Abstract
IMPORTANCE
Endoscopic airway surgery is a frequently used procedure in the management of laryngotracheal stenosis (LTS); however, no established outcome measures are available to assess treatment response.
OBJECTIVE
To assess acoustics and aerodynamic measures and voice- and dyspnea-related quality of life (QOL) in adult patients with LTS who undergo endoscopic airway surgery.
DESIGN, SETTING, AND PARTICIPANTS
This case series compared preoperative measures and postoperative outcomes among adult patients who underwent endoscopic airway surgery for LTS from September 1, 2013, to September 30, 2015, at the tertiary care Johns Hopkins Voice Center. Patients were excluded if they did not undergo balloon dilation or if they had multilevel or glottic stenosis. The Phonatory Aerodynamic System was used to quantify laryngotracheal aerodynamic changes after surgery. Final follow-up was completed 2 to 6 weeks after surgery.
MAIN OUTCOMES AND MEASURES
The voice-related QOL instrument (V-RQOL), Dyspnea Index, and Clinical Chronic Obstructive Pulmonary Disease Questionnaire were completed before and after endoscopic surgery. Consensus auditory perceptual evaluation of voice, acoustic measurements, and aerodynamic outcomes were also assessed.
RESULTS
Fourteen patients (1 man and 13 women; mean [SD] age, 45.4 [4.3] years) were enrolled. The mean postoperative V-RQOL scores (n = 14) increased from 74.3 to 85.5 (mean of difference, 11.3; 95%CI, 2.2 to 20.3). The mean postoperative Dyspnea Index (n = 14) decreased from 26.9 to 6.6 (mean of difference, −20.3; 95%CI, −27.9 to −12.7); the mean postoperative Clinical Chronic Obstructive Pulmonary Disease Questionnaire scores (n = 9) decreased from 3.2 to 1.0 (mean of difference, −2.2; 95% CI, −3.4 to −0.9). Postoperative mean vital capacity (n = 14) increased from 2.5 to 3.1 L (mean of difference, 0.6 L; 95%CI, 0.3–1.0 L), whereas mean laryngeal resistance (n = 14) decreased from 73.9 to 46.4 cm H2O/L/s (mean of difference, −27.5 cm H2O/L/s; 95%CI, −44.8 to −10.3 cm H2O/L/s) postoperatively.
CONCLUSIONS AND RELEVANCE
Patients demonstrate statistically clinically significant improvement in dyspnea-related QOL, whereas a few patients showed a clinically significant improvement in V-RQOL. Dyspnea-related QOL outcomes should be added to airway surgeons’ regular assessment of patients with LTS to measure treatment response and inform the decision to perform a second operation, whereas V-RQOL outcomes need additional prospective study with a larger sample size. The Phonatory Aerodynamic System is not an optimal method to quantify changes in laryngotracheal aerodynamics after intervention in LTS.
Laryngotracheal stenosis (LTS) is a fibrotic disease characterized by significant narrowing of the larynx and/or trachea. Stenosis can occur at any level along the larynx and trachea and can present at a single level or at multiple levels. Common causes of LTS are prolonged intubation or tracheostomy placement, idiopathic autoimmune disease, and more rarely external trauma or irradiation.1 Laryngotracheal stenosis may be treated with medical, endoscopic, and open surgical therapies.2 At present, endoscopic surgical therapy represents the most common surgical treatment, sometimes in combination with local medical therapy such as injectable corticosteroids. More definitive surgery, such as tracheal or cricotracheal resection, is an option when the glottis or supraglottis is not significantly involved. The common goal of treatment is to alleviate airway obstruction and provide symptomatic relief while maintaining quality of life (QOL).3 Outcome metrics that assess the effect of stenosis on QOL, the effect of treatment, and when patients require surgical intervention are beginning to be elucidated.
Laryngotracheal stenosis has been associated with dysphonia, primarily through retrospective studies.4–13 A few studies4–6 have retrospectively demonstrated improved voice QOL after endoscopic balloon dilation in patients with LTS. None of the published studies evaluating voice outcomes in LTS have been prospective. In addition, when patients were stratified by level of stenosis, those with isolated subglottic stenosis (SGS) or tracheal stenosis demonstrated more significant improvement after dilation than those with multilevel stenoses involving the glottis or with posterior glottic stenosis alone.6 Instrumental voice measures such as acoustic and aerodynamic evaluations are less well studied in the population with LTS. One recent retrospective study assessed acoustic and aerodynamic changes before and after balloon dilation in a cohort of patients with idiopathic SGS but arrived at no significant conclusion.5 Nevertheless, the utility of these outcome measures warrants further investigation in the study of LTS.
In addition to voice-related QOL improvement, related clinical research in LTS has begun to quantify dyspnea related QOL after balloon dilation. The Dyspnea Index (DI)14 and Clinical Chronic Obstructive Pulmonary Disease Questionnaire (CCQ)15 are patient-centered outcome metrics that quantify disease burden related to dyspnea, and the CCQ in particular has been validated for use in demonstrating improvement after intervention.
Although emerging literature uses outcome measures to assess responses to therapy for LTS, a dearth of prospective analysis remains. Our objective is to use prospectively collected data to evaluate QOL outcomes, acoustic measures, and aerodynamic outcomes in evaluating the efficacy of endoscopic laryngeal treatment for LTS.
Methods
Study Design
We prospectively collected data on adult patients who underwent endoscopic treatment of LTS from September 1, 2013, to September 31, 2015, at the Johns Hopkins Voice Center, Baltimore, Maryland. Patients were included if they underwent balloon dilation with cryodestruction, cold-instrument lysis, and/or corticosteroid injection of a single-level SGS or proximal tracheal stenosis. Patients were excluded from the study if they did not undergo balloon dilation or if they had multilevel or glottic stenosis. This study was approved by the institutional review board of the Johns Hopkins University School of Medicine. Informed written consent was obtained from all participants before data collection in this prospective study.
Procedure
After providing informed consent, patients were brought to the operating room and were positioned supine on the operating table. Next, bag-mask ventilation was verified and general anesthesia was administered, while a nasal trumpet was placed in the left nasal cavity to enable high-flow oxygenation for periods of apnea.16 A laryngoscope was then inserted that exposed the larynx, and the patient was placed in suspension. Cold excision of stenosis was performed using a cryoprobe (Erbe Elektromedizin GmbH) or a cold knife in 3 to 4 locations circumferentially. Patients then underwent a first balloon dilation of the SGS or tracheal stenosis, followed by a submucosal injection of dexamethasone sodium phosphate (Decadron), 10 mg/mL. After this procedure, a second dilation was performed, after which the patient was taken out of suspension and the laryngoscope was removed, ending the operation. Dilation was performed using sizes 12F to 15F semicompliant pulmonary balloon dilators (CRE; Boston Scientific) for a duration of 1 to 2 minutes. Balloon size was chosen by the laryngologist and estimated based on the size of patient larynx or trachea. Dilation after corticosteroid administration was performed in similar fashion, with inflation of the balloon to a smaller diameter to provide equal distribution of the corticosteroid volume within the lamina propria (Figure).
Figure. Treatment of Subglottic Stenosis (SGS) in a Representative Patient.
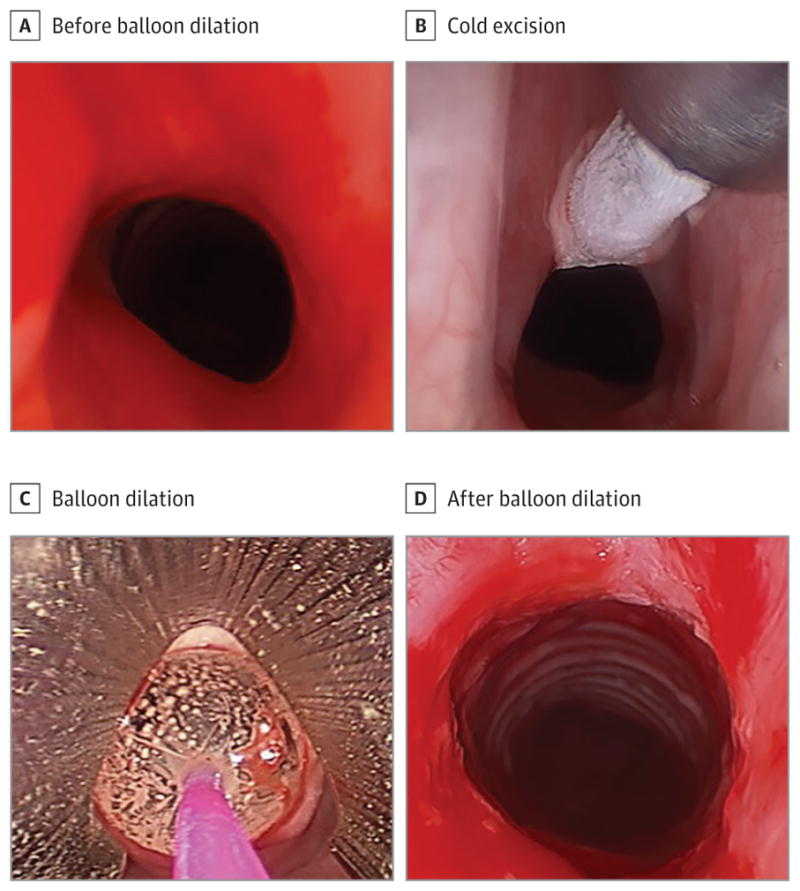
A, Endolaryngeal view of SGS in situ before balloon dilation. B, Endolaryngeal view of SGS in situ with cold excision using the cryoprobe. C, Endolaryngeal view of SGS with a pulmonary balloon dilator. The balloon was inflated in situ. D, Endolaryngeal view of SGS in situ following balloon dilation.
Data Collection
All assessments were performed at preoperative and postoperative clinic visits. If multiple assessments were collected, the most recent complete data set before airway surgery was treated as preoperative data. Evaluations and data collected from 2 to 6 weeks after surgery were treated as postoperative data. The immediate 2-week period after surgery was excluded from data collection to allow any temporary dysphonia due to inflammation to subside.
Patient demographics, including age and sex, were recorded. We assessed QOL with a voice-related QOL scale (V-RQOL) (range, 0–100, with higher scores indicating better QOL),17–19 the DI (range, 0–40, with higher scores indicating worse dyspnea-related symptoms),14 and the CCQ (range, 0–6, with higher scores indicating worse bronchopulmonary symptoms).15 The V-RQOL is a patient-completed questionnaire that has previously been validated. The V-RQOL is used to assess and compare a patient’s voice against itself over time, in this case before and after balloon dilation. The DI and the CCQ are also validated clinical assessment tools used to assess the degree of symptomatic airway disease burden and subsequent effect on QOL. Calculations for V-RQOL and DI were based on the entire study population of 14 patients, whereas the CCQ was added to the study late and matched CCQ data were only available for 9 patients. To establish clinically meaningful significance in our patients, minimal clinically important difference scores were obtained from published literature for the V-RQOL and CCQ.19–21 Because this information was not available for the DI, the minimally important difference score was calculated using the distribution-based method described by Norman et al.22 Briefly, the SD of the original DI patient data from the validation study was calculated using the method described by Gartner-Schmidt et al14 and divided in half. This statistically accepted method has been shown to be a valid predictor of clinically significant disease.23
Perceptual voice assessment was evaluated using the Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V) tool (range, 0–100, with higher scores indicating perceptually worse voice quality).24 The CAPE-V assessment is a previously validated, subjective evaluation of a patient’s recorded voice, performed by 2 blinded speech language pathologists with expertise in voice disorders (K.T. and M.S.). Preoperative and postoperative voice recordings were deidentified and randomized before being scored independently by the 2 evaluators, and then the mean of these scores was calculated to form a single score before undergoing statistical analysis. In the event that scores differed significantly between the 2 evaluators, further statistical analyses, including interrater reliability, were in place as a tie-breaking strategy.
Acoustic and aerodynamic measures assessed included maximum phonation time, vital capacity, mean phonation expiratory airflow, maximal high pitch, phonatory peak pressure, laryngeal resistance, fundamental frequency, noise to harmonic ratio, jitter, and shimmer. We measured maximum phonation time by asking the patient to sustain the vowel/a/as long as possible after a maximal inhalation.25 The best of 3 attempts was recorded and the time was measured in seconds. We recorded vital capacity as the volume of air a patient could exhale during maximal exhalation. Phonatory peak pressure, laryngeal resistance, and phonation expiratory airflow were measured using the Phonatory Aerodynamic System (PAS) (model 6600; KayPENTAX Corp). Patients were asked to hold a mask firmly around their mouth, with the intraoral tube between their lips, and phonate. The device was able to calculate an approximation of laryngeal resistance by measuring phonatory peak air pressure divided by phonation expiratory airflow. Phonation expiratory airflow results were compared against previously published age- and sex-matched normative data for reference.26 An external microphone was used to capture data for maximal high pitch, fundamental frequency, noise to harmonic ratio, jitter, and shimmer analyses.
Statistical Analysis
Statistical analysis was performed using GraphPad software (GraphPad Software, Inc). Comparisons of preoperative and postoperative outcomes were performed using a paired t test. Absolute mean differences between preoperative and postoperative scores and 95% CIs were reported.
Results
Patient Demographics
Fourteen patients met inclusion criteria and consented to participate in this study. Participants ranged in age from 19 to 66 years (mean [SD] age, 45.4 [14.3] years). Thirteen patients (93%) were women; 1 (7%) was a man. Eleven patients (79%) were white and 3 patients (21%) were other races. The distribution of causes of LTS in this cohort was idiopathic SGS in 9 patients (64%), followed by iatrogenic SGS in 4 patients (29%) and an autoimmune case in 1 patient (7%). Ten patients (71%) had prior endoscopic dilation surgery, whereas 4 patients (29%)had no history of laryngeal surgery before study participation. One patient (7%) had a history of tracheostomy placement, whereas no patients had any history of open laryngotracheal reconstruction.
Voice Outcomes
Mean V-RQOL scores significantly improved from 74.3 to 85.5 (mean of difference, 11.3; 95% CI, 2.2 to 20.3). Individually, 7 patients (50%) had V-RQOL scores of less than 80 (abnormal), whereas 7 patients (50%) had scores of at least 80 (normal) preoperatively. Postoperatively only 3 patients (21%) had V-RQOL scores that remained less than 80, whereas 11 patients (79%) had scores of at least 80. Overall, 13 patients (93%) had scores that stayed the same or improved after surgery, whereas only 1 patient (7%) had a score that worsened slightly (97.5 to 92.5). A clinically meaningful change was seen in 3 patients (21%) who demonstrated a change greater than 19 points.19,20 Mean CAPE-V scores deteriorated slightly from 20.9 preoperatively to 25.4 postoperatively, although this change was not statistically significant (mean of difference, 4.5; 95% CI, −3.7 to 12.8). Scores were so similar between the 2 reviewers that no further statistical analysis was necessary. The maximum phonation times were longer postoperatively (14.1 seconds) than preoperatively (10.9 seconds), although this change was not statistically significant (mean of difference, 3.2; 95% CI, −0.4 to 6.8). Mean maximal high pitch improved from 678.6 Hz preoperatively to 750.5 Hz postoperatively (Table 1); however this change was not statistically significant (mean of difference, 2.9 Hz; 95% CI, −5.3 to 170.6 Hz).
Table 1.
Voice and Acoustic Outcomes
| Patient No. | V-RQOL Scorea | CAPE-V Scoreb | MPT, s | Maximal High Pitch, Hz | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |
| 1 | 42.5 | 50.0 | 62.3 | 53.8 | 7.8 | 7.9 | 308 | 587 |
|
| ||||||||
| 2 | 37.5 | 95.0 | 4.5 | 13.3 | 5.8 | 19.9 | 621 | 811 |
|
| ||||||||
| 3 | 72.5 | 82.5 | 26.5 | 38.3 | 10.4 | 14.5 | 845 | 902 |
|
| ||||||||
| 4 | 97.5 | 92.5 | 29.0 | 25.5 | 10.8 | 5.3 | 619 | 481 |
|
| ||||||||
| 5 | 75.0 | 80.0 | 7.0 | 10.0 | 7.9 | 6.7 | 531 | 524 |
|
| ||||||||
| 6 | 90.0 | 100.0 | 5.5 | 3.8 | 11.5 | 14.0 | 677 | 703 |
|
| ||||||||
| 7 | 95.0 | 100.0 | 24.0 | 7.8 | 24.0 | 20.1 | 569 | 750 |
|
| ||||||||
| 8 | 95.0 | 95.0 | 7.0 | 10.8 | 8.7 | 11.2 | 918 | 974 |
|
| ||||||||
| 9 | 85.0 | 90.0 | 25.5 | 15.0 | 13.0 | 11.5 | 744 | 996 |
|
| ||||||||
| 10 | 67.5 | 77.5 | 15.8 | 39.5 | 18.1 | 24.0 | 779 | 559 |
|
| ||||||||
| 11 | 52.5 | 80.0 | 35.8 | 69.0 | 3.5 | 7.4 | 570 | 672 |
|
| ||||||||
| 12 | 95.0 | 95.0 | 12.3 | 15.8 | 15.9 | 15.5 | 573 | 514 |
|
| ||||||||
| 13 | 40.0 | 60.0 | 11.5 | 34.5 | 8.4 | 17.4 | 995 | 1176 |
|
| ||||||||
| 14 | 95.0 | 100.0 | 26.5 | 19.5 | 6.2 | 21.9 | 752 | 1009 |
|
| ||||||||
| Mean | 74.3 | 85.5 | 20.9 | 25.5 | 10.9 | 14.1 | 678.6 | 750.5 |
Abbreviations: CAPE-V, Consensus Auditory-Perceptual Voice Evaluation; MPT, maximal phonation time; V-RQOL, voice-related quality of life.
Scores range from 0 to 100, with higher scores indicating better voice quality of life.
Scores range from 0 to 100, with higher scores indicating perceptually worse voice quality.
Although measures for mean noise to harmonic ratio, jitter, shimmer, and fundamental frequency all improved from preoperative to postoperative measurement, none of these changes were statistically significant. Unfortunately we were unable to collect these data for 1 patient postoperatively owing to lack of a clear signal.
Airway Outcomes
Mean DI scores improved from 26.86 to 6.57 (mean of difference, − 20.3; 95%CI, −27.9 to −12.7). A minimally important difference score of 5.4 was calculated for the DI. Mean CCQ scores improved from 3.2 to 1.0 (mean of difference, −2.2; 95% CI, −3.4 to −0.9). Mean vital capacity improved from 2.60 L preoperatively to 3.14 L postoperatively (mean of difference, 0.6 L; 95% CI, 0.3–1.0L). Mean peak phonation expiratory flow rates were unchanged after surgery from 0.16 to 0.16 L/s (mean of difference, −0.002 L/s; 95% CI, −0.04 to 0.03 L/s). Mean laryngeal resistance improved after balloon dilation from 73.91 to 46.40 cm H2O/L/s (mean of difference, −27.5 cm H2O/L/s; 95% CI, −44.8 to −10.3 cmH2O/L/s). Mean phonatory peak pressure decreased from 7.88 to 7.44 cm H2O; however, this change was not statistically significant (mean of difference, −0.4 cm H2O; 95% CI, −2.2 to 1.3 cm H2O) (Table 2). A summary of patient outcomes is given in Table 3.
Table 2.
Breathing Outcomes
| Patient No. | DI Scorea | Vital Capacity, L | Phonation Expiratory Air Flow Rate, L/s | Phonatory Peak Pressure, cm H2O | Laryngeal Resistance, cm H2O/L/s | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |
| 1 | 39 | 9 | 1.20 | 2.83 | 0.01 | 0.10 | 6.98 | 13.18 | 105.71 | 61.66 |
|
| ||||||||||
| 2 | 37 | 0 | 2.58 | 3.68 | 0.09 | 0.12 | 5.81 | 4.83 | 40.30 | 39.00 |
|
| ||||||||||
| 3 | 29 | 16 | 1.91 | 3.65 | 0.09 | 0.11 | 8.86 | 7.55 | 123.00 | 52.20 |
|
| ||||||||||
| 4 | 19 | 14 | 2.50 | 2.14 | 0.21 | 0.20 | 5.67 | 8.17 | 30.31 | 24.77 |
|
| ||||||||||
| 5 | 19 | 17 | 3.53 | 3.77 | 0.37 | 0.39 | 8.84 | 9.59 | 32.72 | 29.00 |
|
| ||||||||||
| 6 | 30 | 0 | 3.31 | 4.36 | 0.15 | 0.20 | 5.12 | 4.53 | 55.83 | 33.79 |
|
| ||||||||||
| 7 | 25 | 0 | 2.08 | 3.32 | 0.08 | 0.14 | 6.31 | 5.06 | 53.20 | 32.35 |
|
| ||||||||||
| 8 | 16 | 2 | 2.32 | 2.57 | 0.23 | 0.17 | 6.29 | 5.09 | 60.97 | 47.86 |
|
| ||||||||||
| 9 | 38 | 8 | 3.13 | 3.00 | 0.17 | 0.19 | 7.16 | 5.88 | 52.00 | 33.50 |
|
| ||||||||||
| 10 | 31 | 0 | 2.63 | 3.09 | 0.15 | 0.12 | 6.17 | 5.14 | 45.00 | 36.10 |
|
| ||||||||||
| 11 | 39 | 20 | 1.70 | 2.31 | 0.21 | 0.11 | 25.90 | 17.59 | 239.90 | 130.00 |
|
| ||||||||||
| 12 | 2 | 2 | 2.46 | 2.64 | 0.14 | 0.15 | 5.47 | 5.37 | 64.36 | 32.55 |
|
| ||||||||||
| 13 | 39 | 0 | 2.64 | 3.36 | 0.15 | 0.15 | 5.76 | 5.77 | 47.00 | 26.55 |
|
| ||||||||||
| 14 | 13 | 4 | 3.14 | 3.24 | 0.25 | 0.12 | 5.95 | 6.45 | 84.50 | 70.25 |
|
| ||||||||||
| Mean | 26.86 | 6.57 | 2.60 | 3.14 | 0.16 | 0.16 | 7.88 | 7.44 | 73.91 | 46.40 |
Abbreviation: DI, Dyspnea Index.
Scores range from 0 to 40, with higher scores indicating worse dyspnea-related symptoms.
Table 3.
Outcomes Summary
| Measure | Score, Mean (SD) [Range] | Mean Difference (95% CI) | |
|---|---|---|---|
| Preoperative | Postoperative | ||
| V-RQOLa | 74.3 (22.7) [37.5–97.5] | 85.5 (15.2) [50.0 100.0] | 11.3 (2.2 to 20.3) |
| DIb | 26.9 (11.6) [2–39] | 6.6 (7.4) [0–20] | −20.3 (−27.9 to −12.7) |
| CCQc | 3.2 (1.6) [0.8–4.9] | 1.0 (0.2) [0.7–1.3] | −2.2 (−3.4 to −0.9) |
| CAPE-Vd | 20.9 (15.6) [7.0–62.3] | 25.5 (19.2) [3.8–53.8] | 4.5 (−3.7 to 12.8) |
| MPT, s | 10.9 (5.5) [3.5–18.1] | 14.1 (6.0) [5.3–21.9] | 3.2 (−0.4 to 6.8) |
| Vital capacity, L | 2.6 (0.7) [1.2–3.5] | 3.1 (0.6) [2.1–4.4] | 0.6 (0.3 to 1.0) |
| Phonation expiratory flow rate, L/s | 0.16 (0.09) [0.01–0.37] | 0.16 (0.74) [0.10–0.39] | −0.002 (−0.04 to 0.03) |
| Laryngeal resistance, cm H2O/L/s | 73.9 (54.7) [30.3–239.9] | 46.4 (27.6) [24.8–130.0] | −27.5 (−44.8 to −10.3) |
| Phonatory peak pressure, cm H2O | 7.9 (5.3) [5.1–25.9] | 7.4 (3.8) [4.5–17.6] | −0.4 (−2.2 to 1.3) |
| Maximal high pitch, Hz | 678.6 (176.7) [308–995] | 750.5 (226.1) [481–1176] | 82.46 (−5.3 to 170.6) |
| Fundamental frequency, Hz | 184.2 (28.9) [123–232] | 187.1 (28.6) [133–217] | 2.9 (−6.7 to 12.6) |
| Noise to harmonic ratio | 0.10 (0.03) [0.07–0.20] | 0.10 (0.03) [0.06–0.10] | −0.01 (−0.035 to 0.006) |
| Jitter, % | 1.5 (1.1) [0.3–4.1] | 1.5 (0.9) [0.3–2.9] | −0.01 (−0.09 to 0.09) |
| Shimmer, % | 0.4 (0.2) [0.2–0.7] | 0.3 (0.1) [0.1–0.5] | −0.06 (−0.15 to 0.03) |
Abbreviations: DI, Dyspnea Index; CAPE-V, Consensus Auditory-Perceptual Evaluation of Voice; CCQ, Clinical Chronic Obstructive Pulmonary Disease Questionnaire; MPT, maximum phonation time; V-RQOL, voice-related quality of life.
Scores range from 0 to 100, with higher scores indicating better voice quality of life.
Scores range from 0 to 40, with higher scores indicating worse dyspnea-related symptoms.
Scores range from 0 to 6, with higher scores indicating worse bronchopulmonary symptoms.
Scores range from 0 to 100, with higher scores indicating perceptually worse voice quality.
Discussion
This study prospectively evaluated voice and dyspnea QOL measures, perceptual voice evaluation, acoustics, and aerodynamic measures in patients who underwent endoscopic airway surgery for LTS. Patients demonstrated significant improvement in laryngeal resistance, vital capacity, and V-RQOL and DI surveys, whereas no change in acoustic or perceptual evaluation of voice quality was found. These results confirm findings from multiple retrospective studies on voice and dyspnea outcomes after endoscopic airway surgery6,7 and contribute to the development of a panel of outcome measures in the evaluation and treatment of LTS.
The shape of the subglottis reduces turbulence in airflow before reaching the vocal folds during phonation.27 However, LTS disrupts the natural shape of the airway, leading to turbulent airflow and subsequent dysphonia. The association of LTS with dysphonia has been demonstrated through multiple studies,4–13 because patients with glottic or multilevel stenosis have worse voice-related QOL than patients with isolated SGS or tracheal stenosis.6 The altered and narrowed airway tract may result in patients’ subjective expression of “running out of air,” which is captured in the Voice Handicap Index5 and V-RQOL assessment. The QOL score improvement seen in a few retrospective studies may be owing to reduced phonatory effort from the increased subglottic cross-sectional area after dilation. Our voice data suggest that V-RQOL likely improves after airway intervention, which may be clinically meaningful in a subset of patients who have lower (<80) preoperative V-RQOL scores; however, improvement after intervention is less likely to be clinically meaningful in patients with higher (>80) preoperative V-RQOL scores. Perceptual voice quality evaluation using the CAPE-V assessment did not demonstrate improvement in our sample population, a result that is consistent with those of previous perceptual voice evaluation studies.5,6 The lack of concordance between improvement in CAPE-V and V-RQOL scores may be because the patient’s self-rated V-RQOL improvement reflects that they no longer run out of breath or that they are less anxious and/or depressed about their airflow related to voice, rather than the quality of voice grade, roughness, breathiness, asthenia, or strain that perceptual raters assess. Acoustic and aerodynamic measures evaluated largely remained unchanged, although maximum phonation time and maximal high-pitch frequency improved after the airway intervention. The voice data presented herein suggest that further studies involving a larger sample size are needed to better assess voice QOL, acoustics, and aerodynamics in patients with LTS.
Our results using the 2 dyspnea indices support the studies by Gartner-Schmidt et al14 and Nouraei et al15 that found the CCQ and DI are useful in quantifying improvement in patient symptoms after surgery to treat LTS. A clinically meaningful difference was achieved in 7 of 9 patients (78%) who underwent preoperative and postoperative CCQ evaluation based on previous studies of the minimal clinically important difference of 0.4 in respiratory disease.21 Although no published data on a minimal clinically important difference for the DI are available to our knowledge, we calculated a minimally important difference score of 5.4 for the DI, which resulted in 11 of 14 patients (79%) having a clinically meaningful improvement. Dyspnea-related QOL outcomes can be very informative in the evaluation of patients with LTS, and were commend using one of these instruments in regular clinical practice to assist with patient counselling for surgical decision making and to quantify the effectiveness of balloon dilation on symptoms.
Laryngeal aerodynamic results demonstrated improvements in vital capacity and laryngeal resistance after endoscopic airway surgery. The increase seen in vital capacity after balloon dilation was an unexpected finding. We hypothesize that because vital capacity is effort dependent, patients may have exerted greater effort after surgery with the subsequent improvement in large airway diameter. Furthermore, aerodynamic testers may not have been as insistent on forced expiration in contrast to forced vital capacity measures during pulmonary function testing (PFT). On the other hand, laryngeal resistance was reduced owing to an increase in the cross-sectional area, which subsequently caused a drop in subglottal phonatory pressure. After balloon dilation enlarges the airway, patients likely have a reduced effort to breathe (because they are no longer moving air through a narrowed airway), which in turn lowers subglottal pressure during phonation.
Previous studies28,29 have demonstrated the value of measuring resistance in patients with stenosis. Wassermann et al29 studied trans-stenotic resistance in sedated patients undergoing flexible bronchoscopy by passing a pressure catheter beyond the stenosis. Their findings suggested a threshold, based on inspiratory resistance, to determine when surgical intervention would be beneficial. In our study, the PAS does not measure resistance directly but derives laryngeal resistance from measurements of peak airway pressure and airflow. Furthermore, during phonatory measurements taken using the PAS, the glottic aperture is then arrowest portion of the airway rather than the subglottis.30 Finally, PAS measurement of peak phonation expiratory flow did not demonstrate significance, which is in contradistinction to PFT outcomes in patients with LTS. Specifically, Kraft et al31 demonstrated that 4 metrics, including peak expiratory flow rate, improved significantly after endoscopic incision and dilation in a cohort of 17 patients with idiopathic SGS. We note that peak expiratory flow rate measured during forced expiration in the study by Kraft et al31 is not the same as the PAS measurement of phonation expiratory air flow rate in the present study, and the lack of correlation with PFT outcomes suggests limitations to the PAS system of measuring peak flows and laryngeal resistance.
Other limitations to this study include the lack of PFT measurements in our laryngology clinic, which have since been introduced. Recent literature has indicated that some of the metrics measured within PFTs may aide in differentiating LTS from other bronchopulmonary abnormalities32 and may be useful in objectively assessing outcomes in LTS after airway surgery.31,33 Further limitations include the sex distribution and sample size of our patient population. Future prospective studies would benefit from having more robust numbers that include more male patients to achieve an equal general distribution, data such as distance of stenosis from the glottis, length of stenosis, and percentage of stenosis to help stratify outcome differences between patients. Complete data sets should include PFT for thorough comparison.
Conclusions
To our knowledge, this study is the first to use a panel of metrics to prospectively assess patient outcomes after endoscopic airway surgery for SGS and LTS. Patients demonstrated statistically and clinically significant improvements in 2 dyspnea QOL surveys after balloon dilation. Three patients demonstrated clinically significant improvement in V-RQOL scores. Although patients also demonstrated reduced laryngeal resistance, the lack of concordance with PFT peak flow measures suggest that the PAS is not an optimal method to quantify changes in laryngotracheal aerodynamics after interventions in LTS. These results support the inclusion of dyspnea QOL surveys as part of the regular assessment of patients with LTS to help assess surgical benefit and inform the decision to perform a second operation, while further studies on voice QOL that include a larger sample size are needed to draw conclusions.
Key Points.
Question
Which quality-of-life (QOL), acoustic, and aerodynamic outcomes should be added to airway surgeons’ assessment of endoscopic laryngeal treatment for laryngotracheal stenosis (LTS)?
Findings
This case series involved 14 adult patients with LTS who completed QOL assessments and acoustic and aerodynamic testing before and after endoscopic airway surgery. Patients demonstrated clinically significant improvements in a dyspnea QOL measure after dilation, and 2 patients demonstrated clinically significant improvement in voice-related QOL.
Meaning
Voice- and dyspnea-related QOL measures should be included in routine assessment of patients with LTS to help measure surgical benefit and inform the decision to perform a second operation.
Acknowledgments
Funding/Support: This study was supported by award 1K23DC014082 from the National Institute of Deafness and Other Communication Disorders, National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Drs Samad and Akst are co–first authors. Dr Hillel had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Akst, Karataylı-Özgürsoy, Teets, Best, Hillel.
Acquisition, analysis, or intepretation of data: Samad, Akst, Teets, Simpson, Sharma, Hillel.
Drafting of the manuscript: Samad, Akst, Karataylı-Özgürsoy, Sharma, Hillel.
Critical revision of the manuscript for important intellectual content: Samad, Akst, Teets, Simpson, Best, Hillel.
Statistical analysis: Samad.
Obtaining funding: Hillel.
Administrative, technical, or material support: Akst, Teets, Best, Hillel.
Study supervision: Best, Hillel.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Lorenz RR. Adult laryngotracheal stenosis: etiology and surgical management. Curr Opin Otolaryngol Head Neck Surg. 2003;11(6):467–472. doi: 10.1097/00020840-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014;124(3):736–741. doi: 10.1002/lary.24410. [DOI] [PubMed] [Google Scholar]
- 3.Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope. 2006;116(9):1553–1557. doi: 10.1097/01.mlg.0000228006.21941.12. [DOI] [PubMed] [Google Scholar]
- 4.Hatcher JL, Dao AM, Simpson CB. Voice outcomes after endoscopic treatment of laryngotracheal stenosis. Ann Otol Rhinol Laryngol. 2015;124(3):235–239. doi: 10.1177/0003489414551980. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman MR, Brand WT, Dailey SH. Effects of balloon dilation for idiopathic laryngotracheal stenosis on voice production. Ann Otol Rhinol Laryngol. 2016;125(1):12–19. doi: 10.1177/0003489415595425. [DOI] [PubMed] [Google Scholar]
- 6.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis: impact of dilation and level of stenosis. Ann Otol Rhinol Laryngol. 2015;124(5):413–418. doi: 10.1177/0003489414564249. [DOI] [PubMed] [Google Scholar]
- 7.Tirado Y, Chadha NK, Allegro J, Forte V, Campisi P. Quality of life and voice outcomes after thyroid ala graft laryngotracheal reconstruction in young children. Otolaryngol Head Neck Surg. 2011;144(5):770–777. doi: 10.1177/0194599810391198. [DOI] [PubMed] [Google Scholar]
- 8.Smith ME, Mortelliti AJ, Cotton RT, Myer CM., III Phonation and swallowing considerations in pediatric laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 1992;101(9):731–738. doi: 10.1177/000348949210100903. [DOI] [PubMed] [Google Scholar]
- 9.Smith ME, Marsh JH, Cotton RT, Myer CM., III Voice problems after pediatric laryngotracheal reconstruction: videolaryngostroboscopic, acoustic, and perceptual assessment. Int J Pediatr Otorhinolaryngol. 1993;25(1–3):173–181. doi: 10.1016/0165-5876(93)90051-4. [DOI] [PubMed] [Google Scholar]
- 10.MacArthur CJ, Kearns GH, Healy GB. Voice quality after laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1994;120(6):641–647. doi: 10.1001/archotol.1994.01880300055008. [DOI] [PubMed] [Google Scholar]
- 11.Hartley BE, Rutter MJ, Cotton RT. Cricotracheal resection as a primary procedure for laryngotracheal stenosis in children. Int J Pediatr Otorhinolaryngol. 2000;54(2–3):133–136. doi: 10.1016/s0165-5876(00)00360-8. [DOI] [PubMed] [Google Scholar]
- 12.George M, Monnier P. Long-term voice outcome following partial cricotracheal resection in children for severe subglottic stenosis. Int J Pediatr Otorhinolaryngol. 2010;74(2):154–160. doi: 10.1016/j.ijporl.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Ettema SL, Tolejano CJ, Thielke RJ, Toohill RJ, Merati AL. Perceptual voice analysis of patients with subglottic stenosis. Otolaryngol Head Neck Surg. 2006;135(5):730–735. doi: 10.1016/j.otohns.2006.06.1249. [DOI] [PubMed] [Google Scholar]
- 14.Gartner-Schmidt JL, Shembel AC, Zullo TG, Rosen CA. Development and validation of the Dyspnea Index (DI): a severity index for upper airway-related dyspnea. J Voice. 2014;28(6):775–782. doi: 10.1016/j.jvoice.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Nouraei SA, Randhawa PS, Koury EF, et al. Validation of the Clinical COPD Questionnaire as a psychophysical outcome measure in adult laryngotracheal stenosis. Clin Otolaryngol. 2009;34(4):343–348. doi: 10.1111/j.1749-4486.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 16.Samad I, Phelps M, Pandian V, et al. High-flow oxygen, a primary oxygenation technique for endolaryngeal airway surgery: our experience with 10 patients. Clin Otolaryngol. 2016;41(3):286–289. doi: 10.1111/coa.12497. [DOI] [PubMed] [Google Scholar]
- 17.Kupfer RA, Hogikyan EM, Hogikyan ND. Establishment of a normative database for the voice-related quality of life (V-RQOL) measure. J Voice. 2014;28(4):449–451. doi: 10.1016/j.jvoice.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Franic DM, Bothe AK. Psychometric evaluation of condition-specific instruments used to assess health-related quality of life, attitudes, and related constructs in stuttering. Am J Speech Lang Pathol. 2008;17(1):60–80. doi: 10.1044/1058-0360(2008/006). [DOI] [PubMed] [Google Scholar]
- 19.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL) J Voice. 1999;13(4):557–569. doi: 10.1016/s0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 20.Rubin AD, Wodchis WP, Spak C, Kileny PR, Hogikyan ND. Longitudinal effects of Botox injections on voice-related quality of life (V-RQOL) for patients with adductory spasmodic dysphonia: part II. Arch Otolaryngol Head Neck Surg. 2004;130(4):415–420. doi: 10.1001/archotol.130.4.415. [DOI] [PubMed] [Google Scholar]
- 21.Kocks JW, Tuinenga MG, Uil SM, van den Berg JW, Ståhl E, van der Molen T. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir Res. 2006;7:62. doi: 10.1186/1465-9921-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 23.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–23. e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Zraick RI, Kempster GB, Connor NP, et al. Establishing validity of the Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V) Am J Speech Lang Pathol. 2011;20(1):14–22. doi: 10.1044/1058-0360(2010/09-0105). [DOI] [PubMed] [Google Scholar]
- 25.Maslan J, Leng X, Rees C, Blalock D, Butler SG. Maximum phonation time in healthy older adults. J Voice. 2011;25(6):709–713. doi: 10.1016/j.jvoice.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zraick RI, Smith-Olinde L, Shotts LL. Adult normative data for the KayPENTAX Phonatory Aerodynamic System Model 6600. J Voice. 2012;26(2):164–176. doi: 10.1016/j.jvoice.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Oren L, Khosla S, Murugappan S, King R, Gutmark E. Role of subglottal shape in turbulence reduction. Ann Otol Rhinol Laryngol. 2009;118(3):232–240. doi: 10.1177/000348940911800312. [DOI] [PubMed] [Google Scholar]
- 28.Brouns M, Jayaraju ST, Lacor C, et al. Tracheal stenosis: a flow dynamics study. J Appl Physiol (1985) 2007;102(3):1178–1184. doi: 10.1152/japplphysiol.01063.2006. [DOI] [PubMed] [Google Scholar]
- 29.Wassermann K, Koch A, Warschkow A, Mathen F, Müller-Ehmsen J, Eckel HE. Measuring in situ central airway resistance in patients with laryngotracheal stenosis. Laryngoscope. 1999;109(9):1516–1520. doi: 10.1097/00005537-199909000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Smith SL, Thomson SL. Influence of subglottic stenosis on the flow-induced vibration of a computational vocal fold model. J Fluids Struct. 2013;38:77–91. doi: 10.1016/j.jfluidstructs.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraft SM, Sykes K, Palmer A, Schindler J. Using pulmonary function data to assess outcomes in the endoscopic management of subglottic stenosis. Ann Otol Rhinol Laryngol. 2015;124(2):137–142. doi: 10.1177/0003489414548915. [DOI] [PubMed] [Google Scholar]
- 32.Nouraei SA, Nouraei SM, Patel A, et al. Diagnosis of laryngotracheal stenosis from routine pulmonary physiology using the expiratory disproportion index. Laryngoscope. 2013;123(12):3099–3104. doi: 10.1002/lary.24192. [DOI] [PubMed] [Google Scholar]
- 33.Nouraei SM, Franco RA, Dowdall JR, et al. Physiology-based minimum clinically important difference thresholds in adult laryngotracheal stenosis. Laryngoscope. 2014;124(10):2313–2320. doi: 10.1002/lary.24641. [DOI] [PubMed] [Google Scholar]


