Abstract
OBJECTIVES
The increasing use of complementary and alternative medicines (CAMs) has been associated with a rising incidence of CAM-induced drug-induced liver injury (DILI). The aim of this study was to examine the clinical features and outcomes among patients with acute liver failure (ALF) and acute liver injury (ALI) enrolled in the Acute Liver Failure Study Group database, comparing CAM-induced with prescription medicine (PM)-induced DILI.
METHODS
A total of 2,626 hospitalized patients with ALF/ALI of any etiology were prospectively enrolled between 1998 and 2015 from 32 academic transplant centers. Only those with CAM or PM-induced ALI/ALF were selected for analysis.
RESULTS
A total of 253 (9.6%) subjects were found to have idiosyncratic DILI, of which 41 (16.3%) were from CAM and 210 (83.7%) were due to PM. The fraction of DILI-ALF/ALI cases due to CAM increased from 1998–2007 to 2007–2015 (12.4 vs. 21.1%, P=0.047). There was no difference in the type of liver injury—hepatocellular, cholestatic, or mixed—between groups as determined by R score (P=0.26). PM-induced DILI showed higher serum alkaline phosphatase levels compared with the CAM group (median IU/L, 171 vs. 125, P=0.003). The CAM population had fewer comorbid conditions (1.0 vs. 2.0, P<0.005), higher transplantation rates (56 vs. 32%, P<0.005), and a lower ALF-specific 21-day transplant-free survival (17 vs. 34%, P=0.044).
CONCLUSIONS
CAM-induced DILI is at least as severe in presentation as that observed due to PM with higher rates of transplantation and lower transplant-free survival in those who progress to ALF. This study highlights the increasing incidence of CAM-induced liver injury and emphasizes the importance of early referral and evaluation for liver transplantation when CAM-induced liver injury is suspected.
INTRODUCTION
Idiosyncratic drug-induced liver injury (DILI) is a relatively rare event with an estimated incidence of 14–19 events per 100,000 individuals (1,2). It accounts for 13% of all cases of acute liver failure (ALF) in the United States, second only to acetaminophen-induced liver failure (3). DILI manifests in a variety of clinical presentations and is associated with a 13–17% risk of unresolved or chronic injury at 6 months, a 2–4% transplantation rate, and a 6–8% mortality rate (4,5). For those who progress to ALF, transplant-free survival at 3 weeks is only 27.1% with an overall survival rate of 66.2% (6). Hundreds of prescription medications (PMs) have been implicated as causing DILI, with antimicrobials being the most frequently implicated group (4,6,7). Complementary and alternative medications (CAMs) including multivitamins, herbals, dietary supplements, bodybuilding agents, and weight loss supplements are the second most common category of agents responsible for DILI (5,6). A recent population-representative study found that CAM accounts for nearly one in five of all cases of DILI (8).
Although PM must undergo vigorous clinical trials evaluating medication safety and are subject to strict US Food and Drug Administration (FDA) regulation, CAMs, on the other hand, are subject to less stringent regulation. These agents are regulated under the Dietary Supplement Health and Education Act of 1994 and the Final Rule for Current Good Manufacturing Practices for Dietary Supplements of 2007. Manufacturers are responsible for ensuring the safety of, labeling of, and purity of their products without any requirement to prove efficacy (9). CAM agents can be marketed at any concentration, as long as daily recommended values (if applicable) are on the label, and often have significant product-to-product variability (10,11). Contaminants can be often found in dietary supplements with reports of DILI attributed to these medications (12–14).
In recent years, the use of CAM has been rising as nearly half of the US population reports using at least one dietary supplement (15–17). This increase has been associated with a corresponding rise in the incidence of DILI attributed to these agents from 7 to 20% between 2004 and 2012 (18). This same study showed that CAM that was not body building supplements typically presented as severe hepatocellular injury and required transplantation more frequently when compared with PM (13 vs. 3%) (18). The high liver transplantation rate from this study suggests that CAM-induced DILI may be more severe than PM-induced DILI.
The Acute Liver Failure Study Group (ALFSG) was established in 1997 to prospectively study patients with all causes of ALF, and expanded their registry in 2008 to include those with acute liver injury (ALI) to better understand the epidemiology of disease and to elucidate the pathogenesis, mechanism, and outcomes of liver injury. The purpose of this study was to better characterize CAM-induced liver injury using data from the US ALFSG, and compare this type of liver injury with that caused by PM.
METHODS
Study design
From 20 January 1998 to 1 April 2015, 2,626 subjects meeting entry criteria for ALF or ALI were prospectively enrolled at up to 32 academic liver transplant centers participating in the National Institutes of Health (NIH)-funded ALFSG (see Supplementary Methods online for complete listing). All patients with ALI were enrolled after September 2008, when the ALFSG began collecting these data. Written informed consent was obtained from patients with ALI or their next-of-kin for those with ALF. All centers complied with their local Institutional Review Boards’ requirements and the Health Insurance Portability and Accountability Act (HIPAA).
Inclusion criteria
Patients were at least 18 years of age at the time of enrollment. All patients were hospitalized. ALF was defined as hepatic encephalopathy (altered mentation to any degree) and moderately severe coagulopathy (international normalized ratio (INR) ≥1.5) and acute onset of illness <26 weeks; whereas ALI was defined as acute hepatic illness of <26 weeks with INR ≥2.0, alanine aminotransferase (ALT) ≥10× upper limit of normal, total bilirubin ≥3.0 mg/dl, and the absence of hepatic encephalopathy.
The principal investigator at each site was responsible for collecting a detailed history including demographic data, medical history, social history, and medication history but not limited to prescription drugs, over-the-counter medications, dietary supplements, herbal supplements, xenobiotics, CAM, and illicit substances. Relevant clinical, biochemical, serologic, imaging, and in some cases, histologic data were obtained to elucidate the etiology of liver injury. This included serological testing hepatitis A, B, C, and E, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, and autoimmune hepatitis as well as the metabolic marker ceruloplasmin for Wilson’s disease. Subjects with preexisting cirrhosis were excluded.
Causality assessment
Non-acetaminophen DILI was diagnosed if the patient was taking a drug or substance with a strong association with idiosyncratic DILI and if competing causes of ALF/ALI were excluded by rigorous evaluation of aforementioned diagnostic work-up. From 1998 to 2014, DILI was diagnosed by an experienced hepatologist at the local site and then each case was scrutinized and independently confirmed as a case of DILI by the authors at the ALFSG central site. From 2014 to 2015, DILI was diagnosed according to DILIN guidelines, only including those cases determined to be definite (>95% probability), highly likely (75–95%), or probably (50–75%) (19). Subjects deemed as possible or unlikely (probability <50%) were re-adjudicated as indeterminate. If multiple CAM agents were used concurrently, then this was adjudicated as a single case of DILI. Putative agents were then categorized after the causality indicated a >50% likelihood that the implicated agent was responsible for the case of ALI/ALF.
Characterizing DILI
DILI was characterized as hepatocellular, cholestatic, or mixed based on the “R” ratio as calculated by the ratio of serum alanine aminotransferase (ALT, as a multiple of its upper limit of normal) to the relative elevation of alkaline phosphatase (as a multiple of its upper limit of normal) on day 1 of enrollment (20).
Categorizing patients and outcomes
Subjects with DILI were divided into two categories: CAM and PM. To avoid overlap, if PM was implicated in addition to CAM, these subjects were excluded from the study (n=2). Data for both groups were compared including demographics, comorbidities, presenting symptoms, laboratory values, and calculated Model of End-Stage Liver Disease (MELD) score (21). Lab values from the day of presentation were used for analysis. Outcomes at 21 days of enrollment were defined as transplant-free (spontaneous) survival, liver transplantation, or death. Patients who were transplanted but did not have known alive/dead status at day 21 were included in all other analysis for transplant-free survival but excluded from overall survival and mortality analysis. Subgroup analysis was performed on all of those with ALF, including those who progressed from ALI to ALF.
Statistical analysis
Descriptive statistics were used to present demographics, clinical characteristics, and outcomes. Continuous variables were reported as medians with interquartile ranges, due to the non-normal distribution of the data. Differences in continuous variables between the CAM and PM groups, as well as CAM patients with a transplant-free survival and CAM patients, who received a transplant or died, were tested using the Wilcoxon rank-sum test. Differences in categorical variables between the groups were tested using a Chi-Square test or Fisher’s exact test where applicable (expected cell sizes <5). A univariate logistic model was created as admission MELD predictive of transplant-free survival. A receiver operating characteristic curve was generated using the SAS Macro %ROCPLOT to find an optimal cut point, by maximizing sensitivity and specificity, of admission-MELD. All analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC) and evaluated at the 0.05 significance level.
RESULTS
Cases of liver injury
Between January 1998 and April 2015, the ALFSG enrolled 2,626 subjects with 253 (9.6%) adjudicated as DILI by methods as described above. Among those, two cases were excluded as both a CAM and a non-CAM agent (acetaminophen and montelukast) were identified as potential causes of liver injury. Of the remaining 251 cases eligible for analysis, 210 (83.7%) were due to PM and 41 (16.3%) from CAM. Herbal supplements accounted for 63.4% of all CAM cases (Table 1). Antimicrobials and antivirals were the most frequently implicated PM (48.6%) with isoniazid being the most common single agent (n=27). Between 1998 and 7 July 2007, there were 154 cases of DILI, 17 of which were due to CAM (12.4%). From 8 July 2007–2015, of the 138 cases of DILI, 24 cases were attributed to CAM (21.1%), a statistically significant increase in the percent of DILI cases due to CAM (P=0.047).
Table 1.
Implicated agents of drug-induced liver injury
| Complementary and alternative medicines |
Prescription medications |
|---|---|
| Herbal medications (26) | Antimicrobials and antivirals (102) |
| Black cohosh (2) | Anti-tuberculin agents (37) |
| Eurycoma longifolia and oyster extract | Isoniazid (27) |
| Fenugreek | Trimethoprim-sulfamethoxazole (14) |
| Herbalife cell activator | Nitrofurantoin (10) |
| Horny goat weed | Anti-retroviral therapy (7) |
| Kava kava | Penicillin or cephalosporin (7) |
| Ma huang | Antifungal agent (6) |
| Qat | Doxycycline (5) |
| Usnic acid | Neurologic medications (20) |
| Uva-ursi and buchu leaf | Phenytoin (7) |
| Valerian | Cholesterol lowering agents (11) |
| Multiple herbals (14) | Statin (8) |
| Dietary supplements (12) | Antithyroid agents (8) |
| C4 energy | Propylthiouracil (7) |
| Hydroxycut (2) | Chemotherapeutic agents (8) |
| Lipokinetix | Nonsteroidal anti-inflammatories (8) |
| Oxyelite | Psychiatric medications (8) |
| Reumofan plus | Toxins (8) |
| Ripped fuel extreme | Disease modifying agents (7) |
| Rockstar energy drink | Sulfasalazine (4) |
| Slimquick | Thiazolidinediones (6) |
| Stacker 2 Fat Burner | Disulfiram (6) |
| Therma Slim | Cardiac agents (3) |
| Xtreme supplement | Halothanes (2) |
| Anabolic steroids (3) | Other agents (16) |
Demographics
Patient demographics and comorbidities are shown in Table 2. There was no statistical significant difference between the CAM and PM groups in regards to age, sex, BMI, or race. Among both groups, subjects were predominantly Caucasian, male, and overweight. There was no difference in alcohol use within the preceding 6 months between groups. Subjects in the CAM group had significantly less comorbidities than the PM subjects (median 1.0 vs. 2.0, P<0.001). As compared with the CAM group, comorbidities were more common among the PM in all categories including collagen/vascular, chronic liver, endocrine, psychiatric, neurologic, heart, renal, pulmonary, gastrointestinal, and immunodeficiency disease, though only statistically significant in regards to psychiatric and pulmonary disease.
Table 2.
Demographics, comorbidities, and presenting symptoms
| n | CAM-induced liver injury | n | PM-induced liver injury | P value | |
|---|---|---|---|---|---|
| Age, median | 41 | 41.0 | 210 | 46.0 | 0.39 |
| Sex (%) | 41 | 210 | 0.41 | ||
| Female | 16 (39) | 68 (32) | |||
| Male | 25 (61) | 142 (68) | |||
| Race (%) | 41 | 210 | 0.16 | ||
| Caucasian | 27 (66) | 136 (65) | |||
| African American | 5 (12) | 46 (22) | |||
| Asian | 6 (15) | 12 (6) | |||
| Other | 3 (7) | 16 (7) | |||
| Body mass index (BMI), median | 37 | 29.6 | 171 | 28.6 | 0.69 |
| Alcohol use in past 6 months (%) | 12 | 6 (50) | 42 | 11 (26) | 0.16 |
| Comorbidities (%) [IQR] | 41 | 210 | |||
| None | 5 (12) | 3 (1) | <0.01 | ||
| Collagen/vascular disease | 2 (5) | 13 (6) | 0.75 | ||
| Chronic liver disease | 1 (2) | 9 (4) | 0.58 | ||
| Endocrine/diabetes | 5 (12) | 49 (23) | 0.11 | ||
| Psychiatric disease | 2 (5) | 44 (21) | 0.02 | ||
| Neurologic/seizure disease | 2 (5) | 26 (12) | 0.16 | ||
| Hypertension | 7 (17) | 51 (24) | 0.32 | ||
| Heart disease | 2 (5) | 22 (10) | 0.27 | ||
| Renal disease | 0 (0) | 12 (6) | 0.12 | ||
| Pulmonary disease | 0 (0 | 27 (13) | 0.02 | ||
| Substance abuse | 2 (5) | 20 (10) | 0.34 | ||
| GI disease | 3 (7) | 31 (15) | 0.20 | ||
| HIV/AIDs | 0 (0) | 10 (5) | 0.15 | ||
| IDU at any time | 1 (2) | 6 (3) | 0.88 | ||
| Other | 15 (37) | 104 (50) | 0.13 | ||
| Median comorbidities | 1.0 [0.0–1.0] | 2.0 [1.0–3.0] | <0.01 | ||
| Presenting symptoms (%) [IQR] | 41a | 210a | |||
| Nausea/Vomiting | 33 (80) | 135 (67) | 0.19 | ||
| Abdominal pain | 21 (51) | 117 (57) | 0.77 | ||
| Rash | 5 (12) | 46 (23) | 0.10 | ||
| Headache | 9 (22) | 41 (21) | 0.95 | ||
| Malaise | 33 (80) | 162 (79) | 0.90 | ||
| Fever | 12 (29) | 65 (32) | 0.88 | ||
| Joint pains | 3 (7) | 36 (18) | 0.16 | ||
| Jaundice | 37 (95) | 183 (94) | 0.90 | ||
| Onset of jaundice, days | 11.0 [4.0–19.0] | 10.5 [5.0–22] | 0.63 |
CAM, complementary and alternative medicine; GI, gastrointestinal; IDU, intravenous drug use; IQR, interquartile range; PM, prescription medication.
Incomplete data on all patients. Sample size ranges for subset of data: CAM n=37–41 and PM n=182–210.
Presenting symptoms
There was no difference in presenting symptoms between the groups (Table 2). Jaundice was the most common presenting symptom for both groups, being present in >90% of subjects. There was also no difference in the onset of jaundice between CAM and PM groups. Malaise and nausea/vomiting were the next most common presenting symptoms.
Liver injury pattern
There was no difference in the type of liver injury (hepatocellular, cholestatic, or mixed injury) between groups as determined by R score (P=0.26) (Table 3). Hepatocellular injury (R score >5) was the most common injury pattern in both the CAM (80.0%) and PM (72.9%) groups. Thirty-two (78.1%) patients in the CAM group met criteria for ALF as compared with 186 (88.6%) in the PM though this was not statistically significant (P=0.16). On day 1 of enrollment, PM-induced DILI showed higher serum alkaline phosphatase levels compared with the CAM group (mean IU/l, 215.1 vs. 156.2, P=0.005); there were no differences between both cohorts in median ALT/AST (aspartate aminotransferase), INR, platelets, bilirubin, MELD score or coma grade.
Table 3.
Admission laboratory values, pattern, and degree of liver injury
| n | CAM-induced liver injury |
n | PM-induced liver injury | P value | |
|---|---|---|---|---|---|
| Laboratories, median [IQR] | 41a | 209a | |||
| ALT (IU/l) | 769 [293–1,769] | 659 [269–1,592] | 0.50 | ||
| AST (IU/l) | 579 [335–1,609] | 649 [269–1,349] | 0.80 | ||
| Alkaline phosphate (IU/l) | 125 [103–182] | 171 [123–232] | <0.01 | ||
| INR | 2.7 [1.9–3.8] | 2.4 [1.9–3.5] | 0.28 | ||
| Platelet count (×1,000/mm3) | 150 [115–223] | 139.5 [91–209] | 0.28 | ||
| Bilirubin (mg/dl) | 18.2 [9.9–28.1] | 19.0 [12.1–26.6] | 0.80 | ||
| MELD | 31.7 [26.4–37.1] | 31.5 [26.6–36.9] | 0.99 | ||
| Pattern of injury/R score (%) | 40 | 203 | |||
| Hepatocellular | 32 (80) | 148 (73) | |||
| Mixed | 7 (18) | 33 (16) | 0.26 | ||
| Cholestatic | 1 (2) | 22(11) | |||
| Degree of injury—ALI/ALF (%)b | 41 | 210 | |||
| ALI | 5 (12) | 19 (9) | |||
| ALI progressed to ALF | 4 (10) | 5 (2) | 0.16 | ||
| ALF | 32 (78) | 186 (89) |
ALF, acute liver failure; ALFSG, Acute Liver Failure Study Group; ALI, acute liver injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAM, complementary and alternative medicine; INR, international normalized ratio; IQR, interquartile range; MELD, Model of End-Stage Liver Disease; PM, prescription medication.
Incomplete data on all patients. Sample size ranges for subset of data: CAM n=39–41 and PM n=203–209.
ALFSG began collecting data on patients with ALI in 2008.
CAM vs. PM outcomes
More patients with CAM-induced DILI were listed for liver transplantation vs. those with PM-induced DILI (65.9 vs. 47.1%, P=0.28) (Table 4). At 21 days, 23 patients of the CAM cohort underwent liver transplantation, which was significantly greater than that of the PM cohort (56.1 vs. 31.9%, P<0.005). Additionally, nine patients with CAM-induced liver injury died, five of which had received transplants. There was no difference in overall mortality between CAM and PM-induced DILI (22.0 vs. 32.9%, P=0.17). Ten patients (25.0%) within the CAM group survived without receiving a liver transplant, which was a lower fraction than observed for the PM group (37.9%), though not statistically significant (P=0.100). On subgroup analysis of those with ALF, there was no difference in mortality (25.0 vs. 35.1%, P=0.250), but there was a significantly higher transplantation rate (61.1 vs. 35.6%, P<0.005) and lower 21-day transplant-free survival (17.4 vs. 34.4%, P=0.044) when comparing CAM with PM.
Table 4.
Total outcomes at 21 days
| n | CAM-induced liver injury (%) |
n | PM-induced liver injury (%) |
P value | |
|---|---|---|---|---|---|
| Alive | 41 | 32 (78) | 210 | 141 (67) | 0.18 |
| Dead | 9 (22) | 69 (33) | |||
| Listed for transplant | 41 | 27 (66) | 206 | 97 (47) | 0.03 |
| Transplanted | 41 | 23 (56) | 207 | 66 (32) | <0.01 |
| Total transplant-free survival | 40 | 10 (25) | 203 | 77 (38) | 0.12 |
| ALF transplant-free survivala | 35 | 6 (17) | 203 | 64 (34) | 0.04 |
ALF, acute liver failure; CAM, complementary and alternative medicine; PM, prescription medication.
Subgroup analysis of those with ALF only.
CAM subgroup analysis
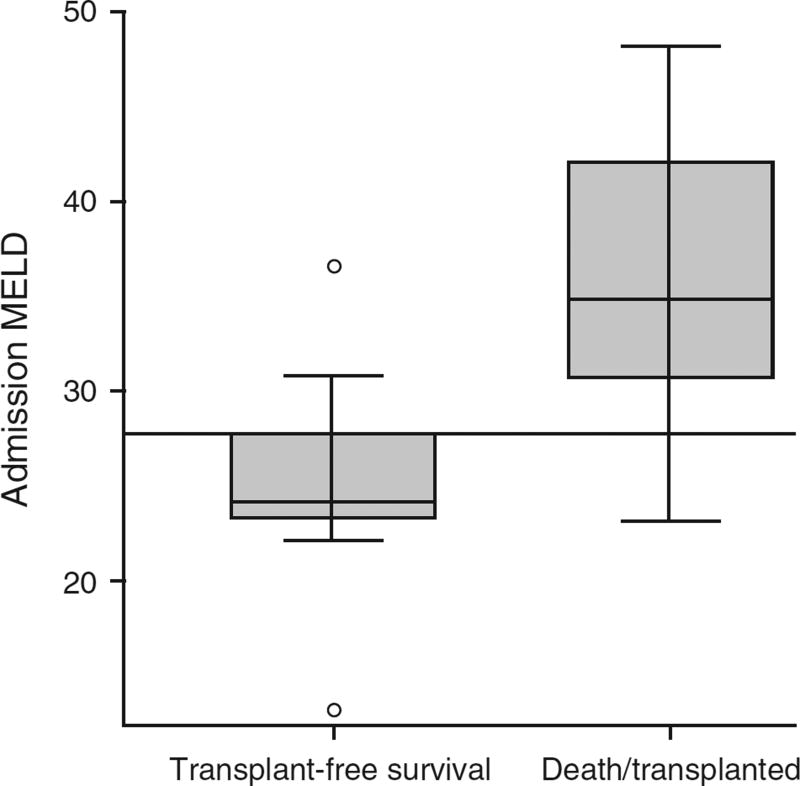
Within the CAM group, those with 21-day transplant-free survival were more likely to have ALI than ALF when compared with those transplanted or dead (Table 5). Additionally, this subset of patients had significantly lower median BMIs (25.4 vs. 30.8, P=0.031), median INRs (1.8 vs. 3.2, P<0.005) and subsequently lower MELD scores (24.2 vs. 33.9, P=0.005). Comparing those who survived spontaneously with those who died or required transplant, a MELD of 27.76 optimized the receiver operating characteristic area under the curve (0.882, Figure 1). Using this cutoff, four patients who died or were transplanted fell below this number and two patients who spontaneously survived were above this number, demonstrating a sensitivity and a specificity in our population of 85.7 and 80%, respectively. No differences were observed in median ALT/AST, alkaline phosphatase, platelet count or bilirubin levels. There was also no difference in liver injury pattern between these groups.
Table 5.
CAM subgroup analysis: transplant-free survival vs. death/transplanted
| n | Transplant-free survival | n | Death/transplanted | P value | |
|---|---|---|---|---|---|
| Age, median | 10 | 46.0 | 30 | 40.0 | 0.66 |
| Sex (%) | 10 | 30 | |||
| Female | 5 (50) | 19 (63) | 0.48 | ||
| Male | 5 (50) | 11 (37) | |||
| Race (Caucasian) (%) | 10 | 4 (40) | 30 | 22 (73) | 0.11 |
| Body mass index (BMI), median | 6 | 25.4 | 20 | 30.8 | 0.03 |
| Laboratories, median [IQR] | 10 | 30a | |||
| ALT (IU/l) | 1,149 [849–2,447] | 602.5 [269–1,145] | 0.08 | ||
| AST (IU/l) | 1,200.5 [549–1,948] | 558.0 [205–1,537] | 0.21 | ||
| Alkaline phosphate (IU/l) | 148 [104–250] | 121 [102–168] | 0.37 | ||
| INR | 1.8 [1.7–2.1] | 3.2 [2.6–4.3] | <0.01 | ||
| Platelet count (×1,000/mm3) | 190.5 [118–290] | 141 [107–202] | 0.11 | ||
| Bilirubin (mg/dl) | 15.4 [9.9–23.4] | 18.6 [12.3–28.4] | 0.44 | ||
| MELD | 24.2 [23.3–27.8] | 33.9 [29.4–39.5] | 0.01 | ||
| Pattern of injury/R score (%) | 10 | 28 | 0.11 | ||
| Hepatocellular | 9 (90) | 22 (79) | |||
| Mixed | 0 (0) | 6 (21) | |||
| Cholestatic | 1 (10) | 0 (0) | |||
| Degree of injury—ALI/ALF (%) | 10 | 30 | 0.01 | ||
| ALI | 4 (40) | 1 (3) | |||
| ALI progressed to ALF | 1 (10) | 3 (10) | |||
| ALF | 5 (50) | 26 (87) |
ALF, acute liver failure; ALI, acute liver injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAM, complementary and alternative medicine; INR, international normalized ratio; IQR, interquartile range; MELD, Model of End-Stage Liver Disease.
Incomplete data on all patients. Sample size ranges for subset of data Death/Transplanted n=28–30.
Figure 1.
CAM subgroup analysis: transplant-free survival MELD (n=10) vs. death/transplant MELD (n=28); P=0.005. Horizontal line represents optimized receiver operating characteristic area under the curve (0.882) of MELD 27.78. CAM, complementary and alternative medicine; MELD, Model of End-Stage Liver Disease.
DISCUSSION
DILI secondary to CAM is becoming increasingly prevalent. ALF/ALI from CAM appears to be at least as severe in presentation as that observed in DILI secondary to conventional PM. As observed with other forms of liver failure, the MELD score was roughly concordant with outcomes for patients with ALF/ALI from CAM with respect to death or transplant. The higher rate of liver transplantation in patients with CAM-induced DILI is possibly explained by the fact that these patients had fewer comorbidities that might preclude transplantation. However, despite being a healthier cohort of patients, subgroup analysis of those with CAM-induced ALF demonstrated a lower 21-day transplant-free survival than those with PM-induced ALF, supporting our hypothesis that DILI from CAM is as severe if not more severe than that seen with PM.
The clinical significance of higher alkaline phosphatase levels related to PM-induced DILI is unclear and was not described in a previous study (18). All cases had abdominal imaging to exclude a biliary source of cholestasis. Cholestatic DILI has been well described. In fact, patients with jaundiced DILI have roughly a 10% short-term mortality (22). However, there was no difference in bilirubin levels or difference in overall survival between groups in this study.
All of the patients in this study were hospitalized at tertiary-care centers with liver transplant services. This selective registry is only representative of the most severe cases of CAM-induced DILI: those that reach the threshold of ALI or ALF. It is likely that milder cases are under-recognized and under-reported. Although this study was confined to 21-day outcomes and did not examine long-term consequences of DILI, other studies have shown that the course can be protracted and some patients are left with sequelae of chronic liver disease (4). A major limitation of this study was the lack of latency data and medication dosage for most cases. When available, these data were collected but as the data were lacking for most cases, it was not included. Although most cases of DILI are idiosyncratic, including these data in future studies may help identify those agents that are truly hepatotoxic.
Exactly why CAM-induced DILI is more severe than PM is unclear. The mechanism of DILI is likely multifactorial and related to direct hepatoxicity, host factors, and drug–host interactions. Current research efforts are underway to better understand the mechanism of DILI with a focus on the potential role of the immune system, mitochondrial injury, and impairment of bile salt excretion (23,24). Using histology and the emerging field of pharmacogenetics, researchers are working on ways to better diagnose and predict DILI (25,26). Research has yielded apparent underlying genetic predispositions to DILI involving human leukocyte antigen genotypes and genes involved in drug metabolism (25,27). Cell-based assays are being developed using immune-related gene expression to help predict the risk of drug-specific DILI (28). How this research will translate into clinical practice remains to be seen, but research efforts focused on the mechanisms involved in CAM-induced DILI may ultimately produce in vitro testing that predicts the risk of DILI and thereby reduce or prevent CAM-induced liver injury. A clinical tool currently available is a recently published prognostic model that uses platelet count and total bilirubin to help predict patients with DILI at greatest risk for ALF (29).
The use of CAM is grossly under-reported. Nearly half of CAM users fail to report the use of these medications to their health-care provider and often fail to disclose all CAM even when they do so (30,31). Physicians should have a high suspicion for CAM in otherwise healthy patients with unexplained liver enzyme abnormalities. Unfortunately, physician knowledge on the clinical use and safety profile of CAM is poor, which discourages an open dialogue about the use of these therapies with patients (32). This lack of knowledge also results in under-reporting of CAM-related adverse events. In fact, almost three in four physicians do not know how or where to report medication-related adverse events (33). MedWatch (http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm) is one tool clinicians can use to report adverse events to the FDA (34). More specific to DILI, www.LiverTox.nih.gov, an NIH-funded database with information on hundreds of compounds (including a few CAM) implicated in DILI can be used by physicians to research culprit substances when DILI is suspected (35).
In conclusion, 16.3% of the idiosyncratic DILI associated with ALI/ALF in the ALFSG database are due to CAM. These patients had significantly higher transplantation rates compared with idiosyncratic DILI from PM, probably reflecting a more severe clinical presentation and less comorbidities that precluded transplantation. Given the significant potential for morbidity and mortality, patients presenting with suspected CAM-induced severe liver injury or liver failure should be considered for early referral and rapid evaluation at a liver transplant center. It is of concern that the proportion of idiosyncratic DILI cases secondary to CAM seem to be increasing in recent years, in all likelihood due to more prevalent use of CAM. We also propose that stricter reporting of CAM-induced DILI and better characterization of the mechanisms of hepatotoxicity from these compounds would help limit the serious outcomes associated with such over-the-counter products.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
The use of complementary and alternative medicine (CAM) is rising among the US population.
-
✓
CAM has been more frequently implicated in acute liver injury and failure.
WHAT IS NEW HERE
-
✓
Incidence of CAM-induced severe liver injury and acute liver failure is rising.
-
✓
Clinical features of CAM-induced injury as compared with prescription-induced injury are relatively uncharacterized.
-
✓
CAM-induced liver injury has higher transplantation rates and lower transplant-free survival than that observed with severe liver injury due to prescription medications.
Acknowledgments
Financial support: The Acute Liver Failure Study Group is funded principally by the National Institutes of Health, under grant U01 DK058369.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantors of the article: Luke Hillman, MD and Daniel Ganger, MD.
Specific author contributions: Statistical analysis: Luke Hillman, Michelle Gottfried, and Daniel Ganger. All authors participated in data acquisition, analysis or interpretation of data. All authors were responsible for drafting, critical revision, and approval of the final manuscript.
Potential competing interests: William M. Lee provides consulting services to Novartis, Sanofi and Lilly and receives research support from BMS, Gilead and Merck. The remaining authors declare no conflict of interest.
References
- 1.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–5. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. doi: 10.1053/j.gastro.2013.02.006. 1425.e1–3; quiz e19–20. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148 doi: 10.1053/j.gastro.2015.03.006. 1340–52.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. doi: 10.1053/j.gastro.2008.09.011. 1934.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuben A, Koch DG, Lee WM, et al. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148 doi: 10.1053/j.gastro.2015.02.050. 1353–61.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17. doi: 10.1111/apt.12109. [DOI] [PubMed] [Google Scholar]
- 10.Administration USFaD . Guidance for Industry A Dietary Supplement Labeling Guide: Chapter IV. In: (FDA) USFaDA, editor. Nutritional Labeling. 2005. [Google Scholar]
- 11.Harkey MR, Henderson GL, Gershwin ME, et al. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–6. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 12.Lai V, Smith A, Thorburn D, et al. Severe hepatic injury and adulterated Chinese medicines. BMJ. 2006;332:304–5. doi: 10.1136/bmj.332.7536.304-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wai CT, Tan BH, Chan CL, et al. Drug-induced liver injury at an Asian center: a prospective study. Liver Int. 2007;27:465–74. doi: 10.1111/j.1478-3231.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 14.Office UGA. Herbal Dietary Supplements: Examples of Deceptive or Questionable Marketing Practices and Potentially Dnagerous Advice. 2010. May 26, [Google Scholar]
- 15.Lindstrom AOC, Lynch ME, Blumenthal M. Herb supplement sales increase 5.5% in 2012: herbal supplement sales rise for 9th consecutive year; turmeric sales jump 40% in natural channel. HerbalGram. 2013:60–5. [Google Scholar]
- 16.Radimer K, Bindewald B, Hughes J, et al. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–49. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 17.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–6. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399–408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 21.Kamath PS, Kim WR. Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 22.Rangnekar AS, Fontana RJ. An update on drug induced liver injury. Minerva Gastroenterol Dietol. 2011;57:213–29. [PubMed] [Google Scholar]
- 23.Aleo MD, Luo Y, Swiss R, et al. Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology. 2014;60:1015–22. doi: 10.1002/hep.27206. [DOI] [PubMed] [Google Scholar]
- 24.Urban TJ, Shen Y, Stolz A, et al. Limited contribution of common genetic variants to risk for liver injury due to a variety of drugs. Pharmacogenet Genomics. 2012;22:784–95. doi: 10.1097/FPC.0b013e3283589a76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis. 2014;34:123–33. doi: 10.1055/s-0034-1375954. [DOI] [PubMed] [Google Scholar]
- 26.Foureau DM, Walling TL, Maddukuri V, et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin Exp Immunol. 2015;180:40–51. doi: 10.1111/cei.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aithal GP. Pharmacogenetic testing in idiosyncratic drug-induced liver injury: current role in clinical practice. Liver Int. 2015;35:1801–8. doi: 10.1111/liv.12836. [DOI] [PubMed] [Google Scholar]
- 28.Oda S, Matsuo K, Nakajima A, et al. A novel cell-based assay for the evaluation of immune- and inflammatory-related gene expression as biomarkers for the risk assessment of drug-induced liver injury. Toxicol Lett. 2015;241:60–70. doi: 10.1016/j.toxlet.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Lo Re V, 3rd, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy's law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13:2360–8. doi: 10.1016/j.cgh.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hensrud DD, Engle DD, Scheitel SM. Underreporting the use of dietary supplements and nonprescription medications among patients undergoing a periodic health examination. Mayo Clin Proc. 1999;74:443–7. doi: 10.4065/74.5.443. [DOI] [PubMed] [Google Scholar]
- 31.Tarn DM, Karlamangla A, Coulter ID, et al. A cross-sectional study of provider and patient characteristics associated with outpatient disclosures of dietary supplement use. Patient Educ Couns. 2015;98:830–6. doi: 10.1016/j.pec.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemper KJ, Amata-Kynvi A, Dvorkin L, et al. Herbs and other dietary supplements: healthcare professionals' knowledge, attitudes, and practices. Altern Ther Health Med. 2003;9:42–9. [PubMed] [Google Scholar]
- 33.Cellini M, Attipoe S, Seales P, et al. Dietary supplements: physician knowledge and adverse event reporting. Med Sci Sports Exerc. 2013;45:23–8. doi: 10.1249/MSS.0b013e318269904f. [DOI] [PubMed] [Google Scholar]
- 34.MedWatch. [cited 22 September 2015];The FDA Safety Information and Adverse Event Reporting Program: Reporting Serious Problems to FDA. 2015 Jan 20; Available from http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm.
- 35.United States National Library of Medicine National Institute of Diabetes and Digestive and Kidney Diseases. [cited 2015];LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2015 Jun 23; Available from http://livertox.nih.gov/ [PubMed]