Abstract
The study presented herein constitutes an extensive investigation of constituents in Hydrastis canadensis L. (Ranunculaceae) leaves. It describes the isolation and identification of two previously unknown compounds, 3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid (1) and 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2), along with the known compounds (±)-chilenine (3), (2R)-5,4′-dihydroxy-6-C-methyl-7-methoxy-flavanone (4), 5,4′-dihydroxy-6,8-di-C-methyl-7-methoxy-flavanone (5), noroxyhydrastinine (6), oxyhydrastinine (7) and 4′,5′-dimethoxy-4-methyl-3′-oxo-(1,2,5,6-tetrahydro-4H-1,3-dioxolo-[4′,5′:4,5]-benzo[1,2-e]-1,2-oxazocin)-2-spiro-1′-phtalan (8). Compounds 3-8 have been reported from other sources, but this is the first report of their presence in H. canadensis extracts. A mass spectrometry based assay was employed to demonstrate bacterial efflux pump inhibitory activity against Staphylococcus aureus for 2, with an IC50 value of 180 ± 6 μM. This activity in addition to that of other bioactive compounds such as flavonoids and alkaloids, may explain the purported efficacy of H. canadensis for treatment of bacterial infections. Finally, this report includes high mass accuracy fragmentation spectra for all compounds investigated herein which were uploaded into the Global Natural Products Social molecular networking library and can be used to facilitate their future identification in H. canadensis or other botanicals.
Keywords: Hydrastis canadensis, goldenseal, alkaloids, flavonoids, efflux pump inhibitors
1. Introduction
The medicinal plant Hydrastis canadensis L. (Ranunculaceae) has a long history of use for the treatment of infections. Native Americans, particularly the Cherokee, used goldenseal roots to treat skin and eye infections, while other populations have used goldenseal tonics to treat gastrointestinal irritation (Foster et al., 2000). H. canadensis roots have been extensively profiled (Le et al., 2013; Bharathi et al., 2012; McNamara et al., 2004), although only a few reports have described the composition of H. canadensis leaves (Junio et al., 2011; Douglas et al., 2010). H. canadensis has been of recent interest due to its ability to inhibit the growth of pathogenic bacteria, including Staphylococcus aureus (Cech et al., 2012). This activity was originally attributed to the antimicrobial alkaloid berberine and to other alkaloids that the plant contains (Knight, 1999; Scazzocchino et al., 2001; Hwang et al., 2003). Recently, it has been shown that the activity of H. canadensis leaves is more complex. Three flavonoids, sideroxylin, 6-desmethyl sideroxylin and 8-desmethyl sideroxylin (Junio et al., 2011) were shown to synergistically enhance the antimicrobial activity of goldenseal alkaloids. These flavonoids act as bacterial efflux pump inhibitors, facilitating accumulation of berberine within bacterial cells and thereby reducing the necessary quantity of berberine (or other alkaloids) to achieve antimicrobial activity (Junio et al., 2011).
Botanicals are chemically complex and contain many compounds that may possess diverse structures and biological activities. On the basis of the previously reported interesting biological activity of H. canadensis leaves, we endeavored to conduct more in-depth chemical profiling of this botanical. With these studies, we sought to identify efflux pump inhibitors from H. canadensis, and to generate a more comprehensive profile of chemical compounds in this botanical than has previously been published.
2. Results and Discussion
2.1. Structures of isolated compounds
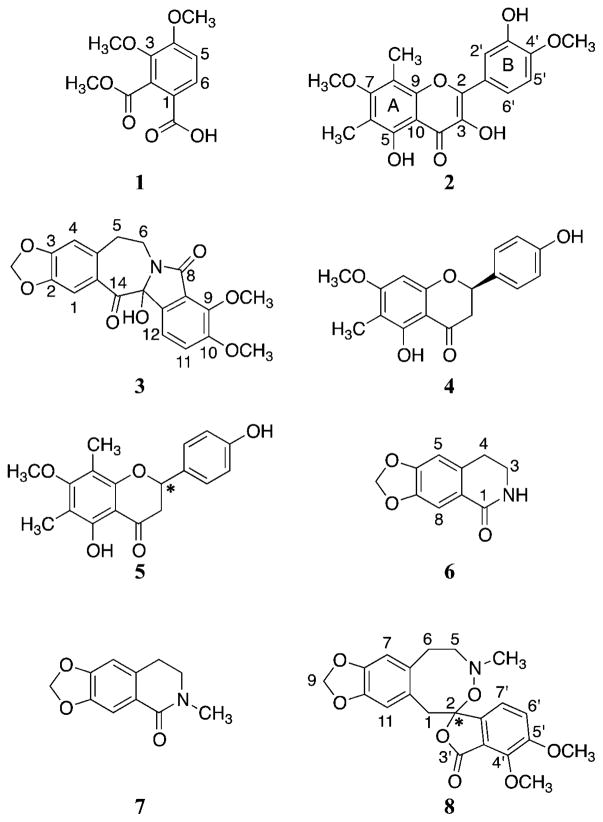
Investigation of Hydrastis canadensis leaves led to isolation of two new compounds, 3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid (1) and 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2), together with six additional compounds (3-8) that are known but new to Hydrastis canadensis (Fig. 1). (±)-Chilenine (3), an isoindolobenzazepine alkaloid, was previously reported from Berberis enpetrifolia (Fajardo et al., 1982); flavonones (2R)-5,4′-dihydroxy-6-C-methyl-7-methoxy-flavanone (4) and 5,4′-dihydroxy-6,8-di-C-methyl-7-methoxy-flavanone (5) were isolated from leaf wax of Callistemon coccineus (Wollenweber et al., 2000); and the isoquinolone derivatives noroxyhydrastinine (6) and oxyhydrastinine (7) were obtained from Thalictrum minus and Hypecoum erectum, respectively (Doskotch et al., 1969; Zhang et al., 1995). Compound 8, 4′,5′-dimethoxy-4-methyl-3′-oxo-(1,2,5,6-tetrahydro-4H-1,3-dioxolo-[4′,5′:4,5]-benzo[1,2-e]-1,2-oxazocin)-2-spiro-1′-phtalan, was reported previously as a product of β-hydrastine-N-oxide under reflux conditions (Klötzer and Oberhänsli, 1973). Given that β-hydrastine is an abundant constituent of H. canadensis (Le et al., 2014), it is possible that compound 8 is an isolation artifact and not a constituent of H. canadensis. Additionally, nine compounds known to be constituents of H. canadensis were also isolated. These include berberine (9) (Qiu et al., 2008), (−)-canadine (10) (Malhotra et al., 1989), sideroxylin (11), 6-desmethyl-sideroxylin (12), 8-desmethyl-sideroxylin (13) (Junio et al., 2011), β-hydrastine (14) (Seger et al., 2004), (−)-8-oxocanadine (15), 8-oxotetrahydrothalifendine (16) (Pinho et al., 1992), and oxyberberine (17) (Singh et al., 2010). The structures of these known compounds were determined by comparing their spectroscopic data with those reported in the literature.
Fig. 1.
Structures of compounds 1-8, which are reported for this first time in this report as constituents of goldenseal (Hydrastis canadensis). The configuration at locations with asterisks are unknown.
Compound 1 was obtained as white amorphous powder. High resolving power electrospray ionization mass spectrometry (HRESIMS) analysis indicated an ion at m/z 241.0702 [M+H]+ (calcd for C11H13O6+, 241.0707), suggesting six degrees of unsaturation. The NMR spectral data (Table 1) allowed the assignment of two aromatic protons (δH = 6.98 and 7.87) and three methoxy groups (δH = 3.87, 3.94 and 3.95). The HMBC analysis allowed correlation of two of the methoxy groups, (δH = 3.87 and 3.95) with carbon C-3 and C-4 (δC = 145.9 and 157.4), respectively. Additionally, the correlation between H-5 (δH = 6.98) and the resonance at δC = 145.9 (C-3), and between H-6 (δH = 7.87) with δC = 157.4 (C-4) supported the placement of the methoxy groups. The HMBC correlations between H-5 (δH = 6.98) and δC = 118.7, and H-6 (δH = 7.87) with δC = 131.8 supported the assignment of the carboxylic group at C-1 and the methoxycarbonyl group at C-2 (Fig. 2). Therefore, the structure of 1 was established as 3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid.
Table 1.
1H (400 MHz) and 13C (100 MHz) NMR spectroscopic dataa for 3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid (1).
| Position | δC | δH, m (J in Hz) | HMBCb |
|---|---|---|---|
| 1 | 118.7 | ||
| 2 | 131.8 | ||
| 3 | 145.9 | ||
| 4 | 157.4 | ||
| 5 | 112.2 | 6.98, d (8.8) | C-1, C-3 |
| 6 | 128.4 | 7.87, d (8.8) | C-2, C-4, C-1a |
| 1a | 168.6 | ||
| 2a | 167.5 | ||
| 3-OCH3 | 61.9 | 3.87, s | C-3 |
| 4-OCH3 | 56.3 | 3.95, s | C-4 |
| 2a-OCH3 | 53.0 | 3.94, s | C-2a |
H and C chemical shifts with reference to CDCl3 (δH = 7.26 ppm) and CDCl3 (δC = 77.16 ppm), respectively.
HMBC correlations are from the proton stated to the indicated carbon.
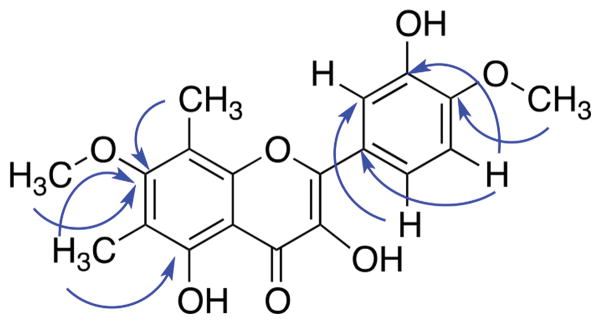
Fig. 2.
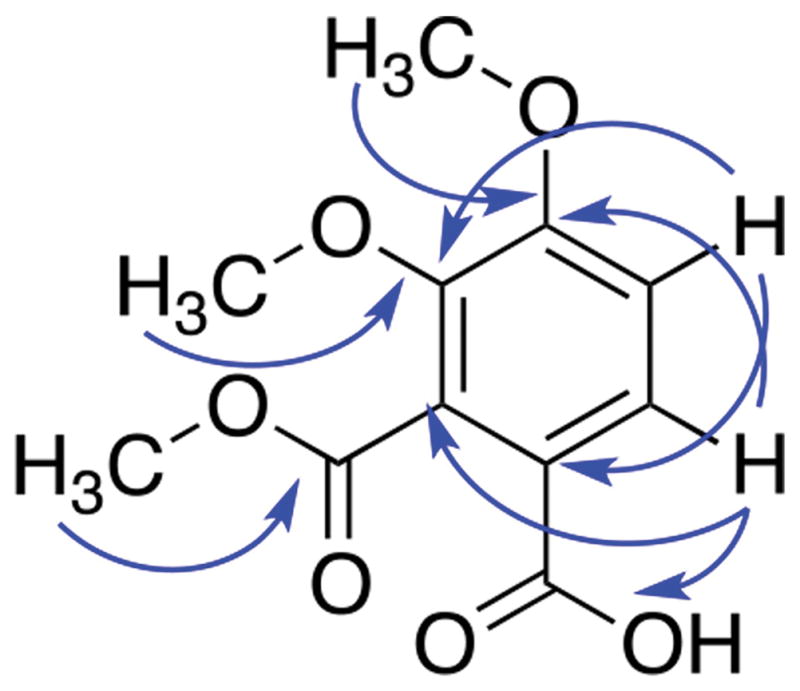
HMBC correlations of 1.
Compound 2, obtained as a yellow amorphous powder, showed in HRESIMS an ion at m/z 359.1129 [M+H]+ (calcd for C19H19O7, 359.1125). The UV maxima absorption bands at λmax 377, 346 and 258 nm were suggestive of a flavone skeleton, given that the absorbance maxima of flavonols are generally at longer wavelengths (350–385 nm) (Tsimogiannis et al., 2007). The 1H-NMR data (Table 2) indicated the presence of two aromatic methoxy groups (δH = 3.80 and 4.00), two C-methyls (δH = 2.41 and 2.23), and three aromatic protons (δH = 7.87, 7.82 and 7.00). Analysis of the 13C-NMR spectrum (Table 2) showed the presence of a α,β-unsaturated carbonyl (δC = 175.9) and a signal at δC = 136.2, which together with the proton signals of the aromatic rings corresponding to a flavonol skeleton. HMBC correlations of 7-OCH3 (δH = 3.80), 6-CH3 (δH = 2.23) and 8-CH3 (δH = 2.41) methyl protons with C-7 (δC = 163.0) support the position of the substituents in ring A (Fig. 3). In addition, the methoxy and hydroxy group in the B ring were assigned based on the correlation between 4′-OCH3 (δH = 4.00) with the C-4′ (δC = 148.4), and the correlations between 5′-H (δH = 7.00) with C-1′ (δC = 124.6) and C-3′ (δC = 145.8) in the HMBC spectrum. Additionally, the HMBC correlations between 2′-H (δH = 7.82) and 6′-H (δH = 7.87) with C-2 (δC = 145.8) supported the connectivity of ring B to C-2 (ring C) (Fig. S10). On the basis of this evidence, the compound was determined to be 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone.
Table 2.
1H (700 MHz) and 13C (175 MHz) NMR spectroscopic dataa for 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2).
| Position | δC | δH, m (J in Hz) | HMBCb |
|---|---|---|---|
| 2 | 145.8 | ||
| 3 | 136.2 | ||
| 4 | 175.9 | ||
| 5 | 156.0 | ||
| 6 | 113.4 | ||
| 7 | 163.0c | ||
| 8 | 109.4 | ||
| 9 | 152.4 | ||
| 10 | 105.8 | ||
| 1′ | 124.6 | ||
| 2′ | 113.7 | 7.82, d (2.1) | C-2, C-6′ |
| 3′ | 145.8 | ||
| 4′ | 148.4 | ||
| 5′ | 110.7 | 7.00, d (9.1) | C-1′, C-3′ |
| 6′ | 121.1 | 7.87, dd (9.1, 2.1) | C-2, C-2′ |
| 6-CH3 | 8.4 | 2.23, s | C-5, C-6, C-7 |
| 8-CH3 | 8.7 | 2.41, s | C-7, C-8, C-9 |
| 7-OCH3 | 60.8 | 3.80, s | C-7 |
| 4′-OCH3 | 56.2 | 4.00, s | C-4′ |
| 3-OH | 6.64 | C-3, C-4 | |
| 3′-OH | 5.71 | C-2′, C-3′ | |
| 5-OH | 11.80 | C-5, C-10 |
H and 13C chemical shifts with reference to CDCl3 (δH = 7.26 ppm) and CDCl3 (δC = 77.16 ppm), respectively.
HMBC correlations are from the proton stated to the indicated carbon.
obtained from HMBC
Fig. 3.
HMBC correlations of 2.
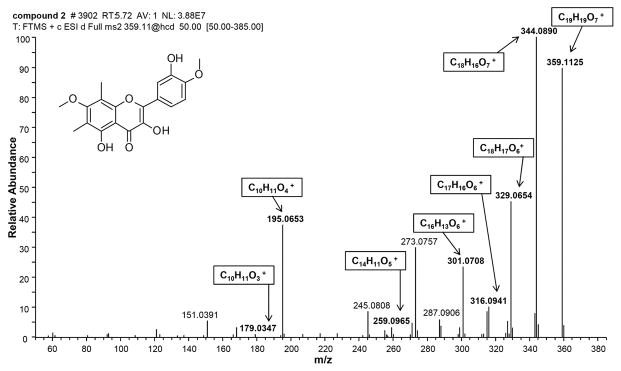
Reisolation of known compounds from botanical extracts is a common problem when seeking to identify novel compounds from botanicals such as H. canadensis. To facilitate future identification of compounds 1–17 in botanical mixtures, tandem high resolving power electrospray ionization mass spectrometry (HRESIMS-MS) was employed to collect fragmentation spectra of all seventeen compounds in both the positive ion mode (Table S1) and the negative ion mode (Table S2). Notably, these fragmentation spectra were collected with high mass accuracy (<10 ppm), enabling the confirmation of molecular formulae of many of the fragments. An example of such a high resolution fragment spectrum is provided in Fig. 4 for 2. High mass accuracy measurements of fragmentation data enable assignment of molecular formulae not just for the intact molecule, but also for its fragments. The fragments with m/z of 344.0890, 329.0654, and 316.0941 represent rearrangements and losses from the C ring, and those with m/z of 301.0708 and 259.0965 represent a partial loss of the C ring with bonds formed with the hydroxyl group at carbon 3. The fragments with m/z of 195.0653 and 179.0347 represent the remaining A ring along with the ketone at carbon 4. An additional fragmentation spectrum of berberine (9), the most abundant alkaloid present in goldenseal (Le et al., 2014) can be found as Supporting Information (Fig. S26). Spectra for 2, berberine, and the remaining H. canadensis compounds identified herein were uploaded into the Global Natural Product Social molecular networking library to facilitate identification of these compounds or their structural analogues by other researchers (Wang et al., 2016). Fragment masses can also be found in Tables 1S and 2S.
Fig. 4.
Accurate mass fragmentation spectrum of 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2) in the positive polarity. Fragments and molecular formulas were predicted using ACD MS fragmenter (Advanced Chemistry Development, Inc. Toronto, Canada) and compared to experimental data. Bold ions had a mass error within 10 ppm of the associated molecular formula.
2.2. Efflux pump inhibitory activity of isolated compounds
The two new compounds isolated as part of this study [3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid (1) and 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2)], as well as the compounds 4 and 16 were tested for biological activity. Specifically, a mass spectrometry based assay was employed to evaluate the ability of these compounds to inhibit efflux of an efflux pump substrate (ethidium bromide) from Staphylococcus aureus cells (Brown et al., 2015). The rationale for evaluating efflux pump inhibitory activity is that drug efflux constitutes a major form of antibiotic resistance in bacteria (Kaatz, 2005). Thus, compounds that prevent efflux of toxins from cells have the potential of contributing to antimicrobial activity. Efflux inhibitory activity against Staphylococcus aureus is particularly relevant given that this pathogen is responsible for approximately 50% of all skin infections, and Hydrastis canadensis is traditionally used in the treatment of such infections (McCaig et al., 2006).
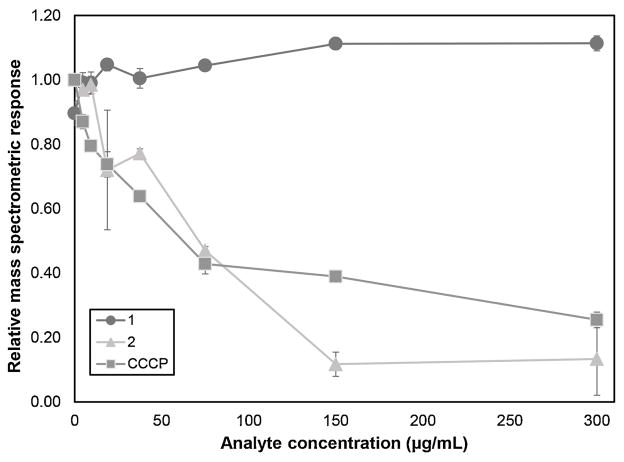
Compound 2 demonstrated moderate inhibitory activity of efflux from Staphylococcus aureus with an IC50 value of 180 ± 6 μMCompounds 1, 4 and 16 were inactive. Fig. 5 shows the raw experimental data evaluating the efflux pump inhibitory activities of compounds 1, 2, and the positive control, carbonyl cyanide m-chloro phenylhydrazone (CCCP), which had an IC50 of 270 ± 50 μM. The data shown are relative quantities (as measured by mass spectrometric peak area) of ethidium ion in the spent bacterial media after exposure to an increasing amount of the test compound or control. As demonstrated for both CCCP and 2, when efflux is blocked, the quantity of ethidium present in the media decreases (Fig. 5). Ethidium concentration remains high regardless of concentration for the compound that does not possess efflux inhibitory activity (Compounds 1, 4 & 16).
Fig. 5.
Efflux pump inhibition assay data for 3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid (1) and 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2). The positive control for this assay is carbonyl cyanide m-chloro-phenylhydrazone (CCCP), a compound that inhibits efflux by collapsing the proton motive force across the cell membrane (Hopfer et al., 1967). Each data point is the mean of triplicate measurements from separate bacterial cultures (biological replicates) and error bars represent standard error of the mean.
4. Experimental
4.1. General experimental procedures
Optical rotations at the sodium D-line wavelength of pure compounds were measured with a Rudolph Research Autopol (II) Polarimeter. 1D and 2D NMR spectra were recorded using a JEOL ECS-400 NMR spectrometer equipped with a high sensitivity JEOL Royal probe operating at 400 MHz for 1H and 100 MHz for 13C, or an Agilent 700 MHz NMR spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a cryoprope, operating at 700 MHz for 1H and 175 MHz for 13C. Chemical shifts are reported as δ values (ppm), and coupling constants (J) were measured in Hz. HRESIMS was performed on a Thermo LTQ Orbitrap XL mass spectrometer equipped with an electrospray ionization source. HPLC was carried out using a Varian ProStar HPLC system equipped with ProStar 210 pumps and a ProStar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2). For preparative HPLC, a Phenomenex Gemini-NX C18 Column (5 μm; 250 × 21.2 mm) was used at a 21 mL/min flow rate. Flash chromatography was performed on a Teledyne ISCO CombiFlash® Rf using 80 g or 120 g RediSep® RF Silica Column (35 – 70 μm particle size) and 12 g RediSep® Rf Gold HP Silica Columns (20 – 40 μm particle size, Teledyne ISCO, Lincoln, NE, USA); and monitored by UV and evaporative light-scattering detectors. UV spectra were measured with a ProStar 335 photodiode array UV detector (PDA) and the reported λmax values were collected from the spectra for relevant compounds eluting from the HPLC. All other reagents and solvents were obtained from Fisher Scientific and were used without further purification.
4.2. Plant material
Hydrastis canadensis L. (Ranunculaceae) was collected in Hendersonville, North Carolina (NC, N 3524.2770, W 08220.9930, 702.4 m elevation), in July 2013. The plants were cultivated in their native environment, a hardwood forest understory. A voucher specimen (NCU583414) was deposited in the University of North Carolina Herbarium, Chapel Hill, NC and the identity was verified by herbarium director Dr. Alan S. Weakley.
4.3. Extraction and isolation
The isolation scheme is provided as Supporting Information (Fig. S1). Batches of dried H. canadensis plant were pulverized into fine powder using a commercial coffee grinder (Kitchen Aid). H. canadensis powder was percolated in MeOH overnight, and the MeOH extract was concentrated in vacuo and subjected to liquid-liquid partition, as described previously (Junio et al., 2011). This concentrated extract was defatted by partitioning between 10% aqueous MeOH and hexane (1:1), and the aqueous MeOH fraction was partitioned further between EtOAc:MeOH:H2O (4:1:5). The organic layer was washed with 1% saline solution to remove tannins.
The first stage of normal-phase flash chromatography (120 g silica gel column) was conducted with a Hex/CHCl3/MeOH gradient, yielding 8 primary fractions (FI-FVIII). Fraction FII, FIII and FIV were subjected to a second stage of normal-phase flash chromatography (80 g silica gel column) with a Hex/EtOAc/MeOH gradient to give 5 (FII1-FII5), 3 (FIII1-FIII3) and 6 (FIV1-FIV6) subfractions, respectively.
The compounds were purified using reversed-phase preparative HPLC with a Phenomenex Gemini-NX C18 column at a 21 mL/min flow rate. Fraction FII2 (32.5 mg) (eluents A: H2O 0.1% formic acid, B: CH3CN, gradient: B 45% at time 0, B 75% at time 20 min, B 100% at time 25 min) yielded the compounds (2R)-5,4′-dihydroxy-6-C-methyl-7-methoxy-flavanone (4; 1.5 mg), 5,4′-dihydroxy-6,8-di-C-methyl-7-methoxy-flavanone (5; 0.7 mg), and 3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2; 0.6 mg).
Fractions FII3 (30.1 mg), FII4 (7.3 mg) and FIII1 (26.9 mg) were purified (eluents A: H2O 0.1% formic acid, B: CH3CN, gradient: B 35% at time 0, B 90% at time 27 min, B 100% at time 30 min) to obtain oxyhydrastinine (7; 2.0 mg), chilenine (3; 0.8 mg) for the first fraction, noroxyhydrastinine (6; 0.9 mg) for the second fraction, and (2R)-5,4′-dihydroxy-6-C-methyl-7-methoxy-flavanone (4; 0.5 mg) for the last fraction.
Fraction FIV3 (96.3 mg) (eluents A: H2O 0.1% formic acid, B: CH3CN, gradient: B 30% at time 0, B 90% at time 20 min, B 100% at time 22 min) and fraction FIV5 (42.3 mg) (eluents A: H2O 0.1% formic acid, B: CH3CN, gradient: B 30% at time 0, B 65% at time 20 min, B 100% at time 22 min) yielded chilenine (3; 1.0 mg) and 3,4-dimethoxy-2-(methoxycarbonyl) benzoic acid (1; 1.2 mg), respectively.
3,4-dimethoxy-2-(methoxycarbonyl)benzoic acid (1): white powder; UV λmax 208 and 259 nm; 1H (400 MHz) and 13C NMR (100 MHz) data see Table 1. HRESIMS m/z 241.07021 [M+H]+ (calcd for C11H13O6 241.0707).
3,5,3′-trihydroxy-7,4′-dimethoxy-6,8-C-dimethyl-flavone (2): yellow powder; UV λmax 217, 258, 346 and 377 nm; 1H (700 MHz) and 13C NMR (175 MHz) data see Table 2. HRESIMS m/z 359.11287 [M+H]+ (calcd for C19H19O7 359.1125).
(±)-chilenine (3): white powder; (c 0.22, MeOH); UV λmax 215 and 318 nm; 1H-NMR (400 MHz, CDCl3) δ: 7.36 (1H, d, J = 8.4 Hz, H-11), 7.05 (1H, d, J = 8.4 Hz, H-12), 6.71 (1H, s, H-1), 6.66 (1H, s, H-4), 5.95 (2H, dd, J = 7.2, 1.2 Hz, OCH2O), 4.26 (1H, m, H-6), 3.99 (3H, s, OCH3-9), 3.87 (3H, s, OCH3-10), 3.56 (1H, m, H-5), 3.30 (1H, m, H-6), 3.11 (1H, m, H-5); 13C-NMR (100 MHz, CDCl3) δ: 199.8 (C-14), 166.7 (C-8), 154.5 (C-10), 151.6 (C-3), 147.1 (C-2), 146.7 (C-9), 133.7 (C-4a), 131.7 (C-12a), 130.7 (C-14a), 124.1 (C-9a), 120.6 (C-12), 116.3 (C-11), 109.4 (C-4), 109.2 (C-1), 101.9 (OCH2O), 94.9 (C-13), 62.6 (OCH3-9), 56.6 (OCH3-10), 38.9 (C-6), 30.7 (C-5); HRESIMS m/z 384.10751 [M+H]+ (calcd for C20H18NO7 384.1078).
(2R)-5,4′-dihydroxy-6-C-methyl-7-methoxy-flavanone (4): yellow oil; (c 0.3, MeOH); UV λmax 216, 291 and 338 nm; 1H-NMR (400 MHz, CDCl3) δ: 12.1 (OH-5), 7.35 (2H, d, J = 8.4 Hz, H-2′ and H-6′), 6.89 (1H, d, J = 8.4 Hz, H-3′ and H-5′), 6.07 (1H, s, H-8), 5.35 (1H, dd, J = 13.2, 2.8, H-2), 3.83 (3H, s, OCH3-7), 3.09 (1H, dd, J = 17.2, 13.2 Hz, H-3), 2.77 (1H, dd, J = 17.2, 2.8 Hz, H-3), 2.01 (1H, s, CH3-6); 13C-NMR (100 MHz, CDCl3) δ: 196.2 (C-4), 165.8 (C-7), 161.3 (C-9), 160.5 (C-5), 156.2 (C-4′), 130.9 (C-1′), 128.1 (C-2′ and C-6′), 115.8 (C-3′ and C-5′), 106.1 (C-6), 102.9 (C-10), 90.9 (C-8), 79.2 (C-2), 55.9 (OCH3-7), 43.5 (C-3), 7.0 (CH3-6); HRESIMS m/z 301.10709 [M+H]+ (calcd for C17H17O5 301.10780).
5,4′-dihydroxy-6,8-di-C-methyl-7-methoxy-flavanone (5): yellow oil; (c 0.12, MeOH); UVλmax 192, 222, 282 and 361 nm; 1H-NMR (400 MHz, CDCl3) δ: 12.0 (OH-5), 7.35 (2H, d, J = 8.4 Hz, H-2′ and H-6′), 6.89 (1H, d, J = 8.4 Hz, H-3′ and H-5′), 5.34 (1H, dd, J = 12.8, 2.8 Hz, H-2), 3.74 (3H, s, OCH3-7), 3.06 (1H, dd, J = 17.2, 12.8 Hz, H-3), 2.83 (1H, dd, J = 17.2, 2.8 Hz, H-3), 2.10 (3H, s, CH3-6), 2.07 (3H, s, CH3-8); 13C-NMR (100 MHz, CDCl3) δ: 197.7 (C-4), 162.5 (C-7), 160.2 (C-5), 158.1 (C-4′), 156.4 (C-9), 131.5 (C-1′), 127.8 (C-2′ and C-6′), 119.5 (C-8),116.2 (C-3′ and C-5′), 108.2 (C-6), 104.0 (C-10), 79.7 (C-2), 63.5 (OCH3-7), 40.7 (C-3), 9.9 (CH3-8), 9.3 (CH3-6); HRESIMS m/z 315.12292 [M+H]+ (calcd for C18H19O5 315.1227).
Noroxyhydrastinine (6): yellow powder; UV λmax 222, 261 and 306 nm; 1H-NMR (400 MHz, CDCl3) δ: 7.50 (1H, s, H-8), 6.66 (1H, s, H-5), 6.01 (2H, s, OCH2O), 3.53 (2H, t, J = 6.8, 6.4 Hz, H-3), 2.91 (1H, t, J = 6.8, 6.4 Hz, H-4); 13C-NMR (100 MHz, CDCl3) δ: 166.5 (C-1), 151.5 (C-6), 147.2 (C-7), 135.0 (C-5a), 121.5 (C-8a), 108.0 (C-8), 107.5 (C-5), 101.8 (OCH2O), 40.4 (C-3), 28.3 (C-4); HRESIMS m/z 192.06526 [M+H]+ (calcd for C10H10NO3 192.0655).
Oxyhydrastinine (7): yellow pale powder; UV λmax 222, 264 and 304 nm; 1H-NMR (400 MHz, CDCl3) δ: 7.54 (1H, s, H-8), 6.61 (1H, s, H-5), 5.99 (2H, s, OCH2O), 3.51 (2H, t, J = 6.8, 6.8 Hz, H-3), 2.90 (1H, t, J = 6.8, 6.8 Hz, H-4), 3.13 (3H, s, N-CH3); 13C-NMR (100 MHz, CDCl3) δ: 164.7 (C-1), 150.4 (C-6), 146.9 (C-7), 133.6 (C-5a), 123.5 (C-8a), 108.3 (C-8), 107.0 (C-5), 101.6 (OCH2O), 48.3 (C-3), 35.3 (N-CH3), 28.1 (C-4); HRESIMS m/z 206.08127 [M+H]+ (calcd for C11H12NO3 206.08117).
4′,5′-dimethoxy-4-methyl-3′-oxo-(1,2,5,6-tetrahydro-4H-1,3-dioxolo-[4′,5′:4,5]-benzo[1,2-e]-1,2-oxazocin)-2-spiro-1′-phtalan (8): yellow pale powder; (c 0.17, MeOH); UV λmax 218, 245 and 300 nm; 1H-NMR (400 MHz, CDCl3) δ: 7.04 (2H, d, J = 8.4 Hz, H-6′), 6.75 (1H, s, H-7), 6.36 (1H, s, H-11), 6.33 (1H, d, J = 8.4 Hz, H-7′), 6.00 and 5.94 (2H, s, OCH2O), 4.28 (1H, m, H-1), 4.11 (3H, s, OCH3-4′), 3.88 (3H, s, OCH3-5′), 3.35 (1H, m, H-6), 3.07 (2H, m, H-5), 2.76 (3H, s, N-CH3), 2.70 (2H, m, H-1 and H-6); 13C-NMR (100 MHz, CDCl3) δ: 165.8 (C-3′), 154.0 (C-5′), 148.1 (C-4′), 146.9 (C-8), 145.4 (C-10), 138.9 (C-7′a), 135.1 (C-7a), 126.9 (C-11a), 119.7 (C-7′), 119.1 (C-4′a), 118.0 (C-6′), 112.6 (C-11), 111.1 (C-7), 109.5 (C-2), 101.2 (C-9), 62.5 (OCH3-4′), 62.1 (C-5), 56.8 (OCH3-5′), 49.2 (N-CH3), 40.6 (C-1), 36.6 (C-6); HRESIMS m/z 400.1375 [M+H]+ (calcd for C21H22NO7 400.1391).
4.4. Collection of HRESIMS fragmentation data
Each of the 17 isolated compounds were suspended in MeOH at either 1 mg/mL or 0.1mg/mL and subjected to ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) analysis via a Waters Acquity UPLC with an Acquity UPLC column (BEH C18, 1.7 μm, 2.1 × 50 mm), Waters Corporation, Milford, MA) coupled to a Thermo Q Exactive Plus orbitrap mass spectrometer with heated electrospray ionization (Thermo Fisher Scientific, MA, USA). The compounds were eluted from the column at a flow rate of 0.3 mL/min using a binary solvent system with A consisting of water with 0.1% formic acid additive and solvent B consisting of acetonitrile with 0.1% formic acid additive. The gradient was as follows: 95:5 (A:B) from 0 to 1 min, increasing to 90:10 (A:B) from 1 to 2 min, 80:20 (A:B) from 2 to 3 min, 60:40 (A:B) from 3 to 4 min, 70:30 (A:B) from 4 to 5 min, 0:100 (A:B) from 5 to 6 min and held from 6 to 7 min, 95:5 (A:B) from 7 to 8 min and held from 8 to 9 min. Duplicate analyses of each sample were conducted in both positive and negative polarities using the following settings: spray voltage, 3.7 kV; capillary temperature, 350 °C; sheath gas, 25; auxiliary gas, 5; S-lens RF level, 50. Each compound was chosen for fragmentation via high energy collision-induced dissociation (HCD, normalized collision energy set to 50) from an inclusion list for both polarities. To determine the average mass accuracy of the product ions, the fragmentation spectra of 2 was compared to theoretical fragments produced by ACD MS fragmenter (Advanced Chemistry Development, Inc. Toronto, Canada). The resulting accurate mass of the predicted chemical formulas and hypothetical structures were matched with experimental data and were within 10 ppm mass error (Fig. 4).
4.5. Efflux pump inhibition assay
Four of the isolated compounds (1, 2, 4 and 16) were of sufficient purity (91%, 98%, 95% and 100%, respectively, by LC-UV) and quantity for evaluation via an efflux pump inhibition assay, as previously described (Brown et al., 2015). The assay was modified from the previously reported method in the chromatographic gradient, in some of the mass spectrometric conditions, and in the use Staphyloccocus aureus strain SA1199 (Kaatz and Seo, 1995).
The gradient was as follows: 95:5 (A:B) from 0 to 1 min, increasing to 0:100 (A:B) from 1 to 3.5 min, held from 3.5 to 9.5 min, 95:5 (A:B) from 9.5 to 10 min. A divert valve was utilized, with the valve set to waste from 0 to 1.5 min and to inject from 1.5 to 10 min. The mass spectrometric analyses were conducted under the following conditions: spray voltage, 3 kV; capillary temperature, 250°C; vaporizer temperature, 40°C; sheath gas, 40; aux gas, 30; tube lens offset, -112. Mass spectral dose-response data were analyzed with SigmaPlot (Systat Software, San Jose, CA) to calculate IC50 values for each of the active compounds.
Supplementary Material
Acknowledgments
This research was supported by the National Center for Complementary and Integrative Health (NCCIH), a component of the National Institute of Health (NIH), by grant numbers 1 R01 AT006860 (to NBC) and F31 AT009164 (to ERB). We thank Bill Burch for supplying plant material and Noemi Paguigan, Vincent Sica, and Diana Kao for helpful suggestions related to the manuscript. Mass spectrometry data were collected in the Triad Mass Spectrometry facility.
Appendix A. Supplementary data
The isolation scheme and NMR spectra are available as Supporting Information.
References
- Bharathi A, Wang YH, Khan IK. Quantitative determination of alkaloids from roots of Hydrastis canadensis L. and dietary supplements using ultra-performance liquid chromatography with UV detection. J AOAC Int. 2012;95:1398–1405. doi: 10.5740/jaoacint.12-074. [DOI] [PubMed] [Google Scholar]
- Brown AR, Ettefagh KA, Todd DA, Cole PS, Egan JM, Foil DH, Graf TN, Schindler BD, Kaatz GW, Cech NB. A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS One. 2015;10(5):e0124814. doi: 10.1371/journal.pone.0124814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech NB, Junio HA, Ackermann LW, Kavanaugh JS, Horswill AH. Quorum quenching and antimicrobial activity of goldenseal (Hydrastis canadensis) against methicillin-resistant Staphylococcus aureus (MRSA) Planta Med. 2012;78:1556–1561. doi: 10.1055/s-0032-1315042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskotch RW, Schiff PL, Jr, Beal JL. Alkaloids of Thalictrum. X Two new alkaloids from T minus var adiantifolium: noroxyhydrastinine and thalifoline. Tetrahedron. 1969;25:469–475. doi: 10.1016/s0040-4020(01)82640-5. [DOI] [PubMed] [Google Scholar]
- Douglas JA, Follett JM, Parmenter GA, Sansom CE, Perry NB, Littler RA. Seasonal variation of biomass and bioactive alkaloid content of goldenseal, Hydrastis canadensis. Fitoterapia. 2010;81:925–928. doi: 10.1016/j.fitote.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Fajardo V, Elango V, Cassels BK, Shamma M. Chilenine: an isoindolobenzazepine alkaloid. Tetrahedron Lett. 1982;23:39–42. [Google Scholar]
- Foster S. [accessed April 2016];The future of goldenseal. 2000 http://www.stevenfoster.com/education/monograph/goldenseal.html.
- Hopfer U, Lehninger AL, Thompson TE. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci. 1967;59:484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BY, Roberts SK, Chadwick LR, Wu CD, Kinghorn AD. Antimicrobial constituents from goldenseal (the Rhizomes of Hydrastis canadensis) against selected oral pathogens. Planta Med. 2003;69:623–627. doi: 10.1055/s-2003-41115. [DOI] [PubMed] [Google Scholar]
- Junio HA, Sy-Cordero AA, Ettefagh KA, Burns JT, Micko KT, Graf TN, Richter SJ, Cannon RE, Oberlies NH, Cech NB. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis) J Nat Prod. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz GW, Seo SM. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz GW. Bacterial efflux pump inhibition. Curr Opin Investig Drugs. 2005;6:191–198. [PubMed] [Google Scholar]
- Knight SE. Goldenseal (Hydrastis canadensis) versus penicillin: A comparison of effects on Staphylococcus aureus, Streptococcus pyogenes, and Pseudomonas aeruginosa. Bios. 1999;70:3–10. [Google Scholar]
- Klötzer W, Oberhänsli WE. Die struktur des sogenannten anhydro-N-oxy-nornarcein. Helv Chim Acta. 1955;56:223–224. [Google Scholar]
- Le PM, McCooeye M, Windust A. Characterization of the alkaloids in goldenseal (Hydrastis canadensis) root by high resolution Orbitrap LC-MS(n) Anal Bioanal Chem. 2013;405:4487–4498. doi: 10.1007/s00216-012-6539-9. [DOI] [PubMed] [Google Scholar]
- Le PM, McCooeye M, Windust A. Application of UPLC-QTOF-MS in MSE mode for the rapid and precise identification of alkaloids in goldenseal (Hydrastis canadensis) Anal Bioanal Chem. 2014;406:1739–1749. doi: 10.1007/s00216-013-7558-x. [DOI] [PubMed] [Google Scholar]
- McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus–associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12:1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CE, Nigel PB, Follett JM, Parmenter GA, Douglas JA. A new glucosyl feruloyl quinic acid as a potential marker for roots and rhizomes of goldenseal, Hydrastis canadensis. J Nat Prod. 2004;67:1818–1822. doi: 10.1021/np049868j. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Taneja SC, Dhar KL. Minor alkaloid from Coscinium fenestratum. Phytochemistry. 1989;28:1998–1999. [Google Scholar]
- Pinho PM, Pinto MM, Kijjoa A, Pharadai K, Díaz JG, Herz W. Protoberberine alkaloids from Coscinlun fenestratum. Phytochemistry. 1992;31:1403–1407. [Google Scholar]
- Qiu F, Zhu Z, Kang N, Piao S, Qin G, Yao X. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab Dispos. 2008;36:2159–2165. doi: 10.1124/dmd.108.021659. [DOI] [PubMed] [Google Scholar]
- Scazzocchio F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67:561–564. doi: 10.1055/s-2001-16493. [DOI] [PubMed] [Google Scholar]
- Seger C, Sturm S, Strasser EM, Ellmerer E, Stuppner H. 1H and 13C NMR signal assignment of benzylisoquinoline alkaloids from Fumaria officinalis L. (Papaveraceae) Magn Reson Chem. 2004;42:882–886. doi: 10.1002/mrc.1417. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh TD, Singh VP, Pandey VB. Quaternary alkaloids of Argemone mexicana. Pharm Biol (London, U K) 2010;48:158–160. doi: 10.3109/13880200903062622. [DOI] [PubMed] [Google Scholar]
- Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Wang M, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu W, Crüsemann M, Boudreau PD, Esquenazi E, Sandoval-Calderón M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu C, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw C, Yang Y, Humpf H, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, Klitgaard A, Larson CB, Boya PCA, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O’Neill EC, Briand E, Helfrich EJN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard P, Phapale P, Nothias L, Alexandrov T, Litaudon M, Wolfender J, Kyle JE, Metz TO, Peryea T, Nguyen D, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BØ, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC, Bandeira N. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nature Biotechnology. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber E, Wehde R, Dörr M, Lang L, Stevens JF. C-Methyl-flavonoids from the leaf waxes of some Myrtaceae. Phytochemistry. 2000;55:965–970. doi: 10.1016/s0031-9422(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Zhang GL, Rücker G, Breitmaier E, Mayer R. Alkaloids from Hypecoum leptocarpum. Phytochemistry. 1995;40:1813–1816. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.