Abstract
CRISPR/Cas9 technology has transformed our abilities to manipulate the genome and epigenome, as applications have expanded from efficient genomic editing to include the targeted localization of effectors to specific genomic loci. By facilitating the manipulation of DNA- and histone-modifying enzyme activities, activation or repression of gene expression, and targeting of transcriptional regulators to defined loci, it is now possible to directly probe the role of gene-regulatory and epigenetic pathways in order to examine their roles in basic biology and disease processes. Here we discuss these emerging CRISPR-based methodologies, with specific consideration of neurobiological applications using human induced pluripotent stem cell (hiPSC)-based models.
I. Epigenetics in Neuropsychiatric Disease
Increasing evidence supports a strong role for transcriptional and epigenetic abnormalities in the development of neurological (Jakovcevski and Akbarian, 2014) and psychiatric (Peña et al, 2014) diseases. Epigenetic mechanisms, such as DNA and histone post-translational modifications, ncRNA-mediated processes, and higher order chromatin structure dynamics regulate a wide variety of neuronal processes and modulate susceptibility to neuropsychiatric illnesses (McGill and Zoghbi, 2014; Rajarajan et al., 2016). Current strategies to study epigenetic processes in disease biology include the manipulation of gene-expression regulatory factors, alteration of non-coding regulatory regions, and pharmacological inhibition of regulatory cascades. Here we discuss application of CRISPR/Cas9 technologies to advance our understanding of the gene-regulatory and epigenetic mechanisms contributing to neuropsychiatric diseases. First, we will provide an overview of CRISPR/Cas9 technologies and their reported applications to the study of neurodevelopment and neuropsychiatric disease (particularly Glioblastoma multiforme, schizophrenia, and autism spectrum disorder (ASD)). Second, given the utility of applying CRISPR-based approaches to human induced pluripotent stem cell (hiPSC)-based models of neurodevelopment and disease, we highlight current applications and future challenges in combining CRISPR/Cas9 and hiPSCs-based approaches for the study of a variety of conditions, including Parkinson’s disease (PD), ASD, and schizophrenia. Finally, we conclude with a discussion of advancements necessary to facilitate further studies of the aberrant transcriptional and epigenetic mechanisms underlying neuropsychiatric disease.
II. Introduction to CRISPR/Cas9
The clustered, regularly interspaced short palindromic repeats (CRISPR)/Cas (CRISPR-associated protein) system allows targeted and specific genetic manipulation. It was initially discovered as a key defense mechanism against invading viruses and plasmids in several bacterial species and Archaea (well reviewed in Wang et al., 2016). When microorganisms incorporate invading DNA sequences into their genomes, these sequences are transcribed into CRISPR RNAs (crRNAs) (Ishino et al., 1987) that, when expressed with Cas endonucleases, enables targeting of the endonuclease to the invading DNA (Jinek et al., 2012). In type II CRISPR/Cas9 systems, a noncoding trans-activating RNA (tracrRNA) mediates the binding of Cas9-crRNA complexes to target loci (Deltcheva et al., 2011). Provided that the target genetic locus contains a protospacer adjacent motif (PAM) that is compatible with Cas endonuclease binding, the Cas9-crRNA-tracrRNA complex creates double stranded breaks (DSBs) in the target locus (Bolotin et al., 2005; Mojica et al., 2009; Shah et al., 2013). In this way, CRISPR/Cas9 serves as a key component of microbial immune defenses.
This process has been adapted to achieve targeted genome editing in experimental systems and has been simplified through technical advances since its original discovery. Great experimental simplification was achieved with the demonstration that crRNA and tracrRNA can be engineered into a single guide RNA (sgRNA) (Jinek et al., 2012). Furthermore, a variety of Cas9 endonucleases have now been isolated from multiple organisms; each orthologue has different properties that enable adaptive fine-tuning of experimental approaches. The Streptococcus pyogenes Cas9 (Sp Cas9) is the endonuclease used most frequently, largely because its PAM consists of NGG, which occurs approximately every 8 base pairs in the human genome (Sander and Joung, 2014), enabling the targeting of Sp Cas9 to virtually any locus. An additional Cas9 endonuclease was derived from Staphylococcus aureus (Sa Cas9), whose small size of 1,053 amino acids (Ran et al., 2015), compared to that of 1,368 amino acids for Sp Cas9 (Chylinski et al., 2014), offers an advantage over other Cas9s in situations in which delivery of large constructs may be problematic. Beyond the available native Cas9 orthologues, several endonucleases have been further engineered to confer additional properties. Mutation of one of the two Cas9 nuclease domains creates a “nickase Cas9 (nCas9)” that cleaves only one of the target DNA strands (Mali et al., 2013), and thus enhances target specificity, as only adjacent nCas9-mediated cuts will bring about the desired DSB (Mali et al., 2013; Ran et al., 2013; Shen et al., 2014). Finally, mutating both nuclease domains generates a “dead” or “deactivated” Cas9 (dCas9) (Qi et al., 2013) to which a variety of proteins can be fused for numerous applications (e.g., Kiani et al., 2015) (Table 1).
Table 1.
Current applications of CRISPR/dCas9 technologies
| Effector Description | Effect on | Transcription |
|---|---|---|
| VP64 | 4 tandem repeats of herpes simplex virus protein 16, VP 16 | Activation |
| VP160 | 10 tandem repeats of herpes simplex virus protein 16, VP16 | Activation |
| VP192 | 12 tandem repeats of herpes simplex virus protein 16, VP16 | Activation |
| VP64-dSpCas9-BFP-VP64 | 4 tandem repeats of herpes simplex virus protein 16, VP 16 at N-terminus of dCas9, and blue flourescent protein with 4 tandem repeats of VP16 at the N-terminus | Activation |
| SunTag | Repeating peptide array that recruits transcription effector proteins | Activation |
| MCP-VP64 | RNA hairpins from male-specific bacteriophage 2 (MS2) along with MS2 coat protein (MCP) fused to VP64 activation domain | Activation |
| Synergistic activation mediator (SAM) | 2 MS2 hairpins and MCP fused to p65 -HSF1; MCP-p65-HSF1 activators bind the MS2 hairpins as homodimers, resulting in 4 copies of MCP-p65-HSF1 scaffolding onto the SAM sgRNA, which is bound to dSpCas9-VP64 | Activation |
| dSpCas9-VP64-p65-Rta | VP64, p65, and Rta activators fused to dCas9 | Activation |
| dCas9-p300 | dCas9 fused to the histone acetyltransferase p300 to add H3K27ac to target loci | Activation |
| KRAB | Kruppel-associated box domain of Kox1 fused to dCas9 | Inhibition |
| SID4X | 4 tandem repeats of mSin3 domain (SID4X) fused to dCas9 scaffold | Inhibition |
| LSD1-dNmCas9 | dCas9 fusion to histone demethylase LSD1 removes transcriptionally activated H3K4 methylation at target loci | Inhibition |
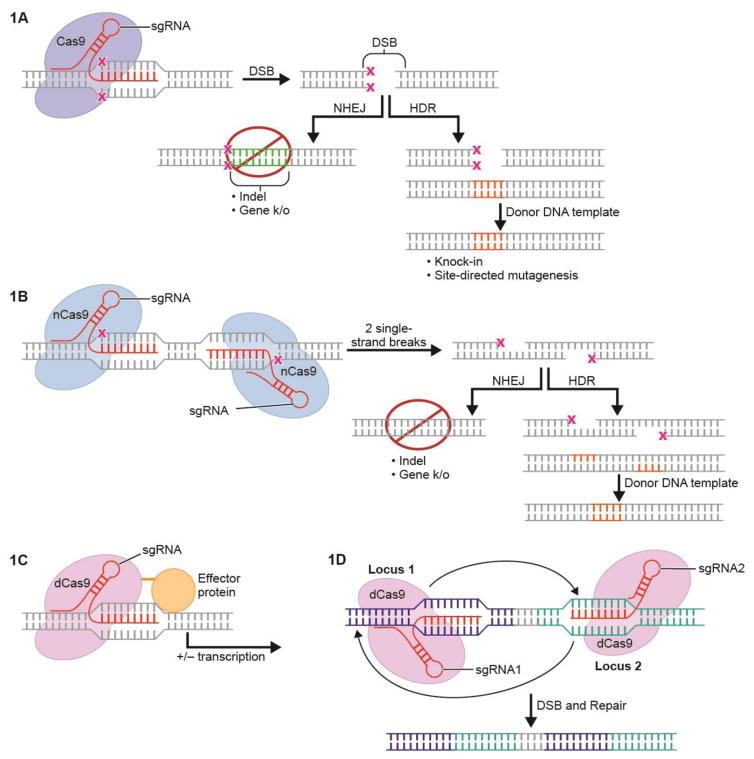
Current CRISPR/Cas9 technologies enable a variety of gene-editing approaches. Following the generation of a DSB by Cas9, the break is repaired through one of two endogenous processes. In the first, non-homologous end-joining (NHEJ) attempts to correct the break, but often creates random insertion or deletion mutations at the DSB site, which in turn can lead to gene-knockout (Lieber, 2010). Second, the DSB repair process known as homology-directed repair (HDR) can be harnessed to generate the replacement of DNA sequence (knock-in) at the site of the DSB using an exogenous DNA template (Doudna and Charpentier, 2014). Because Cas9 endonucleases can manipulate several sites when used with multiple sgRNAs, this approach permits chromosomal inversions, deletions, and translocations (Blasco et al., 2014; Maddalo et al., 2014; Torres et al., 2014). Therefore, CRISPR/Cas9 serves as an improved strategy for DNA deletion, insertion, site-directed mutagenesis, and chromosomal manipulation (see Figure 1 for an illustration of these processes).
Figure 1. General Applications of CRISPR/Cas9.
A. sgRNA guides Cas9 endonuclease to target locus of interest. A double stranded break (DSB) is made and is repaired in one of two ways. In non-homologous end-joining (NHEJ), DNA is deleted or inserted at the site of the DSB and the gene is knocked out. In homology-directed repair (HDR), a template strand is supplied with homology to the cut region; template DNA is then inserted at the site of the DSB.
B. “nickase” Cas9. Cleavage of one of the two nuclease domains of Cas9 results in nickase Cas9 (nCas9). DSBs are made only at a side in which adjacent single-strand cuts are made by nCas9, each guided by their own sgRNA. A double stranded break is made when single-strand cuts are made at adjacent sites. The DSB can then be repaired with either HDR or NHEJ. This allows for improved specificity of endonuclease-mediated cuts.
C. CRISPR/Cas9 effectors. Fusion of “deactivated” or “dead” (dCas9) to a transcriptional effector allows targeted increase or decrease in the expression of a gene of interest
D. CRISPR/Cas9-mediated genomic rearrangements. DSBs at two sites by two separate sgRNAs can lead to inversion of the region in between the two DSBs.
III. Applications of CRISPR/Cas9 to Study Transcriptional and Epigenetic Mechanisms Underlying Disease Processes
CRISPR/Cas9 can be used to modulate expression of histone- and DNA-modifying enzymes, alter DNA-binding sites of factors that regulate transcription, perturb non-coding RNA expression, modify non-coding gene-regulatory regions, and manipulate higher-order chromatin structure. In this section, we focus on the application of CRISPR/Cas9 to study gene regulatory and epigenetic pathways in neurobiology contexts.
Applying CRISPR to Understand Developmental Neurobiology
The development of the nervous system requires the orchestration of complex gene-regulatory pathways, activating those that promote differentiation and suppressing those involved in maintaining the undifferentiated state, and various groups have used CRISPR/Cas9 approaches to study the roles of transcription factors in directing neurodevelopment.
Deletion of key transcription factors can help elucidate their critical functions. For example, the transcription factor Satb2 is necessary for the proper maturation of axonal projections from layers 2 and 3 of the cerebral cortex to the contralateral side of cortex (Alcamo et al., 2008; Britanova et al., 2015); deletion of Satb2 with CRISPR/Cas9 in mice at embryonic day 15.5 ablated proper development of cortical projections (Shinmyo et al., 2016). Additionally, CRISPR/Cas9-mediated knockout of the bHLH transcription factor NeuroD demonstrated its requirement in retinal progenitors cell cycle exit and photoreceptor maturation (Taylor et al., 2015).
In addition to deleting transcription factors, CRISPR/Cas9 can be used to remove specific enhancer regions, thereby disrupting their gene-regulatory functions. For example, Endothelin signaling is essential to the proper differentiation of neural crest cells, promoting differentiation of key craniofacial structures (Pla and Larue, 2003; Ruest et al., 2004). CRISPR/Cas9-mediated deletion of an endothelin-responsive enhancer (Mef2C-F1) in mice resulted in decreased Mef2C expression and embryonic lethality due to palate and craniofacial obstruction (Hu et al., 2015). Moreover, Blimp1 is a zing-finger transcription factor that regulates the development of the proper ratio of bipolar cells and rods in the developing mouse retina; CRISPR/Cas9-mediated deletion of an enhancer ~12kb upstream Blimp1 phenocopied the effects of Blimp1 knockout in the retina (Wang et al., 2014).
By recruiting activators or repressors of transcription, dCas9 fusion proteins can be used to directly increase or decrease gene expression. For example, Frank et al (2015) focused on the role of Grin2c enhancer elements in the maturation of cerebellar granular neurons (CGNs) at three different stages of post-natal development, finding that dCas9-VP64-mediated activation of a specific enhancer region of Grin2c was a key driver of CGN maturation (Frank et al., 2015).
Finally, CRISPR/Cas9-mediated gene-editing can introduce protein tags to target loci, facilitating biochemical and epigenetic studies of endogenous gene products. The transcriptional targets of the zinc-finger transcription factor Rotund have been poorly defined due to a lack of ChIP-validated antibodies, and Li et al. 2015 employed CRISPR/Cas9 to incorporate protein tags in the Rotund gene, finding that Rotund preferentially interacts in heterochromatic regions, particularly at a specific bric-a-bric Rn target locus (Li et al., 2015).
Altogether, these examples highlight the utility of CRISPR/Cas9 approaches to study the roles of gene regulatory processes in neurodevelopment.
Using CRISPR/Cas9 to Study Diseases of the Central Nervous System
Glioblastoma multiforme
The chromatin assembly factor 1 subunit A (CHAF1A) is involved in DNA replication and repair and epigenetic silencing (Kaufman et al., 1995; Moggs et al., 2000; Ridgway and Almouzni, 2000; Dong et al., 2001; Quivy et al., 2001) and has been linked to several cancers (Barbiere et al., 2013;, Barbiere et al., 2014; Wu et al., 2014). Confirming the direct role of CHAF1A in the pathogenesis of Glioblastoma multiforme, CRISPR/Cas9-mediated knockout of CHAF1A in two glioblastoma cell lines suppressed proliferation and increased levels of apoptosis (Peng et al., 2016). Similar effects were obtained by removing regulatory regions rather than genes: CRISPR/Cas9-mediated deletion in a patient-derived gliomasphere line of one downstream locus, a CTCF-bound insulator region of platelet-derived growth factor receptor, alpha polypeptide (PDGFRA), resulted in increased PDGFRA mRNA and protein levels and conferred a growth advantage (Flavahan et al., 2016). Owing to straightforward phenotypic effects such as altered rates of cellular proliferation and/or death, we foresee that CRISPR-based genome-wide screens could soon be applied towards the unbiased study of the molecular mechanisms underlying Glioblastoma multiforme and other malignancies of the nervous system.
Neuropsychiatric conditions
Genes implicated in Autism Spectrum Disorder (ASD) are predominantly involved in synapse formation, chromatin remodeling, and transcriptional regulation (De Rubeis et al,. 2014). Loss-of-function studies of several ASD-associated genes have now been conducted via CRISPR/Cas9 editing. First, deletion in mouse embryonic stem cells (mESCs) of CHD5, a chromatin helicase DNA binding protein that is essential for proper neuronal differentiation (Egan et al., 2013), led to increased expression of the retrotransposon MERVL, with a corresponding decrease in H3K27me3 and increase in histone variant H3.1 and H3.2 deposition at the MERVL locus (Hayashi et al., 2016) Second, introduction of nonsense mutations and deletions of the ASD-associated genes Pten (Goffin et al., 2001) and Katanin P60 subunit A-like 2 (Katnal2) (Neale et al., 2012; O’Roak et al., 2012), led to changes in neuronal cell body size and dendritic morphology in mouse neurons (Williams et al., 2016).
Variants in a locus containing transcription factor 4 (TCF4) have been repeatedly associated with Schizophrenia (Schizophrenia Psychiatrics Genome-Wide Association Study Consortium 2011; Schizophrenia Working Group of the Psychiatric Genomic Consortium, 2014). In utero CRISPR/Cas9 knockout of TCF4 led to decreased neuronal firing and hyperpolarization of cortical neurons (Rannals et al., 2016). Translating ribsosomal affinity purification (TRAP) revealed increased expression of Kcnq1 and Scn10 in TCF4-knockout neurons, and CRISPR/Cas9-mediated deletion of these two genes rescued the hypoexcitable phenotype of TCF4 knockout neurons (Rannals et al., 2016).
Although ASD and schizophrenia are both complex genetic disorders arising through the interplay of highly penetrant rare mutations and common variants of small effect (De Rubeis et al., 2014; Neale et al., 2014; O’Roak et al., 2012; Schizophrenia Psychiatrics Genome-Wide Association Study Consortium 2011; Schizophrenia Working Group of the Psychiatric Genomic Consortium 2014), CRISPR-Cas9 mediated gene editing or regulation of expression will facilitate isogenic molecular and phenotypic characterizations, improving our understanding of the impact of each of these disease-relevant mutations.
IV. Applications of CRISPR/Cas9 Techniques to Human Induced Pluripotent Stem Cell Models
In this section, we provide a brief overview of human induced pluripotent stem cell (hiPSC)-based models and highlight recent advances in the field that have used CRISPR/Cas9 strategies to study gene-regulatory processes in neuropsychiatric disease.
Overview of hiPSC-based models
hiPSC-based models facilitate the study of normal developmental biology and disease mechanisms, and can serve as a component of drug-discovery platforms (Mertens et al., 2015; Brennand et al, 2015; Hartley and Brennand 2016; Xu et al., 2016). Expression of the four Yamanaka factors (C-MYC, KLF4, OCT4, and SOX2) reprograms adult somatic cells into hiPSCs (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Yu et al., 2007), from which neurons can be differentiated by sequential treatment with growth factors or small molecules in a manner recapitulating neuronal development in vivo (Watanabe et al., 2005). Through a related strategy, induced neurons (“iNeurons”) can be directly converted from fibroblasts via overexpression of the transcription factors NEUROD, ASCL1, BRN2, and MYT1L (Vierbuchen et al., 2010; Pang et al., 2011), or through overexpression Ngn2 in hiPSCs (Zhang et al., 2013) or neural progenitor cells (NPCs) (Ho et al., 2016). Because many subtypes of neurons and glia have been implicated in neuropsychiatric disease (Gotter et al., 2001), it is crucial to study defined cell types, both alone and in combination, in order to understand how they function in health and disease. While discussed in great detail by others (reviewed in Ho et al., 2015), a variety of protocols have reported differentiation or induction of glutamatergic (Zhang et al., 2013, Ho et al., 2016), GABAergic (Sun et al., 2016), dopaminergic (Theka et al., 2013), and serotonergic (Lu et al., 2016) neurons, as well as astrocytes (Emdad et al., 2012), oligodendrocytes (Espinosa-Jeffrey et al., 2016; Thiruvalluvan et al., 2016), and microglia-like cells (Muffat et al., 2016).
Limitations of hiPSCs-based models
There remain several limitations of hiPSC-based models. First, current diagnostic criteria for psychiatric disorders are based upon symptomatology alone and do not classify patients according to underlying disease biology (Diagnostic and statistical manual of mental disorders (5th edition), 2013); for this reason, recruitment and selection of patients with psychiatric diagnoses frequently yields heterogeneous samples lacking shared disease etiology. Although this problem may be overcome by studying hiPSCs only from individuals with well-defined genetic lesions and/or disease-relevant endophenotypes (e.g., Wen et al., 2014; Lee et al., 2015; Chailangkarn et al., 2016), such an approach necessarily limits the generalizability of any findings to the broader disorder. Second, because hiPSC-derived neurons most resemble fetal brain cells in temporal and spatial patterning (Mariani et al., 2012; Lancaster et al., 2013; Miller et al., 2013; Vera and Studer, 2015), they better model aspects of disease predisposition than the disease-state itself (Brennand et al., 2015). Even neural organoids (“brain balls”), which provide some three dimensional context to in vitro models (reviewed in Hartley and Brennand, 2016), still most resemble fetal, rather than adult, brain tissue (Pasca et al., 2015). Third, somatic mosaicism - differences in genomic content between cells from the same organism – occurs both in vivo and in hiPSC-based models (McConnell et al., 2013), and the extent to which cell-based models recapitulate the diversity of mosaicism detected in the human brain remains unclear. In fact, reprogramming of fibroblasts and subsequent differentiation to neurons may induce substantial copy number variants (McConnell et al., 2013) not found in the source somatic cells. Because single-cell resolution studies have demonstrated somatic mosaicism in both adult human brain (McConnell et al., 2013) and hiPSCs neurons (Erwin et al., 2016), single-cell approaches may be increasingly necessary to query intercellular variability in genomic content and phenotypes (Bardy et al., 2016). Nevertheless, despite these limitations, many groups have already successfully employed hiPSC-based models for the study of neuropsychiatric disorders (e.g., Brennand et al., 2011; Brennand et al., 2015; Chailangarn et al., 2016; Balachandar et al., 2016).
Using CRISR/Cas9 to probe gene-regulatory pathways in hiPSCs models of neuropsychiatric disease
Parkinson’s Disease (PD)
CRISPR/Cas9-mediated genome editing introduced a PD-associated SNP in the enhancer region of alpha-synuclein (SNCA), which resulted in increased H3K27ac at this enhancer region and increased SNCA expression in hiPSCs-derived neurons (Soldner et al., 2016). Binding of two transcription factors, NKX6-1 and EMX2, was SNP-dependent; both showed increased binding to the protective allele. Altogether, these experiments demonstrate that a PD risk allele in the SNCA enhancer affects the regulatory actions of two transcription factors, leading to over-expressed SNCA, a key pathological hallmark of PD (Soldner et al., 2016).
Fragile X Syndrome and Autism Spectrum Disorder (ASD)
Fragile X Syndrome (FXS), caused by a CGG trinucleotide repeat expansion in the 5-UTR of the FMR1 gene (Verkerk et al 1991), is the most common form of inherited intellectual disability (Folstein and Piven, 1991), and approximately 20% of people with FXS also exhibit ASD-like behaviors (Crawford et al 2001; O’Donnell and Warren 2002). The CGG expansion is associated with suppressed expression of FMR1 via abnormal epigenetic marks at the FMR1 promoter, such as increased DNA cytosine methylation (Coffee et al., 1999; Urback et al 2010), decreased histone tail acetylation (Urback et al 2010), increased H3K9me3 methylation (Avitzour et al, 2014), and loss of H3K4me2 (Avitzour et al, 2014). CRISPR/Cas9-mediated deletion of the CGG repeat in FXS hiPSCs resulted in normalized expression of FMR1, a change that was maintained through subsequent differentiation to NPCs and mature neurons; moreover, hypermethylation of the CpG sites upstream of FMR1 was reversed with CGG deletion (Park et al., 2015). Chromatin immunoprecipitation revealed that CGG repeat deletion led to increased acetylation of the histone 3 tail and H3K4me2 (both transcriptionally activating) and decreased H3K9me2 (transcriptionally repressive). This was the first causal demonstration that removal of CGG expansion was sufficient to reverse the transcriptionally repressed chromatin state of FMR1 in FXS patient-derived cells (Park et al., 2015).
Genetic studies have identified the chromodomain helicase DNA binding protein 8 (CHD8) to be significantly enriched in ASD-associated de novo mutations (Neale et al., 2012; O’Roak et al., 2012a; O’Roak et al., 2012b). The generation of heterozygous CHD8+/− hiPSCs via CRISPR/Cas9 editing and their subsequent differentiation into NPCs and neurons, revealed differential expression of genes involved in neural development and Wnt signaling (Wang et al., 2015), consistent with hiPSC-based models of idiopathic ASD, which have reported similar deficits (Marchetto et al., 2016). While CRISPR/Cas9 hiPSC-based studies of ASD have focused on rare mutations to date, they have unequivocally demonstrated that these highly penetrant mutations are sufficient to produce molecular and functional phenotypes in human neurons in vitro.
Schizophrenia
Many loci have been implicated in the risk architecture of schizophrenia, but only one schizophrenia-linked gene has been manipulated by CRISPR-based methods in hiPSCs thus far. Although not significant in genome wide associate studies, the disrupted in schizophrenia 1 (DISC1) gene was identified in a Scottish family in which a chromosomal 1:11 translocation co-segregated with incidence of schizophrenia and major depressive disorder (Millar et al 2000a; Millar et al. 2000b). DISC1 function has been implicated in neurite outgrowth, neuronal development and migration, synaptic maturation, and cell proliferation (Bradshaw and Porteous, 2012). Srikanth et al., 2015 employed TALENs and CRISPR/Cas9 to delete a critical DISC1 exon in control hiPSCs and showed increased DISC1 nonsense-mediated decay of splice transcripts and altered Wnt signaling (Srikanth et al., 2015). Additionally, Wen et al., 2015 used TALENs to both reconstruct a disease-associated mutation in controls and repair it in DISC1-patient derived hiPSCs (Wen et al., 2015). Across isogenic comparisons, changes in DISC1 genotype produced altered expression of key synaptic genes in hiPSCs-derived neurons and perturbations in synaptic activity (Wen et al., 2015). Altogether, these results convincingly demonstrated a causal role for DISC1 in neural development and synaptic function. Isogenic comparisons made possible via CRISPR/Cas9 gene-editing approaches have helped to elucidate disease mechanisms in hiPSC-derived neurons.
V. Future Directions
In the next section, we discuss ways in which advances in CRISPR/Cas9 technology may further expand our understanding of neuropsychiatric disease. See Figure 2 for an illustration of these processes.
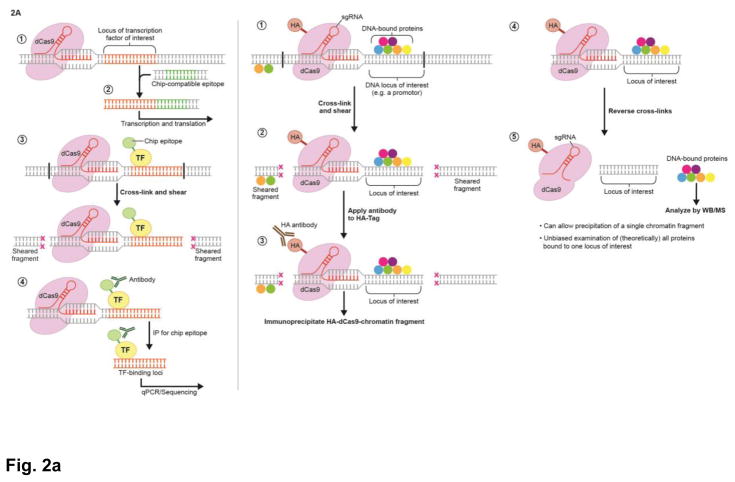
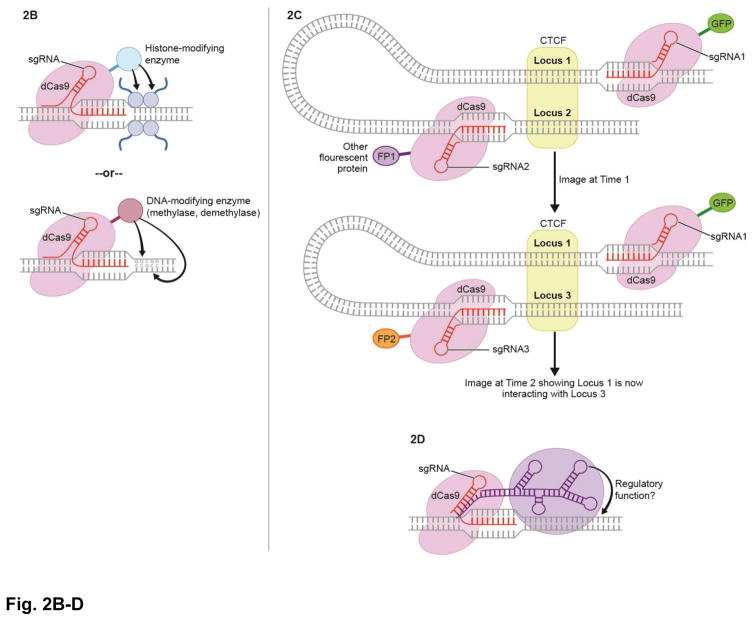
Figure 2. Future applications of CRISPR/Cas9.
A. (Left). Inserting ChIP-compatible epitopes into a DNA-binding protein of interest. A DSB is made adjacent to a gene for a DNA-binding protein of interest and is repaired by HDR using a template strand for a ChIP-compatible epitope. Expression of the fusion protein allows chromatin immunoprecipitation (ChIP) experiments for proteins without ChIP-compatible antibodies. Here, a transcription factor binds to its target regions. The chromatin is cross-linked and sheared. Antibodies to the epitope are then applied to precipitate out fragments of DNA bound to the transcription factor. (Right). Unbiased CRISPR/Cas9 ChIP using dCas9; allows for immunoprecipitation and proteomic characterization of at a specific chromatin region. sgRNA guides dCas9 or an epitope-tagged dCas9 to a region of interest. The protein/DNA complex is cross-linked and sheared. An antibody to either dCas9 or the epitope-tagged dCas9 is used to immunoprecipitate the target chromatin fragment. After reversing cross-links, all of the proteins at that particular region can be analyzed.
B. Fusion of dCas9 to histone and DNA-modifying enzymes; allows such an enzyme to be targeted to a specific region and transcription to be effected accordingly
C. Chromatin dynamics. dCas9 is fused to fluorescent proteins and targeted to two regions of interest to see if they interact with each other (image for both fluorescent proteins). Sequential imaging with different sgRNA-guided fluorescent proteins (FPs) allows tracking of live chromatin interactions
D. A ncRNA can be delivered via dCas9 and its effects can be studied at the target locus.
Advances in chromatin biology
An ongoing challenge of using chromatin immunoprecipitation (ChIP) to study protein-DNA interactions is the paucity of suitable antibodies. To overcome this limitation, it is now possible to use CRISPR/dCas9 to tag a putative DNA-binding protein with a ChIP-compatible epitope, enabling interrogation of DNA-binding patterns of any protein of interest (Savic et al., 2015). By integrating epitope tags in different exons of alternatively spliced genes of interest, this method can also be employed to study DNA-binding patterns of specific splice isoforms of a transcription factor (Li et al., 2015). Even more scalable, as it does not require genetic editing, is the use of sgRNAs to target dCas9 to a locus of interest, and then performing ChIP experiments to dCas9 itself or a protein-tagged dCas9 (Fujii and Fujita, 2015). This approach enables profiling of all proteins interacting at single genomic locus, and has already been used to characterize site-specific proteomic binding patterns by combining dCas9-ChIP with by mass spectrometry (Zhang et al., 2016). The incorporation of selective isotopic labeling of amino acids in cell culture (SILAC) with dCas9-ChIP would further allow investigation of protein turnover rates, at any locus, and over defined periods of time (Fujita and Fujii, 2014).
Moving forward, we expect further development of dCas-epigenetic regulator fusion proteins beyond those that are currently available (see Table 1 for current options). Presently, fusion with VP64, VIPR, and p300a increase gene expression, whereas fusion to KRAB decreases gene expression (reviewed in Vora et al., 2016). In theory, it should be possible to target any known histone- or DNA-modifying enzyme to any specific loci. Already, targeted DNA methylation via dCas9-DNMT3A (DNA methyltransferase 3A) (Vojta et al., 2016; Liu et al., 2016; McDonald et al, 2016) and DNA demethylation via dCas9-TET1 (ten-eleven translocation 1 dioxygenase) (Liu et al., 2016) has been demonstrated. Gene expression can be altered by the addition and removal of a variety of histone post-translational modifications including acetylation, methylation, phosphorylation, sumoylation, and ribosylation, each with different effects depending on the number of specific histone residues modified, and with new “epigenetic editing” tools we expect further elucidation of the function of gene-regulatory complexes as well as targeted exploration of epigenetic modifiers.
Finally, the localizability of dCas9 and amenability to fusion to numerous proteins allows visualization of specific genomic loci, which is a promising approach for further investigation of chromatin dynamics. Already, some groups have tagged TALEs with fluorescent proteins to visualize repetitive elements in living cells (Miyanari et al., 2013; Ma et al., 2013; Thanisch et al., 2013). One report demonstrated imaging of coding and non-coding loci dynamics in living cells using Sp dCas9 fused to GFP (Chen et al., 2013). Strikingly, a different group (Ma et al., 2015), demonstrated multi-color dynamic genome imaging by employing dCas9s from different bacterial species fused to varying fluorescent proteins. Based upon these initial and promising studies, we propose that these and similar approaches will soon permit tracking of the spatial and temporal dynamics of 3D chromatin structures.
Investigation of non-coding gene regulatory regions in neurological and psychiatric disease
It is critical to establish causal roles of non-coding gene-regulatory regions in neuropsychiatric disease. For example, of the 108 loci that are significantly associated with schizophrenia risk (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), many occur in non-protein coding regions (Ripke et al., 2013) and several are enriched in brain-specific promoter and enhancer regions (Roussos et al., 2014), where they likely regulate gene expression (Fromer et al., 2016). CRISPR/Cas9 can be used to perform tiling screens to identify enhancers (Korkmaz et al., 2016) and non-enhancer unmarked regulatory elements (Rajagopal et al., 2016), and to systemically map promoter-enhancer interactions (Fulco et al., 2016). We propose that these kinds of CRISPR/Cas9 techniques will enable identification and investigation of non-coding gene-regulatory regions and their roles in neuropsychiatric disease.
Growing evidence that loci found within insulators, enhancers, and promoter regions contribute to the risk of developing neuropsychiatric diseases implicate a potential role for higher-order chromatin structure in these conditions. In fact, higher-order chromatin structures are key regulators of genome function and gene expression (Bickmore and Van Steensel 2013). CRISPR/Cas9 has been employed to delete, invert, and duplicate sites involved in chromatin looping and to study the effects of such manipulations on gene expression and function (Guo et al., 2015; Lupiáñez et al., 2015; Yang et al., 2016). Increasing evidence implicates higher-order chromatin structure in brain function and cognition (reviewed in Rajarajan et al., 2016); for example, Bharadwaj et al (2014) identified enhancer regions of the NMDA receptor component GRIN2B and demonstrated a neuronal activity-dependent alteration in enhancer-transcription start site interactions. Additionally, CRISPR/Cas9-mediated deletion of loop-interacting regions (including a schizophrenia risk allele in the GRIN2 locus), were associated with altered looping interactions and decreased GRIN2B expression (Bharadwaj et al., 2014). For these reasons, CRISPR/Cas9 editing is an exciting approach for targeting perturbations of chromatin looping and studying the effects of these manipulations on gene expression and neuronal function. Future investigations must apply these approaches to hiPSCs-derived neurons and animal models in order to explore the role of disease-associated gene-regulatory regions in neuropsychiatric conditions.
Investigation of long non-coding RNA function
Long non-coding RNAs (lncRNAs) have important gene-regulatory functions (Quinn and Chang, 2016) and have been implicated in psychiatric (Chubb et al., 2008; Sartor et al., 2012; Le-Niculescu et al., 2013; Liu et al., 2014; Spadaro et al., 2015;), neurodevelopmental (van de Vondervoort II et al., 2013), and neurodegenerative disorders (Faghihi et al., 2008; Szafranski et al., 2015). CRISPR/Cas9-mediated genomic deletion screening has already identified 51 lncRNAs that regulate cancer growth (Zhu et al., 2016), and this approach could be applied to identify lncRNAs with important roles in the regulation of gene expression in hiPSCs-derived neurons. In order to investigate the mechanisms by which individual lncRNAs regulate gene expression, further advancements would need to enable targeting of lncRNAs to specific loci. A recently developed technique, termed “CRISP-Disp,” allows targeting of large, putative regulatory RNAs to genomic loci of interest (Shechner et al., 2015). Using this technique, Shechner et al, 2015 complexed Streptococcus pyogenes (Sp) dCas9-VP64 to increasingly larger RNA cargos, demonstrating that these complexes maintained their transcriptionally activating effects at defined reporter loci. Having established that this was the case for artificial RNA cargos, the investigators next loaded lncRNAs to dCas9-sgRNA complexes, finding that expression of genes at the targeted loci changed in a manner consistent with previously defined gene-regulatory effects of the lncRNAs chosen (Shechner et al., 2015). Such CRISPR-based approaches could theoretically explore functions of regulatory RNAs, providing the means to directly study the consequences of regulatory RNA targeting at any locus of interest.
VI. Summary
Neurological and psychiatric diseases frequently involve pathogenic alterations in gene-regulatory and epigenetic processes. With the advent of CRISPR/Cas9, targeted manipulation of a variety of regulatory factors, non-protein-coding sequences, and non-coding RNAs can now explore the role of these pathways in neuropsychiatric conditions. Future approaches will expand the ability of CRISPR/Cas9 to effect specific perturbations in epigenetic pathways. By applying these new tools to a variety of hiPSC- and animal-based models, CRISPR/Cas9 techniques will help reveal new epigenetic mechanisms underlying the biology of neuropsychiatric disease.
Highlights.
An overview of CRISPR/Cas9 techniques and their applications to the study of neurodevelopment and neuropsychiatric disease.
An introduction to hiPSC-based models of neuropsychiatric disease and CRISPR/Cas9 applications to these models.
The potential for CRISPR/Cas9 approaches in a variety of models, including hiPSCs, to advance understanding of psychiatric illnesses
Acknowledgments
Kristen Brennand is a New York Stem Cell Foundation - Robertson Investigator. This work was partially supported by National Institute of Health (NIH) grants R01 MH101454 (KJB) and R01 MH106056 (SA) and P50 MH096890 (SA), as well as the New York Stem Cell Foundation and the Brain Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal P, Verzi MP, Nguyen T, Hu J, Ehlers ML, McCulley DJ, … Black BL. The MADS box transcription factor MEF2C regulates melanocyte development and is a direct transcriptional target and partner of SOX10. Development. 2011;138(12):2555–2565. doi: 10.1242/dev.056804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57(3):364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anton T, Bultmann S, Leonhardt H, Markaki Y. Visualization of specific DNA sequences in living mouse embryonic stem cells with a programmable fluorescent CRISPR/Cas system. Nucleus. 2014;5(2):163–172. doi: 10.4161/nucl.28488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitzour M, Mor-Shaked H, Yanovsky-Dagan S, Aharoni S, Altarescu G, Renbaum P, … Eiges R. FMR1 epigenetic silencing commonly occurs in undifferentiated fragile X-affected embryonic stem cells. Stem Cell Reports. 2014;3(5):699–706. doi: 10.1016/j.stemcr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar V, Dhivya V, Gomathi M, Mohanadevi S, Venkatesh B, Geetha B. A review of Rett syndrome (RTT) with induced pluripotent stem cells. Stem Cell Investig. 2016;3:52. doi: 10.21037/sci.2016.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri E, De Preter K, Capasso M, Chen Z, Hsu DM, Tonini GP, … Shohet JM. Histone chaperone CHAF1A inhibits differentiation and promotes aggressive neuroblastoma. Cancer Res. 2014;74(3):765–774. doi: 10.1158/0008-5472.CAN-13-1315. [DOI] [PubMed] [Google Scholar]
- Barbieri E, De Preter K, Capasso M, Johansson P, Man TK, Chen Z, … Shohet JM. A p53 drug response signature identifies prognostic genes in high-risk neuroblastoma. PLoS One. 2013;8(11):e79843. doi: 10.1371/journal.pone.0079843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C, van den Hurk M, Kakaradov B, Erwin JA, Jaeger BN, Hernandez RV, … Gage FH. Predicting the functional states of human iPSC-derived neurons with single-cell RNA-seq and electrophysiology. Mol Psychiatry. 2016;21(11):1573–1588. doi: 10.1038/mp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj R, Peter CJ, Jiang Y, Roussos P, Vogel-Ciernia A, Shen EY, … Akbarian S. Conserved higher-order chromatin regulates NMDA receptor gene expression and cognition. Neuron. 2014;84(5):997–1008. doi: 10.1016/j.neuron.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell. 2013;152(6):1270–1284. doi: 10.1016/j.cell.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Blasco RB, Karaca E, Ambrogio C, Cheong TC, Karayol E, Minero VG, … Chiarle R. Simple and rapid in vivo generation of chromosomal rearrangements using CRISPR/Cas9 technology. Cell Rep. 2014;9(4):1219–1227. doi: 10.1016/j.celrep.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151(Pt 8):2551–2561. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- Bradshaw NJ, Porteous DJ. DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology. 2012;62(3):1230–41. doi: 10.1016/j.neuropharm.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Marchetto MC, Benvenisty N, Brustle O, Ebert A, Izpisua Belmonte JC, … Jaenisch R. Creating Patient-Specific Neural Cells for the In Vitro Study of Brain Disorders. Stem Cell Reports. 2015;5(6):933–945. doi: 10.1016/j.stemcr.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, … Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473(7346):221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, Akopov S, Lukyanov S, Gruss P, Tarabykin V. Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. Eur J Neurosci. 2005;21(3):658–668. doi: 10.1111/j.1460-9568.2005.03897.x. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, … Tarabykin V. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57(3):378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Chailangkarn T, Trujillo CA, Freitas BC, Hrvoj-Mihic B, Herai RH, Yu DX, … Muotri AR. A human neurodevelopmental model for Williams syndrome. Nature. 2016;536(7616):338–343. doi: 10.1038/nature19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, … Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155(7):1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13(1):36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Chylinski K, Makarova KS, Charpentier E, Koonin EV. Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res. 2014;42(10):6091–6105. doi: 10.1093/nar/gku241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat Genet. 1999;22(1):98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55(5):585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. doi:10.109700125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, … Buxbaum JD. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515(7526):209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, … Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Lin W, Zhang CK, Xiong H, Fu G, Jin WR, … Huang GM. Genomic sequence and expression analyses of human chromatin assembly factor 1 p150 gene. Gene. 2001;264(2):187–196. doi: 10.1016/s0378-1119(01)00335-3. [DOI] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Egan CM, Nyman U, Skotte J, Streubel G, Turner S, O’Connell DJ, … Bracken AP. CHD5 is required for neurogenesis and has a dual role in facilitating gene expression and polycomb gene repression. Dev Cell. 2013;26(3):223–236. doi: 10.1016/j.devcel.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2012;21(3):404–410. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- Erwin JA, Paquola AC, Singer T, Gallina I, Novotny M, Quayle C, … Gage FH. L1-associated genomic regions are deleted in somatic cells of the healthy human brain. Nat Neurosci. 2016;19(12):1583–1591. doi: 10.1038/nn.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Jeffrey A, Blanchi B, Biancotti JC, Kumar S, Hirose M, Mandefro B, … de Vellis J. Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes. Curr Protoc Stem Cell Biol. 2016;38:2D 18 11–12D 18 27. doi: 10.1002/cpsc.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, … Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14(7):723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty EK, Brennand KJ. Using hiPSCs to model neuropsychiatric copy number variations (CNVs) has potential to reveal underlying disease mechanisms. Brain Res. 2017;1655:283–293. doi: 10.1016/j.brainres.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, … Bernstein BE. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529(7584):110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Piven J. Etiology of autism: genetic influences. Pediatrics. 1991;87(5 Pt 2):767–773. [PubMed] [Google Scholar]
- Frank CL, Liu F, Wijayatunge R, Song L, Biegler MT, Yang MG, … West AE. Regulation of chromatin accessibility and Zic binding at enhancers in the developing cerebellum. Nat Neurosci. 2015;18(5):647–656. doi: 10.1038/nn.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM, … Sklar P. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19(11):1442–1453. doi: 10.1038/nn.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Fujita T. Isolation of Specific Genomic Regions and Identification of Their Associated Molecules by Engineered DNA-Binding Molecule-Mediated Chromatin Immunoprecipitation (enChIP) Using the CRISPR System and TAL Proteins. Int J Mol Sci. 2015;16(9):21802–21812. doi: 10.3390/ijms160921802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Fujii H. Identification of proteins associated with an IFNgamma-responsive promoter by a retroviral expression system for enChIP using CRISPR. PLoS One. 2014;9(7):e103084. doi: 10.1371/journal.pone.0103084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco CP, Munschauer M, Anyoha R, Munson G, Grossman SR, Perez EM, … Engreitz JM. Systematic mapping of functional enhancer-promoter connections with CRISPR interference. Science. 2016;354(6313):769–773. doi: 10.1126/science.aag2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet. 2001;105(6):521–524. doi: 10.1002/ajmg.1477. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xu Q, Canzio D, Shou J, Li J, Gorkin DU, … Wu Q. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell. 2015;162(4):900–910. doi: 10.1016/j.cell.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley BJ, Brennand KJ. Neural organoids for disease phenotyping, drug screening and developmental biology studies. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Maehara K, Harada A, Semba Y, Kudo K, Takahashi H, … Ohkawa Y. Chd5 Regulates MuERV-L/MERVL Expression in Mouse Embryonic Stem Cells Via H3K27me3 Modification and Histone H3.1/H3.2. J Cell Biochem. 2016;117(3):780–792. doi: 10.1002/jcb.25368. [DOI] [PubMed] [Google Scholar]
- Ho SM, Hartley BJ, Tcw J, Beaumont M, Stafford K, Slesinger PA, Brennand KJ. Rapid Ngn2-induction of excitatory neurons from hiPSC-derived neural progenitor cells. Methods. 2016;101:113–124. doi: 10.1016/j.ymeth.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Topol A, Brennand KJ. From “directed differentiation” to “neuronal induction”: modeling neuropsychiatric disease. Biomark Insights. 2015;10(Suppl 1):31–41. doi: 10.4137/BMI.S20066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Verzi MP, Robinson AS, Tang PL, Hua LL, Xu SM, … Black BL. Endothelin signaling activates Mef2c expression in the neural crest through a MEF2C-dependent positive-feedback transcriptional pathway. Development. 2015;142(16):2775–2780. doi: 10.1242/dev.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169(12):5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nat Med. 2012;18(8):1194–204. doi: 10.1038/nm.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PD, Kobayashi R, Kessler N, Stillman B. The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell. 1995;81(7):1105–1114. doi: 10.1016/s0092-8674(05)80015-7. [DOI] [PubMed] [Google Scholar]
- Kiani S, Chavez A, Tuttle M, Hall RN, Chari R, Ter-Ovanesyan D, … Church G. Cas9 gRNA engineering for genome editing, activation and repression. Nat Methods. 2015;12(11):1051–1054. doi: 10.1038/nmeth.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz G, Lopes R, Ugalde AP, Nevedomskaya E, Han R, Myacheva K, … Agami R. Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9. Nat Biotechnol. 2016;34(2):192–198. doi: 10.1038/nbt.3450. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, … Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IS, Carvalho CM, Douvaras P, Ho SM, Hartley BJ, Zuccherato LW, … Brennand KJ. Characterization of molecular and cellular phenotypes associated with a heterozygous CNTNAP2 deletion using patient-derived hiPSC neural cells. NPJ Schizophr. 2015:1. doi: 10.1038/npjschz.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, … Niculescu AB., 3rd Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry. 2013;18(12):1249–1264. doi: 10.1038/mp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Barish S, Okuwa S, Volkan PC. Examination of Endogenous Rotund Expression and Function in Developing Drosophila Olfactory System Using CRISPR-Cas9-Mediated Protein Tagging. G3 (Bethesda) 2015;5(12):2809–2816. doi: 10.1534/g3.115.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, … Jaenisch R. Editing DNA Methylation in the Mammalian Genome. Cell. 2016;167(1):233–247. e217. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li X, Sun N, Xu Y, Meng Y, Yang C, … Zhang K. Microarray profiling and co-expression network analysis of circulating lncRNAs and mRNAs associated with major depressive disorder. PLoS One. 2014;9(3):e93388. doi: 10.1371/journal.pone.0093388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhong X, Liu H, Hao L, Huang CT, Sherafat MA, … Zhang SC. Generation of serotonin neurons from human pluripotent stem cells. Nat Biotechnol. 2016;34(1):89–94. doi: 10.1038/nbt.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupianez DG, Kraft K, Heinrich V, Krawitz P, Brancati F, Klopocki E, … Mundlos S. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161(5):1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A. 2015;112(10):3002–3007. doi: 10.1073/pnas.1420024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Reyes-Gutierrez P, Pederson T. Visualization of repetitive DNA sequences in human chromosomes with transcription activator-like effectors. Proc Natl Acad Sci U S A. 2013;110(52):21048–21053. doi: 10.1073/pnas.1319097110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalo D, Manchado E, Concepcion CP, Bonetti C, Vidigal JA, Han YC, … Ventura A. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature. 2014;516(7531):423–427. doi: 10.1038/nature13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, … Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31(9):833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria KC, … Muotri AR. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, … Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109(31):12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Lindberg MR, Brennand KJ, Piper JC, Voet T, Cowing-Zitron C, … Gage FH. Mosaic copy number variation in human neurons. Science. 2013;342(6158):632–637. doi: 10.1126/science.1243472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JI, Celik H, Rois LE, Fishberger G, Fowler T, Rees R, … Challen GA. Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation. Biol Open. 2016;5(6):866–874. doi: 10.1242/bio.019067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill, Zoghbi . Neurobiology of Mental Illness. 4. Oxford University Press; 2014. Chapter 7: Epigenetics of Psychiatric Diseases. 2013. [Google Scholar]
- Mertens J, Wang QW, Kim Y, Yu DX, Pham S, Yang B, … Yao J. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527(7576):95–99. doi: 10.1038/nature15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Christie S, Semple CA, Porteous DJ. Chromosomal location and genomic structure of the human translin-associated factor X gene (TRAX; TSNAX) revealed by intergenic splicing to DISC1, a gene disrupted by a translocation segregating with schizophrenia. Genomics. 2000a;67(1):69–77. doi: 10.1006/geno.2000.6239. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, … Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000b;9(9):1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, … Studer L. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 2013;13(6):691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanari Y, Ziegler-Birling C, Torres-Padilla ME. Live visualization of chromatin dynamics with fluorescent TALEs. Nat Struct Mol Biol. 2013;20(11):1321–1324. doi: 10.1038/nsmb.2680. [DOI] [PubMed] [Google Scholar]
- Moggs JG, Grandi P, Quivy JP, Jonsson ZO, Hubscher U, Becker PB, Almouzni G. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol Cell Biol. 2000;20(4):1206–1218. doi: 10.1128/mcb.20.4.1206-1218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155(Pt 3):733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, … Jaenisch R. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016;22(11):1358–1367. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, … Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, … Shendure J. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338(6114):1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, … Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, … Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Halevy T, Lee DR, Sung JJ, Lee JS, Yanuka O, … Kim DW. Reversion of FMR1 Methylation and Silencing by Editing the Triplet Repeats in Fragile X iPSC-Derived Neurons. Cell Rep. 2015;13(2):234–241. doi: 10.1016/j.celrep.2015.08.084. [DOI] [PubMed] [Google Scholar]
- Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, … Pasca SP. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12(7):671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña CJ, Bagot RC, Labonté B, Nestler EJ. Epigenetic signaling in psychiatric disorders. J Mol Biol. 2014;426(20):3389–412. doi: 10.1016/j.jmb.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Du B, Jiang H, Gao J. Over-expression of CHAF1A promotes cell proliferation and apoptosis resistance in glioblastoma cells via AKT/FOXO3a/Bim pathway. Biochem Biophys Res Commun. 2016;469(4):1111–1116. doi: 10.1016/j.bbrc.2015.12.111. [DOI] [PubMed] [Google Scholar]
- Pla P, Larue L. Involvement of endothelin receptors in normal and pathological development of neural crest cells. Int J Dev Biol. 2003;47(5):315–325. [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- Quivy JP, Grandi P, Almouzni G. Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO J. 2001;20(8):2015–2027. doi: 10.1093/emboj/20.8.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal N, Srinivasan S, Kooshesh K, Guo Y, Edwards MD, Banerjee B, … Sherwood RI. High-throughput mapping of regulatory DNA. Nat Biotechnol. 2016;34(2):167–174. doi: 10.1038/nbt.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarajan P, Gil SE, Brennand KJ, Akbarian S. Spatial genome organization and cognition. Nat Rev Neurosci. 2016;17(11):681–691. doi: 10.1038/nrn.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, … Zhang F. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, … Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannals MD, Hamersky GR, Page SC, Campbell MN, Briley A, Gallo RA, … Maher BJ. Psychiatric Risk Gene Transcription Factor 4 Regulates Intrinsic Excitability of Prefrontal Neurons via Repression of SCN10a and KCNQ1. Neuron. 2016;90(1):43–55. doi: 10.1016/j.neuron.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway P, Almouzni G. CAF-1 and the inheritance of chromatin states: at the crossroads of DNA replication and repair. J Cell Sci. 2000;113(Pt 15):2647–2658. doi: 10.1242/jcs.113.15.2647. [DOI] [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, … Sullivan PF. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45(10):1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Mitchell AC, Voloudakis G, Fullard JF, Pothula VM, Tsang J, … Sklar P. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9(4):1417–1429. doi: 10.1016/j.celrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest LB, Xiang X, Lim KC, Levi G, Clouthier DE. Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development. 2004;131(18):4413–4423. doi: 10.1242/dev.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, St Laurent G, 3rd, Wahlestedt C. The Emerging Role of Non-Coding RNAs in Drug Addiction. Front Genet. 2012;3:106. doi: 10.3389/fgene.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic D, Partridge EC, Newberry KM, Smith SB, Meadows SK, Roberts BS, … Myers RM. CETCh-seq: CRISPR epitope tagging ChIP-seq of DNA-binding proteins. Genome Res. 2015;25(10):1581–1589. doi: 10.1101/gr.193540.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study C. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Erdmann S, Mojica FJ, Garrett RA. Protospacer recognition motifs: mixed identities and functional diversity. RNA Biol. 2013;10(5):891–899. doi: 10.4161/rna.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner DM, Hacisuleyman E, Younger ST, Rinn JL. Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat Methods. 2015;12(7):664–670. doi: 10.1038/nmeth.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, … Skarnes WC. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods. 2014;11(4):399–402. doi: 10.1038/nmeth.2857. [DOI] [PubMed] [Google Scholar]
- Shinmyo Y, Tanaka S, Tsunoda S, Hosomichi K, Tajima A, Kawasaki H. CRISPR/Cas9-mediated gene knockout in the mouse brain using in utero electroporation. Sci Rep. 2016;6:20611. doi: 10.1038/srep20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, … Jaenisch R. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature. 2016;533(7601):95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro PA, Flavell CR, Widagdo J, Ratnu VS, Troup M, Ragan C, … Bredy TW. Long Noncoding RNA-Directed Epigenetic Regulation of Gene Expression Is Associated With Anxiety-like Behavior in Mice. Biol Psychiatry. 2015;78(12):848–859. doi: 10.1016/j.biopsych.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth P, Han K, Callahan DG, Makovkina E, Muratore CR, Lalli MA, … Young-Pearse TL. Genomic DISC1 Disruption in hiPSCs Alters Wnt Signaling and Neural Cell Fate. Cell Rep. 2015;12(9):1414–1429. doi: 10.1016/j.celrep.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AX, Yuan Q, Tan S, Xiao Y, Wang D, Khoo AT, … Je HS. Direct Induction and Functional Maturation of Forebrain GABAergic Neurons from Human Pluripotent Stem Cells. Cell Rep. 2016;16(7):1942–1953. doi: 10.1016/j.celrep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- Szafranski K, Abraham KJ, Mekhail K. Non-coding RNA in neural function, disease, and aging. Front Genet. 2015;6:87. doi: 10.3389/fgene.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taylor SM, Alvarez-Delfin K, Saade CJ, Thomas JL, Thummel R, Fadool JM, Hitchcock PF. The bHLH Transcription Factor NeuroD Governs Photoreceptor Genesis and Regeneration Through Delta-Notch Signaling. Invest Ophthalmol Vis Sci. 2015;56(12):7496–7515. doi: 10.1167/iovs.15-17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanisch K, Schneider K, Morbitzer R, Solovei I, Lahaye T, Bultmann S, Leonhardt H. Targeting and tracing of specific DNA sequences with dTALEs in living cells. Nucleic Acids Res. 2014;42(6):e38. doi: 10.1093/nar/gkt1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theka I, Caiazzo M, Dvoretskova E, Leo D, Ungaro F, Curreli S, … Broccoli V. Rapid generation of functional dopaminergic neurons from human induced pluripotent stem cells through a single-step procedure using cell lineage transcription factors. Stem Cells Transl Med. 2013;2(6):473–479. doi: 10.5966/sctm.2012-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruvalluvan A, Czepiel M, Kap YA, Mantingh-Otter I, Vainchtein I, Kuipers J, … Copray S. Survival and Functionality of Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes in a Nonhuman Primate Model for Multiple Sclerosis. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Martin MC, Garcia A, Cigudosa JC, Ramirez JC, Rodriguez-Perales S. Engineering human tumour-associated chromosomal translocations with the RNA-guided CRISPR-Cas9 system. Nat Commun. 2014;5:3964. doi: 10.1038/ncomms4964. [DOI] [PubMed] [Google Scholar]
- Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6(5):407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de V, II, Gordebeke PM, Khoshab N, Tiesinga PH, Buitelaar JK, Kozicz T, … Glennon JC. Long non-coding RNAs in neurodevelopmental disorders. Front Mol Neurosci. 2013;6:53. doi: 10.3389/fnmol.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera E, Studer L. When rejuvenation is a problem: challenges of modeling late-onset neurodegenerative disease. Development. 2015;142(18):3085–3089. doi: 10.1242/dev.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, … Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojta A, Dobrinic P, Tadic V, Bockor L, Korac P, Julg B, … Zoldos V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016;44(12):5615–5628. doi: 10.1093/nar/gkw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S, Tuttle M, Cheng J, Church G. Next stop for the CRISPR revolution: RNA-guided epigenetic regulators. FEBS J. 2016;283(17):3181–3193. doi: 10.1111/febs.13768. [DOI] [PubMed] [Google Scholar]
- Wang H, La Russa M, Qi LS. CRISPR/Cas9 in Genome Editing and Beyond. Annu Rev Biochem. 2016;85:227–264. doi: 10.1146/annurev-biochem-060815-014607. [DOI] [PubMed] [Google Scholar]
- Wang P, Lin M, Pedrosa E, Hrabovsky A, Zhang Z, Guo W, … Zheng D. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment. Mol Autism. 2015;6:55. doi: 10.1186/s13229-015-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sengel C, Emerson MM, Cepko CL. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev Cell. 2014;30(5):513–527. doi: 10.1016/j.devcel.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, … Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8(3):288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, … Ming GL. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515(7527):414–418. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Fricano-Kugler CJ, Getz SA, Skelton PD, Lee J, Rizzuto CP, … Luikart BW. A Retroviral CRISPR-Cas9 System for Cellular Autism-Associated Phenotype Discovery in Developing Neurons. Sci Rep. 2016;6:25611. doi: 10.1038/srep25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Cui F, Yu F, Peng X, Jiang T, Chen D, … Peng Z. Up-regulation of CHAF1A, a poor prognostic factor, facilitates cell proliferation of colon cancer. Biochem Biophys Res Commun. 2014;449(2):208–215. doi: 10.1016/j.bbrc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, … Tang H. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22(10):1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Kerschner JL, Gosalia N, Neems D, Gorsic LK, Safi A, … Harris A. Differential contribution of cis-regulatory elements to higher order chromatin structure and expression of the CFTR locus. Nucleic Acids Res. 2016;44(7):3082–3094. doi: 10.1093/nar/gkv1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, … Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hu JF, Wang H, Cui J, Gao S, Hoffman AR, Li W. CRISPR Cas9-guided chromatin immunoprecipitation identifies miR483 as an epigenetic modulator of IGF2 imprinting in tumors. Oncotarget. 2016 doi: 10.18632/oncotarget.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, … Sudhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78(5):785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Liu J, Chen CH, Liao Q, Xu P, … Wei W. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9 library. Nat Biotechnol. 2016;34(12):1279–1286. doi: 10.1038/nbt.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]