Abstract
Botanical pyrethrins and synthetic pyrethroids are highly potent and environmentally safe insecticides that are used to control a wide range of disease vector and pest arthropods. Unfortunately, resistance to these insecticides has been demonstrated in numerous medically important mosquito species. In this study adult Culex pipiens sensu lato were captured in agricultural and urban locations in Fresno County, California, and subsequently exposed to a commercial formulation of pyrethrin insecticide by ultra low volume spraying. Following insecticide exposure, two pyrethroid-like, fluorescent substrates (cis-DCVC and cis-TFMCVC) and CDNB were used to measure esterase and glutathione S-transferase (GST) activities in surviving mosquitoes. Elevated esterase activity (2.5-fold) was found in surviving urban mosquitoes at 12 h post pyrethrin exposure (in comparison to non-insecticide exposed control mosquitoes) when cis-TFMCVC was used as a substrate. Additionally, when CDNB was used as a substrate, 2.8-fold higher GST activity was found. A simple assay was established using our pyrethroid-like, fluorescent substrates that was able to detect low-level esterase activities in homogenates made from individual mosquitoes. The cis-TFMCVC-based assay suggested that esterase activity plays a role in pyrethrin resistance in urban mosquitoes in California.
Keywords: pyrethrin resistance, Culex, esterase, GST, fluorescent substrate
1. Introduction
The common house mosquito Culex pipiens and southern house mosquito Cx. quinquefasciatus are major vectors of arboviruses such as West Nile virus (Sardelis et al. 2001), Rift Valley fever virus (Meegan 1979), and St. Louis encephalitis virus (Tsai et al. 1989). In some locations members of the Cx. pipiens complex are also vectors of filariasis causing nematodes such as Wuchereria bancrofti (Farid et al. 2001) and dog heartworm (Lai et al. 2000). In the U.S., the control of mosquito pests primarily involves targeting the larval stage with habitat management and the use of biological insecticides such as endotoxins produced by Bacillus thuringiensis and juvenile hormone analog insecticides such as methoprene. The adult stage is generally only targeted in response to confirmed outbreaks of disease or in response to a public demand to quickly reduce nuisance mosquitoes. Adult mosquitoes are generally controlled with botanical insecticides such as pyrethrins or synthetic pyrethroids that are applied together with a cytochrome P450 monooxygenase (P450) inhibitor such as piperonyl butoxide (PBO) (Isman 2006; Khambay & Jewess 2005; Macedo et al. 2010).
Resistance to pyrethroids has been detected in numerous insect species including Culex complex mosquitoes in California (McAbee et al. 2003) and elsewhere in the U.S. (Li et al. 2012; Liu et al. 2004; Liu et al. 2006) The two primary mechanisms of pyrethroid resistance involve target site insensitivity and elevation in the activity of detoxification enzymes such as P450s, esterases, and glutathione S-transferases (GSTs) (Hemingway & Ranson 2000; Nkya et al. 2013). Biological assays such as the bottle bioassay (Brogdon & McAllister 1998) are often used to detect insecticide resistance in mosquitoes. Biological assays are relatively inexpensive to perform and can detect resistance resulting from target site insensitivity as well as increases in the activity of detoxification enzymes. Biological assays, however, are slow to produce results, require large numbers of insects, and the interpretation of their end points may not be consistent among different laboratories. Furthermore, since these assays cannot detect low frequency alleles, they do not support early intervention strategies for resistance management. PCR-based assays using various detection systems and/or labeled primers have been developed for the rapid, single-insect detection of target site mutations (Bass et al. 2007; Sarkar et al. 2011). A robust PCR-based assay for quantification of the expression levels of detoxification enzymes, however, is not in common use. This is partly because of the current lack of knowledge of specific isoenzymes that are involved in pyrethroid detoxification in Cx. pipiens s.l. and other mosquitoes.
Numerous substrates that are based on fluorescent, luminescent or colorimetric reporters are available for the detection of P450, esterase, and GST activities. Assays based on fluorescent or luminescent reporters generally provide enhanced sensitivity (allowing for the use of smaller amounts of biological material) and reduced background in comparison to colorimetric reporters. Our laboratory, for example, has developed a rapid luminescent assay for the detection of elevated P450 activity from a single mosquito larva using commercially available P450 substrates (Inceoglu et al. 2009). We have also developed a series of pyrethroid-like, ester-containing, fluorescent substrates for the sensitive detection of esterase activity (Huang et al. 2012). These novel ester substrates are composed of an acid moiety that is found in some commonly used pyrethroids (i.e., permethrin, lambda-cyhalothrin, and resmethrin) and an alcohol moiety consisting of 7-hydroxy-4-methylcoumarin. A unique aspect of these pyrethroid-like compounds is that they are also metabolized by GSTs (Huang et al. 2012). Thus, these pyrethroid-like compounds can function as substrates for the dual detection of esterase and GST activities in the presence of an appropriate GST inhibitor or esterase inhibitor, respectively.
A primary goal of this study was to test whether our pyrethroid-like fluorescent substrates could detect esterase and GST activities from individual mosquitoes that were collected in the field. Specifically, we quantify GST and esterase activities in individual adult Cx. pipiens s.l. using two of pyrethroid-like, fluorescent substrates, cis-DCVC (a mimic of permethrin/cypermethrin) and cis-TFMCVC (a mimic of bifenthrin/cyhalothrin) (Huang et al. 2012). The mosquitoes that were used in this study were captured in an agricultural or urban environment in Central California and then exposed, within a short time after capture, to a commercial formulation of pyrethrins by ultra-low volume (ULV) spraying using a truck-mounted applicator. Mosquitoes that were knocked down or not knocked down at 1 h or 12 h post pyrethrin exposure were individually tested for esterase and GST activities.
2. Materials and methods
2.1. Mosquito collection and colony maintenance
Adult Cx. pipiens complex mosquitoes were captured using modified CO2-baited encephalitis vector survey (EVS) traps (Nelson & Chamberlain 1955) in Riverdale, California, and on the campus of California State University (CSU) Fresno on August 21, 2012. Riverdale is a town of approximately 3,200 residents that is located about 35 miles southwest of Fresno, California, and completely surrounded by agricultural land. CSU Fresno is an urban university campus that is located in the northeast corner of the city of Fresno. Metropolitan Fresno has more than one million residents. In addition, a highly pyrethroid-sensitive laboratory colony of Cx. quinquefasciatus (CQ1) was reared on a diet of ground rodent chow at 27°C under a 14:10 (light:dark) photoperiod as described previously (Inceoglu et al. 2009). The CQ1 strain was initially field-collected in Merced County, California, in the early 1950s and has since been maintained without interruption in the laboratory.
2.2. Open field pyrethrin insecticide exposure
Adult mosquitoes that were captured in Riverdale or CSU Fresno (as well as the control CQ1 strain) were exposed to a commercial formulation of pyrethrins by ULV spraying within 18 h of capture. This was done to mimic typical insecticide exposure regimes that are in use in California, and to enhance the level of detoxification enzymes resulting from insecticide exposure. Riverdale, CSU Fresno, and CQ1 mosquitoes that were not exposed to the pyrethrins were used as untreated controls. For the insecticide exposure assays, cohorts of 20 adults were placed into a modified ULV trial sentinel cage using standard methods (Townzen & Natvig 1973). Subsequently, the cages were placed at a height of 1 m, at distances of 30.5, 61.0, and 91.4 m downwind from the insecticide application made with a truck-mounted, cold aerosol generating ULV sprayer (Cougar model, Clarke, Roselle, IL) traveling at a speed of 10 mph (16 kph). The insecticide formulation, EverGreen EC 60-6 (6% pyrethrins, 60% piperonyl butoxide (PBO); MGK, Minneapolis, MN), was applied undiluted at the maximum label rate of 0.64 ounce per acre (0.0025 lb pyrethrins per acre and 0.025 lb PBO per acre) to a fallow field near Fresno, California. The insecticide was sprayed under a 3.1°C temperature inversion with 3 mph (4.8 kph) crosswinds. At 1 h post spraying, all mosquitoes that were knocked down and a sample of mosquitoes that were not knocked down (still alive) were individually placed within 1.8 ml microfuge tubes on a bed of crushed dry ice. The field-collected, frozen mosquitoes were transferred to a deep freezer (−80°C) in the laboratory and stored there until used for the GST and esterase activity assays. In addition, at 12 h post spraying, mosquitoes that were not knocked down were also individually collected and frozen as described above.
2.3. Colorimetric GST assay with CDNB
General GST activity in homogenates prepared from individual adult mosquitoes was measured using the colorimetric substrate 1-chloro-2,4-dinitrobenzene (CDNB). The mass of each adult was determined prior to homogenization using a Mettler model AE50 analytical balance. Individual adults were manually homogenized in 200 μl of homogenization buffer (100 mM sodium phosphate, pH 6.5, buffer containing 1 mM dithiothreitol (DTT), 1 mM disodium ethylene diamine tetraacetate (EDTA), 0.1 mM 1-phenyl-2-thiourea (PTU), and 0.1% (v/v) ethanol) using a plastic pestle. Following the homogenization, the mixture was centrifuged (16,100 xg, 1 min, 5°C) and 180 μl of the supernatant was collected as the mosquito homogenate. The mosquito homogenate was used immediately or stored at −80°C.
The colorimetric GST assay was routinely performed in a 200 μl reaction containing 10 μl of the mosquito homogenate, 1 mM CDNB, 3 mM reduced glutathione (GSH), 1 mM DTT, 1 mM EDTA, 0.1 mM PTU, and 1.15% (v/v) ethanol in 100 mM sodium phosphate, pH 6.5, buffer. The reaction was preincubated for 2 min at 30°C. The CDNB assays were performed in triplicate in the wells of a clear bottom 96 well plate. Wells containing 10 μl of homogenization buffer in place of the mosquito homogenate were run on each plate as negative controls to account for any chemical hydrolysis of CDNB. The conjugation of GSH and CDNB was monitored at a wavelength of 340 nm for 10 min (with readings at 17 s intervals) on a SpectraMax M2 spectrophotometer (Molecular Devices, Sunnyvale, CA). A molar extinction coefficient of 9.6 mM−1 cm−1 was used to calculate specific activity. The specific activity of the homogenate was calculated in terms of nmol of product formed per min per mg of adult mosquito.
2.4. Fluorescent GST assay with pyrethroid-like substrates
Potential pyrethrin-selective GST activity in the homogenates from individual adult mosquitoes was measured using two pyrethroid-like, ester containing fluorescent substrates 4-methyl-2-oxo-2H-chromen-6-yl, cis-3- (2,2-dichlorovinyl)-2,2-dimethylcyclo propanecarboxylate (cis-DCVC) and 4-methyl-2-oxo-2H-chromen-6-yl, cis-3- ( (Z)-2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate (cis-TFMCVC). The design, synthesis, and validation of these fluorescent substrates have been previously described (Huang et al. 2012). The structures of cis-DCVC and cis-TFMCVC are shown in Fig. 2. When these substrates are metabolized by a GST (or an esterase as described in the following section), a fluorescent coumarin-based metabolite (7-hydroxy-4-methyl coumarin) is released (Huang et al. 2012).
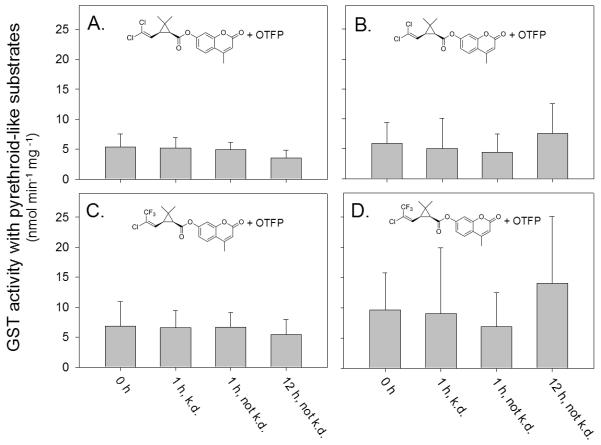
Figure 2.
GST activity of adult Cx. pipiens s.l. complex mosquitoes captured in Riverdale, California (A and C) or on the campus of California State University Fresno (B and D). The GST activity was measured using the pyrethroid-like fluorescent substrates cis-DCVC (A or B) or cis-TFMCVC (C and D) in the presence of the esterase inhibitor OTFP (10 μM). GST activity of individual mosquitoes was measured prior to exposure (0 h), or at 1 h or 12 h post exposure to a commercial formulation of pyrethrins. GST activity was determined in individual mosquitoes that were knocked down (k.d.) or not k.d. Background hydrolysis of the fluorescent substrates was undetectable under the assay conditions used in this study. The error bars indicate the standard deviation of the mean value of each treatment group.
The fluorescent GST assays were routinely performed in a 200 μl reaction containing 10 μl of the mosquito homogenate (described in the previous section), 50 μM fluorescent substrate, 3 mM reduced GSH, 1 mM DTT, 1 mM EDTA, 0.1 mM PTU, 0.5 % (v/v) di (ethylglycerol)ethyl ether, 0.1% (v/v) ethanol, and 5 μM 3-octylthio-1,1,1-trifluoropropan-2-one (OTFP) in 100 mM sodium phosphate, pH 7.4, buffer. In order to inhibit any esterase activity, the mosquito homogenate was preincubated for 5 min at 30°C in homogenization buffer containing 10 μM OTFP, a potent and broadly active inhibitor of esterase activity (Abdel-Aal & Hammock 1985). Following the OTFP preincubation, the fluorescent GST assay was initiated by the addition of a 2-fold concentrated assay buffer solution containing the fluorescent substrate and reduced GSH. The fluorescent assays were performed in triplicate in the wells of a black 96 well plate. Wells containing 10 μl of homogenization buffer (both with and without OTFP) in place of the mosquito homogenate were run on each plate as negative controls to account for any chemical hydrolysis of the fluorescent substrate. The release of 7-hydroxy-4-methyl coumarin was monitored (after an additional 2 min-long incubation at 30°C following the addition of substrate) with an excitation wavelength of 330 nm and emission wavelength of 460 nm for 10 min (with readings at 27 s intervals) on a SpectraMax M2 spectrophotometer. A molar extinction coefficient of 18,000 M−1 cm−1 was used to calculate specific activity. The specific activity of the homogenate was calculated in terms of nmol of product formed per min per mg of adult mosquito.
2.5. Fluorescent esterase assay with pyrethroid-like substrates
Esterase activity in the adult mosquito homogenates was measured with cis-DCVC and cis-TFMCVC. The fluorescent esterase assay was performed as described above for the fluorescent GST assay, except that OTFP treatment was omitted from the reaction. Hydrolysis of the ester of cis-DCVC or cis-TFMCVC results in the formation of 7-hydroxy-4-methyl coumarin, the same metabolite that is generated by the enzymatic activity of GST (Huang et al. 2012). Esterase activity was taken as the difference between the activity that was generated in the absence of OTFP (i.e., total activity) and in the presence of OTFP (i.e., GST activity).
2.6. Statistical analysis
Two-tailed Student’s t tests were used to determine whether the mean values of GST and esterase activities that were generated from two different treatments showed a statistical difference. On average each experimental cohort consisted of 21 individual adults. Some cohorts (e.g., insects that were not knocked down at 12 h post pyrethrin exposure) were composed of fewer insects but never less than 10 individuals.
3. Results and discussion
3.1. Open field pyrethrin exposure experiments and mosquito mass
In this study, esterase and GST activities were quantified in individual, adults of Cx. pipiens s.l. that were captured in agricultural (Riverdale) or urban (Fresno State) environments in Central California. Within 18 h of capture, the mosquitoes were exposed in an open field exposure experiment to a commercial formulation of pyrethrin (EverGreen EC 60-6), an insecticide formulation that contains the monooxygenase inhibitor PBO. The insecticide was released using a truck-mounted ULV sprayer, an application method that is in common use by mosquito and vector control districts throughout the U.S. Laboratory-reared control mosquitoes (CQ1 strain) were exposed to the insecticide in an identical manner. At 1 h post exposure, and at a distance of 30.5 m from the ULV spray source, 84.0%, 94.9%, and 100% of Riverdale, Fresno State, and control CQ1 mosquitoes, respectively, were knocked down. By 12 h post exposure 89.5%, 94.9%, and 100%, of Riverdale, Fresno State, and CQ1 mosquitoes were dead.
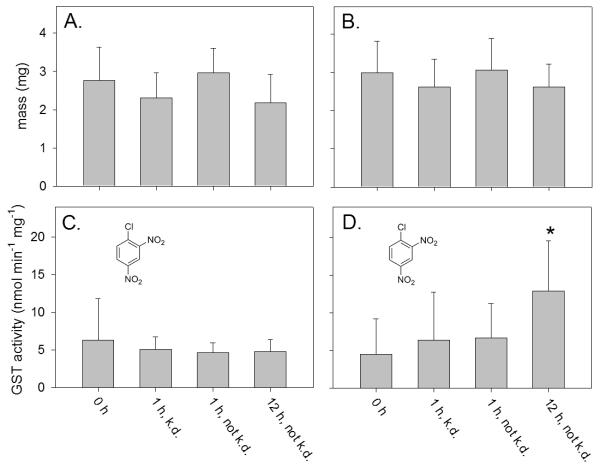
The average mass of untreated adult mosquitoes that were captured in Riverdale (2.8 ± 0.9 mg, n = 20) was not statistically different than that of adult mosquitoes that were captured on the campus of CSU Fresno (3.0 ± 0.8 mg, n = 22). Although the average age of the field-collected adults was unknown, these field-collected adults were about 2-fold heavier than the laboratory-reared 2 day old CQ1 adults (1.5 ± 0.4 mg, n = 40). The average mass of Riverdale and Fresno State adults that were knocked down or not knocked down following insecticide exposure was not statistically different than that of untreated adults (Figure 1A and 1B). This indicated that, at least with the exposure dose that was used in this study, the ability of the field-collected adults to resist knockdown was not dependent upon greater mass.
Figure 1.
Mass and GST activity of Cx. pipiens s.l. complex mosquitoes captured in Riverdale, California (A and C) or on the campus of California State University Fresno (B and D). The mass (A and B) and GST activity (C and D) of individual adult mosquitoes were measured prior to exposure (0 h), or at 1 h or 12 h post exposure to a commercial formulation of pyrethrins. The mass and GST activity were determined in individual mosquitoes that were knocked down (k.d.) or not k.d. GST activity was measured using the general GST substrate CDNB. Background hydrolysis of CDNB was undetectable under the assay conditions used in this study. The error bars indicate the standard deviation of the mean value of each treatment group. The asterisk (*) indicates a significant difference (p<0.001) between the mean value of control mosquitoes (0 h) and pyrethrin exposed mosquitoes.
3.2. Characterization of general GST activity with CDNB
Untreated adults that were captured in Riverdale (Figure 1C) and on the campus of CSU Fresno (Figure 1D) showed similar levels of GST activity when CDNB was used as a substrate to measure activity. This suggested that both Riverdale and Fresno State mosquitoes had similar levels of exposure to environmental chemicals that induce GST activities prior to capture. At 1 h post insecticide exposure (Figs. 1C and 1D), the adults from both populations showed similar GST activity regardless of whether they were knocked down or not knocked down by the insecticide. At 12 h post insecticide exposure, Fresno State adults that were not knocked down showed 2.8-fold higher GST activity in comparison to untreated Fresno State adults (i.e., 0 h control) when CDNB was used to measure activity (Figure 1D). The GST activity of Riverdale adults that were not knocked down, however, was similar to that of untreated control adults at 12 h post insecticide exposure (Figure 1C). Because CDNB is a general substrate for GSTs, it is unclear if the higher CDNB metabolic activity that was found in Fresno State adults that were not knocked down was due to a potential pyrethrin-selective GST or the activity of GSTs in general.
3.3. Characterization of pyrethroid-selective GST activity
Our laboratory has developed a series of pyrethroid-like, ester containing fluorescent substrates that are selectively metabolized by a Delta class GST called CpGSTD1 (Huang et al. 2012). CpGSTD1 originates from a pyrethroid-resistant Cx. pipiens and shows 10- to 170-fold higher specific activity for our pyrethroid-like substrates in comparison to purified GSTs from mouse. In this study, we used two of our fluorescent substrates, cis-DCVC and cis-TFMCVC, to measure GST and esterase activities in individual adult mosquitoes. cis-DCVC and cis-TFMCVC are esters composed of a pyrethroid-like acid and fluorescent coumarin-based alcohol. The coumarin-based alcohol is a good leaving group and produces the same fluorescent metabolite when metabolized by a GST or esterase (Huang et al. 2012). Our substrates can thus be used to measure GST activity when in the presence of an esterase inhibitor such as OTFP (Abdel-Aal & Hammock 1985). Or they can be used to measure esterase activity when in the presence of a GSH depleting agent or GST inhibitor (Samra et al. 2012). When cis-DCVC was used as a substrate, no statistically significant difference in GST activity was found in untreated Riverdale, Fresno State, and CQ1 adults (5.39 ± 2.19, 5.87 ± 3.52, and 4.92 ± 2.64 nmol min−1 mg−1, respectively). Similarly, when cis-TFMCVC was used as a substrate, no statistically significant difference in GST activity was found in untreated Riverdale, Fresno State, and CQ1 adults (6.83 ± 4.12, 9.60 ± 6.19, and 11.58 ± 5.35 nmol min−1 mg−1, respectively). Following pyrethrin exposure (1 h or 12 h post exposure), no statistically significant difference in GST activity was found in Riverdale or Fresno State adults when either cis-DCVC or cis-TFMCVC was used to measure activity (Figure 2). Although the CDNB assay identified a 2.8-fold elevation in general GST activity in Fresno State adults that were knocked down at 12 h post insecticide exposure, no such increase was found when our pyrethroid-like, fluorescent substrates were used (Figure 2B and 2D). This suggested that pyrethrin exposure does not select for mosquitoes with a pyrethrin-selective GST or that such an activity is not present in these mosquitoes or that it is not induced within the time frame of the test. cis-DCVC and cis-TFMCVC, however, are cis-compounds that are not identical mimics of the natural pyrethrins I and II. Thus, the activity of a potential pyrethrin-selective GST may have been gone undetected. Although our results do not exclude the possibility that GSTs potentially function as pyrethrin binding proteins, they suggest that the metabolism of pyrethrins by GSTs does not make a major contribution in conferring pyrethrin resistance.
3.4. Characterization of pyrethroid-selective esterase activity
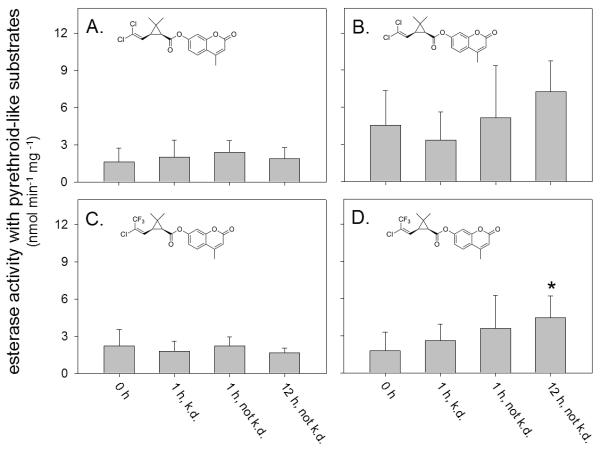
Esterase activity in the field-collected mosquitoes was determined using cis-DCVC and cis-TFMCVC. In order to determine pyrethroid-selective esterase activity, total activity (i.e., GST activity and esterase activity) was first determined using each fluorescent substrate in the absence of OTFP. Subsequently, the difference between the total activity and GST-selective activity (i.e., activity in the presence of OTFP) was taken as the pyrethroid-selective esterase activity. When cis-DCVC was used as a substrate, esterase activity in untreated Fresno State adults (4.55 ± 2.78 nmol min−1 mg−1) was 2.8-and 3.3-fold higher than that found in untreated Riverdale (1.62 ± 1.10 nmol min−1 mg−1) and CQ1 (1.37 ± 0.68 nmol min−1 mg−1) adults, respectively. On the other hand, when cis-TFMCVC was used as a substrate, no statistically significant difference in esterase activity was found in untreated Riverdale (2.23 ± 1.33 nmol min−1 mg−1), untreated Fresno State (1.77 ± 1.50 nmol min−1 mg−1), and CQ1 (1.75 ± 1.04 nmol min−1 mg−1) adults.
Following pyrethrin exposure (1 h or 12 h), no statistically significant difference in esterase activity was found in Riverdale or Fresno State adults in comparison to unexposed mosquitoes when cis-DCVC was used to measure activity (Figure 3A and 3B). Similarly, no statistically significant difference in esterase activity was found in Riverdale mosquitoes adults following pyrethrin exposure when cis-TFMCVC was used to measure activity. However, when cis-TFMCVC was used to measure esterase activity, 2.5-fold higher activity was found in Fresno State adults that were not knocked down at 12 h post pyrethrin exposure in comparison to untreated controls. This suggests that pyrethrin-selective esterase activity, at least partially, helps these mosquitoes to resist pyrethrin knock down.
Figure 3.
Esterase activity of adult Cx. pipiens s.l. complex mosquitoes captured in Riverdale, California (A and C) or on the campus of California State University Fresno (B and D). The esterase activity was measured using the pyrethroid-like fluorescent substrates cis-DCVC (A or B) or cis-TFMCVC (C and D). Esterase activity of individual mosquitoes was measured prior to exposure (0 h), or at 1 h or 12 h post exposure to a commercial formulation of pyrethrins. Esterase activity was determined in individual mosquitoes that were knocked down (k.d.) or not k.d. Background hydrolysis of the fluorescent substrates was undetectable under the assay conditions used in this study. The error bars indicate the standard deviation of the mean value of each treatment group. The asterisk (*) indicates a significant difference (p<0.001) between the mean value of control mosquitoes (0 h) and pyrethrin exposed mosquitoes.
The ester functional group of natural pyrethrins and synthetic pyrethroids is generally resistant to base and enzyme catalyzed hydrolysis. This is because the carbonyl moiety of pyrethrins and most pyrethroids is conjugated through the cyclopropane to a double bond. The bond strain of the cyclopropane also gives an aromatic nature to the cyclopropane. This conjugated system increases the activation energy required for a nucleophile to attack the ester carbonyl and reduces the rate of hydrolysis. Pyrethrins and some pyrethroids are also stabilized to esterase attack by steric hindrance. Allethrin and the natural pyrethirns are secondary alcohols whose hydrolysis is hindered by the bulky cyclopentenone ring. Steric hindrance by an α-cyano alcohol is found in some but not all pyrethroids. In our earlier work, we made chemically stable red-shifted fluorescent alcohol moieties as pyrethroid-like reporters (Shan & Hammock 2001). These fluorescent pyrethroid mimics, however, are turned over too slowly to be useful for monitoring esterase activity in individual mosquitoes. Thus, pyrethroid acids were conjugated to coumarin as a fluorophore resulting in compounds such as cis-DCVC and cis-TFMCVC with ester bonds which are less stable and more readily hydrolzed (Huang et al. 2012). Although these second generation substrates are less similar to α-cyano pyrethroids, coumarin is such a good leaving group that they can be used to monitor esterase and GST activities in individual mosquitoes. Even though coumarin is such a good leaving group, these substrates did not show detectable background base hydrolysis under the assay conditions that were developed in this study.
The rapid, population level detection of insecticide resistance is crucial for maintaining the effectiveness of chemical insecticides against disease vectors. The genome of Cx. quinquefasciatus encodes more than 200 detoxification genes (mostly P450s, but also dozens of carboxylesterases and GSTs) (Arensburger et al. 2010). However, only a few of the proteins expressed by these genes are likely to be involved in the metabolism of permethrin and/or pyrethroid insecticides. Thus, it is important to be able to selectively identify specific isozymes that are involved in permethrin and pyrethroid detoxification. We believe that substrates such as cis-DCVC and cis-TFMCVC that mimic the structure of pyrethroids/pyrethrins are critical for the development of sensitive and efficient assays to detect these detoxification-specific isozymes. The enzyme assays that we developed using our pyrethroid-like substrates were able to efficiently detect elevated pyrethroid-selective esterase activity from individual, field-collected adult mosquitoes that were exposed to a commercial formulation of pyrethrins. Furthermore, our assays were able to distinguish between an increase in general GST activity and GST activity that is selective for pyrethroid-like compounds.
In addition to esterases and potentially GSTs, members of the highly diverse cytochrome P450 monooxygenases family of detoxification enzymes are known to metabolize pyrethroid insecticides (reviewed in Nkya et al. 2013). In this study, the commercial pyrethrin formulation contained the oxygenase inhibitor PBO. Thus, we assumed that cytochrome P450 monooxygenase activity played a limited role in the induction of resistance. On the other hand, our preliminary PCR-based experiments suggest that target site (i.e., sodium channel) insensitivity likely plays a significant role in the knock down resistance that we observed in Riverdale and Fresno State mosquitoes. We are investigating whether there is a correlation between known kdr mutations within the sodium channel gene and increases in esterase/GST activities in mosquitoes that were knocked down following pyrethrin exposure.
4. Conclusions
Resistance to pyrethroid insecticides has been demonstrated in numerous insect species of agricultural and public health importance including those found in Culex. In this study, we exposed field-collected mosquitoes to a commercial pyrethrin insecticide formulation that was applied at a rate and spray methodology that is in common use by vector control districts. Under these conditions, we showed that our fluorescent substrates are a useful surveillance tool that can be further developed into a fast and simple assay for the detection of esterase and GST activities in individual adult mosquitoes.
Acknowledgements
We thank Huazhang Huang for the synthesis of the fluorescent substrates as well as Tac Tsan, Luis A. Perez, and Grant H. Oshita for help with the assays.
Funding
This work was supported by Mosquito Research Foundation under grant #201222676; and the National Institute of Environmental Health Sciences under grant #R01 ES002710.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdel-Aal YAI, Hammock BD. 3-Octylthio-1,1,1-trifluoro-2-propanone, a high affinity and slow binding inhibitor of juvenile hormone esterase from Trichoplusia ni (Hubner) Insect Biochem. 1985;15:111–122. [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–88. doi: 10.1126/science.1191864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, Vontas J, Field LM. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malaria J. 2007;6 doi: 10.1186/1475-2875-6-111. Epub 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4:605–613. doi: 10.3201/eid0404.980410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid HA, Hammad RE, Hassan MM, Morsy ZS, Kamal IH, Weil GJ, Ramzy RMR. Detection of Wuchereria bancrofti in mosquitoes by the polymerase chain reaction: a potentially useful tool for large-scale control programmes. Trans Roy Soc Trop Med Hyg. 2001;95:29–32. doi: 10.1016/s0035-9203(01)90322-0. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Huang H, Yao H, Liu J-Y, Samra AI, Kamita SG, Cornel AJ, Hammock BD. Development of pyrethroid-like fluorescent substrates for glutathione S-transferase. Anal Biochem. 2012;431:77–83. doi: 10.1016/j.ab.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu AB, Waite TD, Christiansen JA, McAbee RD, Kamita SG, Hammock BD, Cornel AJ. A rapid luminescent assay for measuring cytochrome P450 activity in individual larval Culex pipiens complex mosquitoes (Diptera: Culicidae) J Med Entomol. 2009;46:83–92. doi: 10.1603/033.046.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Khambay BPS, Jewess PJ. Comprehensive Molecular Insect Science. Elsevier; Oxford: 2005. Pyrethroids; pp. 1–30. [Google Scholar]
- Lai CH, Tung KC, Ooi HK, Wang JS. Competence of Aedes albopictus and Culex quinquefasciatus as a vector of Dirofilaria immitis after blood meal with different microfilarial density. Vet Parasitol. 2000;90:231–237. doi: 10.1016/s0304-4017(00)00242-9. [DOI] [PubMed] [Google Scholar]
- Li T, Zhang L, Reid WR, Xu Q, Dong K, Liu NN. Multiple mutations and mutation combinations in the sodium channel of permethrin resistant mosquitoes, Culex quinquefasciatus. Sci Rep. 2012;2:781. doi: 10.1038/srep00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HQ, Cupp EW, Micher KM, Guo AG, Liu NN. Insecticide resistance and cross-resistance in Alabama and Florida strains of Culex quinquefaciatus. J Med Entomol. 2004;41:408–413. doi: 10.1603/0022-2585-41.3.408. [DOI] [PubMed] [Google Scholar]
- Liu N, Xu Q, Zhu F, Zhang L. Pyrethroid resistance in mosquitoes. Insect Sci. 2006;13:159–166. [Google Scholar]
- Macedo PA, Schleier JJ, Reed M, Kelley K, Goodman GW, Brown DA, Peterson RKD. Evaluation of efficacy and human health risk of aerial ultra-low volume applications of pyrethrins and piperonyl butoxide for adult mosquito management in response to West Nile virus activity in Sacramento County, California. J Am Mosq Control Assoc. 2010;26:57–66. doi: 10.2987/09-5961.1. [DOI] [PubMed] [Google Scholar]
- McAbee RD, Kang KD, Stanich MA, Christiansen JA, Wheelock CE, Inman AD, Hammock BD, Cornel AJ. Pyrethroid tolerance in Culex pipiens pipiens var molestus from Marin County, California. Pest Manag Sci. 2003;60:359–368. doi: 10.1002/ps.799. [DOI] [PubMed] [Google Scholar]
- Meegan JM. The Rift Valley fever epizootic in Egypt 1977-1978. 1. Description of the epizootic and virological studies. Trans Roy Soc Trop Med Hyg. 1979;73:618–623. doi: 10.1016/0035-9203(79)90004-x. [DOI] [PubMed] [Google Scholar]
- Nelson DB, Chamberlain RW. A light trap and mechanical aspirator operating on dry cell batteries. Mosq News. 1955;15:28–32. [Google Scholar]
- Nkya TE, Akhouayri I, Kisinza W, David JP. Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects. Insect Biochem Molec Biol. 2013;43:407–416. doi: 10.1016/j.ibmb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Samra AI, Kamita SG, Yao HW, Cornel AJ, Hammock BD. Cloning and characterization of two glutathione S-transferases from pyrethroid-resistant Culex pipiens. Pest Manag Sci. 2012;68:764–772. doi: 10.1002/ps.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M, Baruah I, Srivastava RB, Borkotoki A, Bhattacharyya IK. High-throughput approach to detection of knockdown resistance (kdr) mutation in mosquitoes, Culex quinquefasciatus, based on real-time PCR using single-labelled hybridisation probe/melting curve analysis. Pest Manag Sci. 2011;67:156–161. doi: 10.1002/ps.2044. [DOI] [PubMed] [Google Scholar]
- Shan G, Hammock BD. Development of sensitive esterase assays based on α-cyano-containing esters. Anal Biochem. 2001;299:54–62. doi: 10.1006/abio.2001.5388. [DOI] [PubMed] [Google Scholar]
- Townzen KR, Natvig HL. A disposable adult mosquito bioassay cage. Mosq News. 1973;33:113–114. [Google Scholar]
- Tsai TF, Smith GC, Happ CM, Kirk LJ, Jakob WL, Bolin RA, Francy DB, Lampert KJ. Surveillance of St. Louis encephalitis virus vectors in Grand Junction, Colorado, in 1987. J Am Mosq Control Assoc. 1989;5:161–165. [PubMed] [Google Scholar]