistent inverse associations were observed across two studies among aspirin users ≥5 years before prostate cancer diagnosis with statistically significant study-specific multiplicative interactions (PFisher's method<0.001) between the time interval and the exposure. When we meta-analyzed results from two studies, occasional (HR=0.82; 95%CI=0.75 to 0.90) and daily (HR=0.85; 95%CI=0.77 to 0.94) aspirin use ≥5 years before prostate cancer diagnosis were associated with reduced all-cause mortality.
Table 3.
Frequency of pre-diagnostic aspirin use in relation to mortality within strata of the time interval from exposure ascertainment to prostate cancer diagnosis in NIH-AARP and PLCO
| Time interval from exposure ascertainment to prostate cancer diagnosis (year)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <2 years
|
2–4 years
|
≥ 5 years
|
|||||||
| None | Occasional | Daily | None | Occasional | Daily | None | Occasional | Daily | |
| Prostate-specific mortality | |||||||||
|
| |||||||||
| AARP | |||||||||
| No. of deaths, n=709 | 34 | 92 | 53 | 52 | 84 | 66 | 75 | 156 | 97 |
| Model 1, HRs (95%CIs) a | ref | 1.38 (1.05, 1.80) | 1.16 (0.84, 1.62) | ref | 0.96 (0.73, 1.27) | 1.17 (0.88, 1.57) | ref | 0.79 (0.63, 0.99) | 0.94 (0.72, 1.22) |
| Model 2, HRs (95%CIs) b | ref | 1.38 (1.06, 1.82) | 1.00 (0.72, 1.40) | ref | 0.96 (0.73, 1.27) | 1.12 (0.83, 1.50) | ref | 0.80 (0.63, 1.00) | 0.91 (0.70, 1.19) |
| PLCO total | |||||||||
| No. of deaths, n=266 | 45 | 18 | 30 | 42 | 20 | 25 | 35 | 30 | 21 |
| Model 1, HRs (95%CIs) c | ref | 0.99 (0.60, 1.64) | 1.29 (0.86, 1.93) | ref | 1.23 (0.76, 1.98) | 1.16 (0.75, 1.79) | ref | 1.23 (0.82, 1.84) | 0.66 (0.41, 1.05) |
| Model 2, HRs (95%CIs) d | ref | 1.03 (0.62, 1.71) | 1.32 (0.87, 2.00) | ref | 1.23 (0.76, 1.99) | 1.16 (0.74, 1.80) | ref | 1.19 (0.79, 1.79) | 0.62 (0.39, 1.01) |
|
| |||||||||
| Pooled HRs (95%CIs) e | ref | 1.30 (1.02, 1.65) | 1.12 (0.86, 1.45) | ref | 1.02 (0.80, 1.30) | 1.13 (0.88, 1.44) | ref | 0.87 (0.72, 1.07) | 0.83 (0.66, 1.05) |
|
| |||||||||
| All-cause mortality | |||||||||
|
| |||||||||
| AARP | |||||||||
| No. of deaths, n=3640 | 205 | 376 | 310 | 285 | 451 | 444 | 344 | 707 | 518 |
| Model 1, HRs (95%CIs) a | ref | 1.05 (0.92, 1.18) | 1.22 (1.07, 1.39) | ref | 0.80 (0.71, 0.90) | 1.17 (1.04, 1.31) | ref | 0.75 (0.68, 0.83) | 0.95 (0.85, 1.06) |
| Model 2, HRs (95%CIs) b | ref | 1.08 (0.96, 1.22) | 1.06 (0.93, 1.21) | ref | 0.85 (0.76, 0.96) | 1.05 (0.93, 1.18) | ref | 0.81 (0.73, 0.89) | 0.88 (0.79, 0.99) |
| PLCO total | |||||||||
| No. of deaths, n=1122 | 171 | 91 | 157 | 158 | 83 | 132 | 147 | 85 | 98 |
| Model 1, HRs (95%CIs) c | ref | 1.27 (1.02, 1.60) | 1.60 (1.33, 1.92) | ref | 1.08 (0.85, 1.36) | 1.32 (1.09, 1.60) | ref | 0.89 (0.70, 1.12) | 0.81 (0.65, 1.01) |
| Model 2, HRs (95%CIs) d | ref | 1.16 (0.92, 1.45) | 1.47 (1.22, 1.77) | ref | 1.06 (0.84, 1.34) | 1.18 (0.97, 1.44) | ref | 0.91 (0.72, 1.15) | 0.75 (0.60, 0.94) |
|
| |||||||||
| Pooled HRs (95%CIs) e | ref | 1.10 (0.99, 1.22) | 1.18 (1.06, 1.32) | ref | 0.89 (0.80, 0.99) | 1.08 (0.98, 1.20) | ref | 0.82 (0.75, 0.90) | 0.85 (0.77, 0.94) |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference.
Model 1 used age as the time-metric, adjusted for Gleason score (<7 vs. ≥7), tumor stage (localized vs. regional/distant), and primary treatment (no curative treatment, prostatectomy, radiation only, hormone treatment only, radiation and hormone treatment, and other).
Model 2 additionally adjusted for race (Non-Hispanic whites vs. other), marital status (married/cohabiting vs. other), history of cardiovascular disease (ever vs. never), history of diabetes (ever vs. never), BMI (<25, 25–29, and ≥30 kg/m2), smoking status (never, former & quit≥10 years, former & quit<10 years, and current & quit<1 year), history of prostate cancer screening (ever vs. never), and self-reported general health status (excellent/very good, good, and fair or poor).
Model 1 age as the time-metric, adjusted for Gleason score (<7 vs. ≥7), tumor stage (localized vs. regional/distant), primary treatment (prostatectomy, radiation only, hormone treatment only, radiation and hormone treatment, and other), and trial arm (screening vs. standard of care).
Model 2 additionally adjusted for race (Non-Hispanic whites vs. other), marital status (married/cohabiting vs. other), history of cardiovascular disease (ever vs. never), history of diabetes (ever vs. never), BMI (<25, 25–29, and ≥30 kg/m2), and smoking status (never, former & quit≥10 years, former & quit<10 years, and current & quit<1 year).
Pooled HRs and 95%CIs were computed using study-specific results from Model #2 in fixed-effects models.
Table 4 shows associations between post-diagnostic NSAID use and mortality. In AARP, aspirin use was consistently associated with reduced PCSM and all-cause mortality— with or without adjustment for pre-diagnostic aspirin use, although only all-cause mortality reached statistical significance. In PLCO, non-significant inverse associations were also seen between aspirin use and all-cause mortality, but not for PCSM. When we meta-analyzed study-specific results, occasional and daily aspirin use after diagnosis were associated with 17% (HR=0.83; 95%CI=0.72 to 0.95) and 25% (HR=0.75; 95%CI=0.66 to 0.86) reduced all-cause mortality, respectively, independent of pre-diagnostic aspirin use (occasional: HR= 0.93; 95%CI=0.82 to 1.07; daily: HR=1.05; 95%CI=0.91 to 1.20). Comparing with associations of aspirin, associations between non-aspirin NSAID use and all-cause mortality were weaker and did not reach statistical significance. Sensitivity analyses using four-level exposure resulted in similar associations and provided no obvious non-linear pattern (Supplementary Table 7).
Table 4.
Frequency of post-diagnostic NSAID use in relation to mortality in NIH-AARP and PLCO
| Aspirin
|
Non-aspirin NSAID
|
|||||
|---|---|---|---|---|---|---|
| None | Occasional | Daily | None | Occasional | Daily | |
| Prostate cancer-specific mortality | ||||||
|
| ||||||
| AARP | ||||||
| No. of deaths, n=209 | 62 | 59 | 88 | 125 | 71 | 13 |
| Model 1, HRs (95%CIs) a | ref | 0.85 (0.59, 1.22) | 0.81 (0.58, 1.12) | ref | 0.97 (0.72, 1.30) | 0.87 (0.49, 1.54) |
| Model 2, HRs (95%CIs) b | ref | 0.91 (0.63, 1.31) | 0.82 (0.58, 1.16) | ref | 0.95 (0.70, 1.28) | 0.74 (0.41, 1.33) |
| Model 3, HRs (95%CIs) c | ref | 0.87 (0.60, 1.27) | 0.77 (0.54, 1.11) | ref | 0.97 (0.71, 1.33) | 0.78 (0.43, 1.43) |
| PLCO total | ||||||
| No. of deaths, n=35 | 5 | 15 | 15 | 19 | 12 | 4 |
| Model 1, HRs (95%CIs) d | ref | 1.44 (0.51, 4.03) | 1.30 (0.47, 3.61) | ref | 1.58 (0.75, 3.30) | 2.53 (0.84, 7.62) |
| Model 2, HRs (95%CIs) e | ref | 1.56 (0.55, 4.40) | 1.27 (0.44, 3.63) | ref | 1.63 (0.78, 3.43) | 2.22 (0.71, 6.94) |
| Model 3, HRs (95%CIs) f | ref | 1.52 (0.53, 4.33) | 1.26 (0.43, 3.67) | ref | 1.65 (0.77, 3.50) | 2.35 (0.74, 7.51) |
|
| ||||||
| Pooled HRs (95%CIs) g | ref | 0.93 (0.65, 1.33) | 0.81 (0.58, 1.14) | ref | 1.05 (0.79, 1.40) | 0.99 (0.58, 1.68) |
|
| ||||||
| All-cause mortality | ||||||
|
| ||||||
| AARP | ||||||
| No. of deaths, n=1326 | 397 | 344 | 585 | 808 | 425 | 93 |
| Model 1, HRs (95%CIs) a | ref | 0.78 (0.67, 0.90) | 0.80 (0.70, 0.91) | ref | 0.91 (0.81, 1.02) | 1.02 (0.82, 1.26) |
| Model 2, HRs (95%CIs) b | ref | 0.83 (0.72, 0.96) | 0.77 (0.67, 0.87) | ref | 0.92 (0.82, 1.04) | 0.90 (0.72, 1.12) |
| Model 3, HRs (95%CIs) c | ref | 0.85 (0.73, 0.98) | 0.75 (0.66, 0.86) | ref | 0.94 (0.83, 1.06) | 0.89 (0.71, 1.12) |
| PLCO total | ||||||
| No. of deaths, n=208 | 47 | 65 | 96 | 138 | 53 | 17 |
| Model 1, HRs (95%CIs) d | ref | 0.74 (0.51, 1.08) | 0.86 (0.61, 1.23) | ref | 0.97 (0.71, 1.33) | 1.19 (0.71, 1.97) |
| Model 2, HRs (95%CIs) e | ref | 0.73 (0.50, 1.06) | 0.76 (0.53, 1.09) | ref | 1.01 (0.73, 1.39) | 1.27 (0.76, 2.12) |
| Model 3, HRs (95%CIs) f | ref | 0.72 (0.49, 1.05) | 0.76 (0.52, 1.10) | ref | 0.97 (0.70, 1.34) | 1.27 (0.76, 2.14) |
|
| ||||||
| Pooled HRs (95%CIs) g | ref | 0.83 (0.72, 0.95) | 0.75 (0.66, 0.86) | ref | 0.94 (0.84, 1.06) | 0.94 (0.77, 1.16) |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference.
Model 1 used age as the time-metric, adjusted for Gleason score (<7 vs. ≥7), tumor stage (localized vs. regional/distant), and primary treatment (no curative treatment, prostatectomy, radiation only, hormone treatment only, radiation and hormone treatment, and other).
Model 2 additionally adjusted for race (Non-Hispanic whites vs. other), marital status (married/cohabiting vs. other), history of cardiovascular disease (ever vs. never), history of diabetes (ever vs. never), BMI (<25, 25–29, and ≥30 kg/m2), smoking status (never, former & quit≥10 years, former & quit<10 years, and current & quit<1 year), history of prostate cancer screening (ever vs. never), and self-reported general health status (excellent/very good, good, and fair or poor).
Model 3 additionally adjusted for pre-diagnostic aspirin or non-aspirin NSAID use, respectively, from Risk Factor Questionnaire.
Model 1 used age as the time-metric, adjusted for Gleason score (<7 vs. ≥7), tumor stage (localized vs. regional/distant), primary treatment (prostatectomy, radiation only, hormone treatment only, radiation and hormone treatment, and other), and trial arm (screening vs. standard of care).
Model 2 additionally adjusted for race (Non-Hispanic whites vs. other), marital status (married/cohabiting vs. other), history of cardiovascular disease (ever vs. never), history of diabetes (ever vs. never), BMI (<25, 25–29, and ≥30 kg/m2), and smoking status (never, former & quit≥10 years, former & quit<10 years, and current & quit<1 year).
Model 3 additionally adjusted for pre-diagnostic aspirin or non-aspirin NSAID use, respectively, from Baseline Questionnaire for Men.
Pooled HRs and 95%CIs were computed using study-specific results from Model #3 in fixed-effects models
Discussion
Across two large prospective cohort studies of prostate cancer survivors, we did not find statistically significant associations of pre- or post-diagnostic NSAIDs with PCSM. However, aspirin use before (≥five years) and after prostate cancer diagnosis was associated with an approximately 20% reduced all-cause mortality without evidence of a dose-response relationship.
This study adds to the limited evidence of survival benefits of pre-diagnostic NSAID use among prostate cancer survivors. Few studies have investigated pre-diagnostic aspirin use, and even fewer have examined non-aspirin NSAID use. Four studies have reported null associations of aspirin or non-aspirin NSAIDs with PCSM (13–16), and agree with what we found in AARP and PLCO. However, all of these studies had fewer outcomes comparing with this analysis, three restricted to non-metastatic cases only (13–15), and two overlooked over-the-counter NSAID use (13,16). Importantly, these studies have failed to evaluate whether the time interval from exposure ascertainment to prostate cancer diagnosis modifies survival benefits among prostate cancer survivors. Among cancer-free volunteers, a pooled analysis of 7 randomized clinical trials of daily aspirin for prevention of vascular events has demonstrated a delayed protective effect of aspirin on overall cancer death with 5 years of follow-up and a non-significant inverse association with PCSM based on 37 deaths (27). Our study suggests that—for pre-diagnostic exposure—only prolonged (≥ 5 years) aspirin use provides a survival benefit in men with prostate cancer, as supported by consistently reduced mortality due to prostate cancer and all causes, although the association for PCSM did not surpass the nominal statistical significance threshold.
Seven studies have investigated post-diagnostic NSAID use following prostate cancer diagnosis with inconsistent results (14,16–21). Two prescription-based studies in the UK Clinical Practice Research Datalink (aspirin: HR=1.46; 95% CI=1.29 to 1.65) (17) and the Finnish Prostate Cancer Screening Trial (non-aspirin NSAIDs: HR=2.09; 95%CI=1.75 to 2.50) (16) reported that post-diagnostic aspirin or non-aspirin NSAIDs were associated with increased PCSM. However, such associations may be explained by reverse causation from increasing use of NSAIDs in disease progression or palliative care, as the positive association diminished when cohort entry was delayed for 3 years in the Finnish study. In contrast, a study in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) found that ever aspirin use during follow-up after diagnosis with localized prostate cancer was associated with decreased PCSM (HR=0.43, 95%CI=0.21, 0.87) (19), yet this result is limited by survival bias whereby cases who lived longer were more likely to be classified as being exposed. A more recent analysis of the Physicians’ Health Study assessed time-varying regular aspirin use (>3 days/week for ≥1 year) after diagnosis of non-metastatic prostate cancer in relation to PCSM (n=315) and all-cause mortality, yet reported contradictory results for patients who stopped using aspirin (past users; PCSM: HR=1.51, 95%=1.06 to 2.16; all-cause: HR=1.28, 95%CI=1.08 to 1.53) versus those who were using aspirin (current users; PCSM: HR=0.66, 95%CI=0.48 to 0.90; all-cause: HR=0.72, 95%CI=0.61 to 0.84) immediately before the corresponding outcome (21). Similar to our findings, three studies reported null results despite some having more outcomes (14,18,20); one of these in the Cancer Prevention Study-II (CPS-II) reported a reduction in PCSM among high-risk, non-metastatic cases (≥T3 and/or Gleason score ≥8; HR=0.60, 95% CI=0.37 to 0.97) (14) but not in low-risk cases. However, we did not find an inverse association with comparable sample size when restricting to high-grade (Gleason score≥7), high-stage (regional/distant) cases in AARP (daily: HR=0.98; 95%CI=0.51 to 1.86). It remains unclear whether the differences are a result of chance due to multiple comparisons in posthoc analyses or differential time windows of exposure; the CPS-II study used aspirin information one year after diagnosis versus 3 years in AARP, on average. Nevertheless, we found post-diagnostic aspirin use was consistently associated with reduced all-cause mortality in AARP and PLCO. This observation was supported by another analysis in CaPSURE among cases who underwent radical prostatectomy and radiotherapy (28).
Limitations of this study include NSAIDs being self-reported and thus subject to recall accuracy. The limited time-points of exposure ascertainment increases the likelihood of misclassification, yet this should be non-differential for pre-diagnostic exposures given the prospective designs, and the true association may be underestimated. Conversely, post-diagnostic exposures may be affected by severity and treatment of the disease, although distributions of tumor characteristics and primary treatment by exposure status did not differ substantially (Supplementary Table 2) and we adjusted for these factors in all statistical models. Second, there were slight differences in exposure ascertainment including the referent group for pre-diagnostic analysis (none vs. no regular use) and patients’ characteristics between PLCO and AARP (Supplementary Table 1), which may partially explain inconsistencies in associations of occasional and daily use despite adjustment for tumor characteristics and history of cardiovascular disease. Third, we lacked dose information of NSAIDs, although the CPS-II study did not support a dose-response relationship of pre- or post-diagnostic daily aspirin use with PCSM (14). Fourth, we lacked indications for NSAID use, which may share risk factors with prostate cancer survival. Although we adjusted for history of cardiovascular disease and metabolic conditions, residual confounding may have attenuated estimated associations. Lastly, the analytic sample is predominantly non-Hispanic white, and the role of aspirin or non-aspirin NSAIDs among African American prostate cancer survivors is unclear. A small analysis among African American prostate cancer cases treated with radiotherapy reported a reduced risk of distant metastases among aspirin users (29). A recent case-control study supported such a finding reporting a reduced risk of T3/T4 prostate cancer among daily or long-term (>3 years) regular aspirin users, and tentatively longer survival time to disease recurrence for African American men (30).
In conclusion, aspirin or non-aspirin NSAIDs were not associated with prostate cancer survival, despite a nonsignificant inverse association of aspirin use ≥five years before prostate cancer diagnosis. Conversely, overall survival benefits among aspirin users were observed regardless of whether the exposure was pre- or post-diagnostic, highlighting the importance of prevention of comorbidities among prostate cancer survivors. However, the use of aspirin needs to be considered in light of the potential adverse effects, such as gastrointestinal bleeding and hemorrhagic stroke.
Supplementary Material
Acknowledgments
Funding Sources: This research was supported by the Intramural Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
We are indebted to the participants in the NIH-AARP and PLCO cohorts for their outstanding cooperation. The authors also thank Jerome Mabie and Dave S. Campbell at Information Management Services, Inc. for data management and support. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Abbreviations
- BMI
Body Mass Index
- BQM
Baseline Questionnaire for Men
- CaPSURE
Cancer of the Prostate Strategic Urologic Research Endeavor
- CI
Confidence Interval
- COX
Cyclooxygenase
- CPS-II
Cancer Prevention Study-II
- FUQ
Follow-up Questionnaire
- HR
Hazard Ratio
- IQR
Interquartile Range
- NSAID
Nonsteroidal Anti-inflammatory Drug
- PCSM
Prostate Cancer-Specific Mortality
- PLCO
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
- PSA
Prostate-Specific Antigen
- RFQ
Risk Factor Questionnaire
- SQX
Supplemental Questionnaire
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 2.World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report. Diet, Nutrition, Physical Activity, and Prostate Cancer. Available: www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf2014.
- 3.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J Natl Cancer I. 2002;94(4):252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 4.Lange PH, Vessella RL. Mechanisms, Hypotheses and Questions Regarding Prostate Cancer Micrometastases to Bone. Cancer and Metastasis Reviews. 1998;17(4):331–6. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 5.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nature reviews Cancer. 2011;11(2):123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer. 2009;124(8):1773–7. doi: 10.1002/ijc.24016. [DOI] [PubMed] [Google Scholar]
- 7.Helley D, Banu E, Bouziane A, Banu A, Scotte F, Fischer AM, et al. Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol. 2009;56(3):479–84. doi: 10.1016/j.eururo.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56(4):671–6. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, et al. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64(9):3334–43. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- 10.Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol. 2000;164(3 Pt 1):820–5. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 11.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(7 Pt 1):2172–7. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24(18):2723–8. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 13.Flahavan EM, Bennett K, Sharp L, Barron TI. A cohort study investigating aspirin use and survival in men with prostate cancer. Ann Oncol. 2014;25(1):154–9. doi: 10.1093/annonc/mdt428. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs EJ, Newton CC, Stevens VL, Campbell PT, Freedland SJ, Gapstur SM. Daily aspirin use and prostate cancer-specific mortality in a large cohort of men with nonmetastatic prostate cancer. J Clin Oncol. 2014;32(33):3716–22. doi: 10.1200/JCO.2013.54.8875. [DOI] [PubMed] [Google Scholar]
- 15.Stock DC, Groome PA, Siemens DR, Rohland SL, Song Z. Effects of non-selective non-steroidal anti-inflammatory drugs on the aggressiveness of prostate cancer. The Prostate. 2008;68(15):1655–65. doi: 10.1002/pros.20834. [DOI] [PubMed] [Google Scholar]
- 16.Veitonmaki T, Murtola TJ, Maattanen L, Taari K, Stenman UH, Tammela TL, et al. Use of non-steroidal anti-inflammatory drugs and prostate cancer survival in the finnish prostate cancer screening trial. The Prostate. 2015;12(10):23020. doi: 10.1002/pros.23020. [DOI] [PubMed] [Google Scholar]
- 17.Assayag J, Pollak MN, Azoulay L. The Use of Aspirin and the Risk of Mortality in Patients with Prostate Cancer. J Urol. 2014;14(14):04863–0. doi: 10.1016/j.juro.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Cardwell CR, Flahavan EM, Hughes CM, Coleman HG, O'Sullivan JM, Powe DG, et al. Low-dose aspirin and survival in men with prostate cancer: a study using the UK Clinical Practice Research Datalink. Cancer Cause Control. 2014;25(1):33–43. doi: 10.1007/s10552-013-0306-x. [DOI] [PubMed] [Google Scholar]
- 19.Choe KS, Cowan JE, Chan JM, Carroll PR, D'Amico AV, Liauw SL. Aspirin Use and the Risk of Prostate Cancer Mortality in Men Treated With Prostatectomy or Radiotherapy. J Clin Oncol. 2012;30(28):3540–4. doi: 10.1200/JCO.2011.41.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Aspirin Use after a Prostate Cancer Diagnosis and Cancer Survival in a Prospective Cohort. Cancer Prev Res. 2012;5(10):1223–8. doi: 10.1158/1940-6207.CAPR-12-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downer MK, Allard CB, Preston MA, Gaziano JM, Stampfer MJ, Mucci LA, et al. Regular Aspirin Use and the Risk of Lethal Prostate Cancer in the Physicians' Health Study. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 22.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. American journal of epidemiology. 2001;154(12):1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 23.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled clinical trials. 2000;21(6 Suppl):273s–309s. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Raghunathan TE, Lepkowski JM, Hoewyk J, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 25.White IR, Royston P. Imputing missing covariate values for the Cox model. Statistics in medicine. 2009;28(15):1982–98. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raghunathan TE, Solenberger P, Hoewyk JV. IVEware: Imputation and Variance Estimation Software Survey Methodology Program. Survey Research Center, Institute for Social Research, University of Michigan; [Google Scholar]
- 27.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 28.Katz MS, Carroll PR, Cowan JE, Chan JM, D'Amico AV. Association of statin and nonsteroidal anti-inflammatory drug use with prostate cancer outcomes: results from CaPSURE. Bju Int. 2010;106(5):627–32. doi: 10.1111/j.1464-410X.2010.09232.x. [DOI] [PubMed] [Google Scholar]
- 29.Osborn VW, Chen SC, Weiner J, Schwartz D, Schreiber D. Impact of aspirin on clinical outcomes for African American men with prostate cancer undergoing radiation. Tumori. 2016;102(1):65–70. doi: 10.5301/tj.5000424. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Dorsey TH, Tang W, Jordan SV, Loffredo CA, Ambs S. Aspirin Use Reduces the Risk of Aggressive Prostate Cancer and Disease Recurrence in African-American Men. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


