Abstract
In animal models, the physiological systems involved in metabolic homeostasis exhibit a sex difference. Investigators often use male rodents because they show metabolic disease better than females. Thus, females are not used precisely because of an acknowledged sex difference that represents an opportunity to understand novel factors reducing metabolic disease more in one sex than the other. The National Institutes of Health (NIH) mandate to consider sex as a biological variable in preclinical research places new demands on investigators and peer reviewers who often lack expertise in model systems and experimental paradigms used in the study of sex differences. This review discusses experimental design and interpretation in studies addressing the mechanisms of sex differences in metabolic homeostasis and disease, using animal models and cells. We also highlight current limitations in research tools and attitudes that threaten to delay progress in studies of sex differences in basic animal research.
eTOC blurb
Mauvais-Jarvis et al. discuss experimental design and interpretation in studies addressing the mechanisms of sex differences in metabolic homeostasis and disease, using animal models and cells. They highlight current limitations in research tools and attitudes that threaten to delay progress in studies of sex differences in basic animal research.
Introduction
A pervasive attitude today among basic researchers is that most mammalian physiological systems are fundamentally the same in males and females, and therefore studying one sex is usually sufficient to understand the basic principles of tissue function and disease. We challenge this idea for metabolic homeostasis. Metabolic physiological systems are among those that show quite significant differences caused by the inherently different biology of the two sexes. Nevertheless, many investigators frequently rely on exclusively male rodents in research (Zucker and Beery, 2010). Females are avoided as experimental subjects based on the concern that the estrous cycle induces variability in traits that complicate experimental designs. In fact, females have been found to be no more variable than males (Becker et al., 2016; Itoh and Arnold, 2015; Prendergast et al., 2014). In the field of metabolic disease, the use of male rodents may also be motivated by observations that males exhibit more pronounced disease phenotypes than females. For example, males are used in studies in which obesity is induced with high-fat diet, and in studies using streptozotocin to induce insulin-deficient diabetes (Mauvais-Jarvis, 2015b). In these cases, the omission of females is precisely because of an acknowledged biological difference. In our view, studies of these sex differences should be emphasized, rather than ignored, because they present an opportunity to understand novel factors that reduce metabolic disease more in one sex than the other (Mauvais-Jarvis, 2015a).
In virtually any physiological study, the focus on a single sex threatens to limit the impact of research findings, as results may be relevant to only half of the population. To correct this bias, the National Institutes of Health (NIH) has recently mandated researchers to consider sex as a biological variable in preclinical research, by including both sexes in research designs (Clayton and Collins, 2014; NOT-OD-15-102). Policies have been introduced for the design of grants by applicants and for the review of these grants by NIH study sections (Tannenbaum et al., 2016). The importance of studying male and female models is not just a matter of being inclusive. Rather, the comparison of the two sexes raises questions that would otherwise not be asked: What are the forces that are protective more in one sex than the other, and can those forces be harnessed for better therapy (Danska, 2014; Klein et al., 2015; Mauvais-Jarvis, 2015a)? Even if a phenotype does not show an overall sex difference, underlying mechanisms may still differ in the two sexes. For example, diverse sex-specific molecular pathways may have opposite effects and cancel out a sex difference, leading to sexual equivalence of the overt phenotype (De Vries, 2004). The balance between two sex-biased mechanisms may be disrupted by a physiological stress that affects one of the sex-biased mechanisms more than the other, with the result that the sex differences may emerge or disappear as the stress changes. Therefore, the recognition and identification of sex-specific biological processes will lead to better understanding of underlying mechanisms, and drive novel discovery to improve therapy.
The effort to encourage better study of both sexes places new demands on investigators and peer reviewers. Some NIH study sections lack expertise in model systems and experimental paradigms used in the study of sex differences, which places burdens on reviewers and undermines the applicant’s ability to propose research strategies effectively. We conceived this article as a discussion of the design and interpretation of studies to address the mechanisms causing sex differences in metabolic homeostasis and disease, using animal models and cells. We also highlight current limitations in research tools and attitudes that threaten to delay progress in sex differences in basic animal research. For further reading, please refer to valuable resources that address detailed methods for sex- and gender-based basic and clinical research (Becker et al., 2005) or the issue of sex inclusion in basic research (Danska, 2014; Greenspan et al., 2007; Klein et al., 2015; Ouyang et al., 2016; Richardson et al., 2015; Ritz et al., 2014; Tannenbaum et al., 2016).
Sex and gender are distinct
In 2001, the Institute of Medicine published a review that emphasized the need for clear definitions of sex and gender (Wiseman and Pardue, 2001). Sex was defined as “the classification of living things, generally as male or female according to their reproductive organs and functions assigned by chromosomal complement.” In contrast, gender was defined as, “a person’s self-representation as male or female, or how that person is responded to by social institutions on the basis of the individual’s gender presentation. Gender is rooted in biology and shaped by environment and experience.” In our view, sex differences in human traits are caused by a small cluster of inherent biological factors as a consequence of genetic, molecular, cellular, anatomical and physiological events that interact with one another. In contrast, gender refers to male-female differences that are caused by environmental factors related to the social and cultural roles of individuals, and expectations of others (Holdcroft, 2007). The sex-gender dichotomy therefore refers to two major classes of factors that make males and female phenotypically different. These biological and social/environmental factors influence each other, and are completely intertwined. For example, differential social environments of males and females (because of social expectations including body image and body weight, or stress that varies by gender because of gender-biased choice of occupations, etc.) influence their biological phenotypes, including food intake, exercise, and obesity. Moreover, the biology of the individual also influences gender (for example, biological differences in body size and strength lead to gender-biased choices of occupations and environments). Yet, “sex” and “gender” are often used interchangeably and inappropriately in basic research. Some researchers prefer to use the term “gender” rather than “sex” because it sounds more polite or politically correct, or avoids any connotation of the sexual act. Although we acknowledge the importance of environmental and social factors to differentiate male and female humans, here we focus on biological sex differences in preclinical research. The study of animals is motivated often by this focus, because animals (like humans) share genes and hormones that make the two sexes different. Because most animals do not share the complex gendered social environments of humans, studying animals is not informative about human gender (Wiseman and Pardue, 2001). Of course, many animals have social environments too, which are different in the two sexes and contribute to sex differences in phenoptype (e.g., differential grooming contributing to sex differences in rats (Moore and Power, 1992)). However, these socially induced differences are not usually similar to those of humans. Therefore, “sex” applies more appropriately to male-female differences in basic research on rodents, birds, fishes, flies, worms, and cells. “Gender” is best reserved for references to human beings (Wiseman and Pardue, 2001).
It has been proposed to divide sex differences into three categories to inform experimental design (McCarthy et al., 2012). The first category encompasses sex differences that are extreme, leading to “sex dimorphism” in which the trait exists only in two forms, one found exclusively in males and the other exclusively in females. This is rare in metabolism but frequent in reproduction. The second category is the most common type of “sex difference,” in which the trait exists along a range in males and females and shows varying degrees of overlap, but the average is greater in one sex (for example fat distribution). The third case is when there is no observed sex difference in a trait at baseline but the underlying biology influencing this trait is markedly different in males and females such that the sex difference appears during physiological stress. For example, the overall energy balance is similar in males and females, but the survival strategy of male rodents following food deprivation is to reduce the loss of fat stores by increasing energy intake. Conversely, females reduce the loss of fat stores by decreasing energy expenditure (Shi et al., 2007). The underlying neurobiology, that is not apparent under basal conditions, could result from a sex difference in the density of anorexigenic neuropeptide pro-opiomelanocortin (POMC) neuronal fibers in the hypothalamic arcuate nucleus (Nohara et al., 2011a).
The mouse is a tractable metabolic system but is not a human
Most animal models of type 2 diabetes and obesity show some degree of sex differences, often with a more severe phenotype in males. This is true for spontaneous, pharmacologically-induced or genetic diabetic models (associated or not with obesity and insulin resistance) in which males develop β-cell failure but females don’t. Describing all of these models is beyond the scope of this review, but widely used models include the Zucker diabetic fatty (ZDF) rat (Lee et al., 1994), the Akita mouse (Oyadomari et al., 2002; Yoshioka et al., 1997), the Otsuka Long-Evans Tokushima Fatty (OLETF) rat (Kawano et al., 1994), the New Zealand obese (NZO) mouse (Plum et al., 2000), transgenic mice overexpressing the human islet amyloid polypeptide (hIAPP) (Geisler et al., 2002; Janson et al., 1996), and mice with diabetes induced by treatment with streptozotocin (Le May et al., 2006; Puah and Bailey, 1985) or alloxan (Kilic et al., 2014). Most rodent models of diet-induced obesity or insulin resistance also tend to have more severe phenotypes in males (Hevener et al., 2002; Hong et al., 2009). Importantly, sex differences in diabetes and obesity are also observed in humans with a male predominance in diabetes and a female predominance in extreme obesity ((NCD-RiskC), 2016a, b; Kelly et al., 2008; Lovre and Mauvais-Jarvis, 2015; Mauvais-Jarvis, 2015b; Menke et al., 2015).
The purpose of rodent systems is to be experimentally more tractable than humans, so that causality between variables can be proven beyond a doubt. Indeed, the study of rodents yields more accurate information about the basic architecture of biological metabolic systems than can be obtained from the study of humans. In addition, the study of animal models provides a rich source of discoveries and new hypotheses that frame novel questions about human biology, especially because vertebrates share many of the genes and hormones that cause sex differences in traits. However, despite the conserved underlying biological mechanisms to explain sex differences in animals and humans, there is no a priori expectation that studying rodents informs us directly about the human condition. Thus, new ideas arising from animal experiments need to be tested in humans as much as is possible. For example, the master regulator of energy balance, leptin was initially discovered in the mouse and subsequently found to share similar function in humans (Halaas et al., 1995; Zhang et al., 1994). The same is true for the insulin sensitizing adipokine, adiponectin (Scherer et al., 1995; Yamauchi et al., 2001). However, major differences between rodents and humans are not a rationale to avoid studying rodents (Richardson et al., 2015). Indeed, resolving why human physiology is similar to and different from that of animals provides our best chance for understanding human physiology and disease. For example, mice deficient in the insulin receptor (IR) die within the first week of life from diabetic ketoacidosis (Accili et al. 1996; Joshi et al. 1996), whereas humans with mutant or missing IR exhibit relatively mild hyperglycemia (Accili et al. 1992; Taylor 1992). This species difference has allowed the discovery that insulin acting on the IGF-1 receptor is more effective in humans than in mice in promoting glucose homeostasis (Nakae et al., 2001).
The study of sex differences often requires the manipulation of gonadal hormones, which is achieved classically by removing the gonads and replacing the hormones one by one (as will be discussed below). There is a misconception that the study of gonadectomized rodents is less relevant to humans because most humans have gonads. The point of this manipulation, however, is that studying gonadectomized rodents allows one to isolate hormones and demonstrate causality between a hormone and a sex difference in a trait. Humans too can have their gonads surgically removed. For example, men castrated for prostate cancer do not have testes, and even larger numbers of women experience surgical menopause and do not have ovaries. The study of these populations with primary androgen and estrogen deficiencies has been instrumental in understanding the role sex hormones in diabetes prevention in humans (Appiah et al., 2014; Keating et al., 2010; Keating et al., 2006; Mauvais-Jarvis, 2016).
The origin of sex differences
Sex differences in physiology begin during development from the combination of genetic and hormonal events and they continue after puberty. They result from the combination of three major events.
The cell autonomous action of sex chromosomes
All biological sex differences originate with the differences in the number and type of sex chromosomes. One of the most important differences is that the mammalian testis-determining Sry gene on the Y chromosome causes the development of testes in males (Arnold, 2017). In the absence of Sry in XX females, autosomal or X-linked genes induce differentiation of ovaries. The presence vs. absence of Sry, therefore, sets up a lifelong difference in the levels of gonadal hormones, which are primary factors that induce sex differences in many tissues. Because ovarian hormones occur together with XX sex chromosomes, and testicular hormones occur together with XY sex chromosomes, it has historically been difficult to separate the sex-biased effects of sex chromosomes from the effects of gonadal hormones. Although it is widely established that post-pubertal gonadal hormones have sex-specific effects on metabolic homeostasis (Mauvais-Jarvis et al., 2013; Navarro et al., 2015) and that pre-pubertal effects of gonadal hormones program metabolism in adults (as discussed below), it is not generally appreciated that the sex chromosome complement itself (outside the testis-determining Sry gene) contributes to metabolic differences between males and females (Chen et al., 2012). Still, prior to the differentiation of gonads during development, male XY embryos of several species are larger (Burgoyne et al., 2002; Burgoyne et al., 1995) and grow faster (Avery et al., 1992; Pergament et al., 1994; Ray et al., 1995; Xu et al., 1992) than female XX embryos. This suggests that to fully understand metabolic differences between males and females, it is important to consider both the presence of female/male gonads and the effects of XX vs. XY chromosome complement outside of the Sry gene (Fig. 1).
Figure 1. Origins of sex differences.
Sex differences in physiology begin during development from the combination of genetic and hormonal events and they continue after puberty. They result from the combination of the cell autonomous effect of sex chromosomes, the organizational action (masculinization) of the testicular testosterone surge in males and the activational effect of male and females sex hormones acting on their receptors after puberty. T: testosterone; AR, androgen receptor; ER, estrogen receptor; GPER, G protein coupled ER; PR, progesterone receptor.
The testicular testosterone surge
In many mammals the differentiated testis produces two perinatal testosterone surges that will masculinize the reproductive tract and the organization of neural circuits permissive to the activation of male behavior at puberty. In male humans and primates, the predominant testosterone surge occurs prenatally, during the second trimester of pregnancy (Corbier et al., 1992; Forest et al., 1976). In male rodents, the first testosterone surge occurs in late gestation and the second peaks at 2-hours postnatally and returns to basal levels at 6-hours (Corbier et al., 1992; Weisz and Ward, 1980). However, rodent male levels of testosterone are higher than those of females from day 18 of gestation through day 5 post-partum (Weisz and Ward, 1980). Extensive evidence relates sexually dimorphic aspects of physiology to brain masculinization by these testicular testosterone surges in males (Arnold and Gorski, 1984; MacLusky and Naftolin, 1981; Morris et al., 2004; Simerly, 2002). This sexual differentiation is referred to as the “organizational” action of testosterone as it causes the hypothalamus to permanently change its structure and function, leading to sex differences in reproductive behavior and physiology. This organizational effect is said to produce a masculinization if the acquired trait corresponds to a typical male behavior, like the masculinization of underlying neural circuitry responsible for male behaviors such as fighting and urine marking after puberty. It is called a defeminization if the acquired trait corresponds to the loss of a typical female behavior, like the loss of the pituitary ability to mount a pre-ovulatory surge of gonadotropins. The hypothalamus, however, also controls energy balance after receiving signals for the main appetite suppressing hormone, leptin, which acts in the arcuate nucleus (ARC) (Elmquist et al., 1999). In rodents, primates and humans, the organization and wiring of ARC neurons circuitry controlling energy balance also occurs during perinatal life (Bouret et al., 2004; Grayson et al., 2006; Koutcherov et al., 2002). Thus, as in the case of the hypothalamic control of reproduction, the hypothalamic control of energy homeostasis is likely to be sexually dimorphic and to be programmed or masculinized by the same testicular testosterone surge (Fig. 1).
Gonadal hormones after puberty
Most sex differences in glucose and energy homeostasis are believed to be the consequence of the “activational” (reversible) role of gonadal hormones acting on their receptors after the onset of puberty. Although these effects are reversible they are the most potent proximate factors that make male and female tissues different. Therefore, they contribute to sexual differentiation (Arnold, 2017). Testosterone is the main male gonadal hormone and 17β-estradiol (E2) and progesterone (P4) are the main female gonadal hormones. The actions of these sex hormones on metabolic homeostasis in sexually mature males and female animals have been extensively described in recent reviews and will not be discussed here (Mauvais-Jarvis et al., 2013; Navarro et al., 2015). Fig. 1 summarizes the causes of sex differences in physiology.
Methods for the study of sex differences in preclinical studies of metabolism
Apart from sex-related reproductive behavior, reproductive traits and sex-specific hormone-dependent cancers, most diseases differ in the two sexes. As discussed above, this is especially true for animal models of metabolic diseases (Mauvais-Jarvis, 2015b). Therefore, limiting studies to only one sex should require an explicit scientific justification in basic research involving animals and cells with regard to metabolic homeostasis, diabetes or obesity. Arguably, the first question to ask is to what extent there is a sex difference in the trait of interest. If we take the example of laboratory rodents, the phenotypic sex difference should be first observed in adult male and female animals of the same reproductive age with intact gonads. It is important initially to ascertain that the sex difference is present under normal laboratory conditions. Because of the importance of the perinatal nutritional environment in programming the projection of hypothalamic circuits regulating energy homeostasis in laboratory rodents (Coupe and Bouret, 2013), it is also critical that male and female animals studied be littermates in order to be comparable. The use of rodents of the same genetic background but bred in different sites and environments can introduce phenotypic differences in offspring that are unrelated to sex (Ussar et al., 2015). Another important parameter is the number of animals per cage, to compare males and females at the same housing density to avoid nutritional confounding factors. Indeed, because of their aggressive behavior, males are usually separated from one another, resulting to lower numbers per cage, which may affect their food intake, locomotor activity and energy expenditure (Ritz et al., 2014). In fact, rearing rodents in small litters favors nutritionally-induced obesity compared to larger litters (Kennedy, 1957). Once all of these important parameters are controlled, several approaches can be used to find the variables that differentially influence the trait in the two sexes.
The role of gonadal hormones after puberty
The powerful effects of sex hormones make them the top choice of factors that cause sex differences in metabolism after puberty. As discussed previously, multiple rodent models of metabolic diseases show a male predominance. One important question is to what extent this is due to E2 action in females or to testosterone action in males? This question can be first addressed by performing ovariectomy (OVX) in females to suppress ovarian secretions, including E2 and P4, and orchidectomy in males to determine the impact of testicular secretion of testosterone (Fig. 3). Importantly, surgery causes prolonged alterations of the hypothalamic-pituitary adrenal axis, and therefore influences stress hormones [reviewed in (Becker et al., 2005)]. Therefore, if gonadectomy is performed, the control group should undergo sham surgery (without gonadectomy). To take the example of the role of E2, if OVX in females abolishes the sex difference in a particular trait (and makes the female like the male), then E2 replacement therapy, with doses leading to physiological concentrations, should be performed to determine if E2 restores the phenotype of the intact female. This experiment is usually performed in a three group design: gonad-intact controls, OVX with vehicle replacement and OVX with E2 replacement (Figure 2). If E2 restores the phenotype to the level of controls, this supports the concept that E2 contributes to the sex difference in the trait. Depending on whether the sexually dimorphic phenotype is acute or chronic and the length of the desired replacement, several methods of E2 replacement therapy can be used. These include daily subcutaneous (S.C.) injection of E2 in oil (Wong et al., 2010), S.C. implantation of commercially available pellets containing E2 (Kim et al., 2014; Le May et al., 2006), S.C. implantation of Silastic tubes filled with E2 (Kudwa et al., 2009) or peroral E2 administration in fatty paste (Ingberg et al., 2012). The advantages, limitations and serum E2 concentrations achieved for each method have been reviewed (Becker et al., 2005; Ingberg et al., 2012). Once the role of E2 is ascertained, it may be important to determine which estrogen receptor (ER) mediates the effect. One method is to use commercially available selective agonists for ERα (PPT) (Stauffer et al., 2000), ERβ (DPN) (Meyers et al., 2001) or G protein-coupled ER (G1) (Bologa et al., 2006), which can be administered in OVX mice as described for E2. The results of the pharmacological manipulation are then confirmed by genetic elimination of the target ER in female mice with knockout of these receptors globally and in a tissue-specific manner (Figure 2). An in-depth discussion of these models is beyond the scope of this review, except for a few considerations. First, global knockout of gene function from inception can cause developmental defects, and these can be avoided by the use of conditional gene deletion systems. The choice of specific gene deletion conditions is important; however, as for example, the use of tamoxifen as an inducer of Cre recombinase could confound results due to the activity of tamoxifen as an ER antagonist. Finally, recent studies have revealed that sex differences in energy homeostasis could involve E2 recruitment of specific brain regions and neuronal cell types (Correa et al., 2015; Martinez de Morentin et al., 2014; Saito et al., 2016). Therefore, in the future, neural circuit-based approaches need to be integrated in the framework of studying of sex differences in metabolism.
Figure 3. Decision tree to study sex differences.
Diagram showing steps to investigate the sex-biased factors that cause a sex difference in animals. Investigators start by comparing the phenotype of the two sexes, keeping environmental conditions similar, which reveals a sex difference in a trait. The next step is to vary levels of gonadal hormones in adulthood at the time of testing using gonadectomy and replacement of hormones, to determine if gonadal hormones explain the sex difference. If these manipulations show an effect, the investigators then determines the hormone receptor that mediates the effect, and the downstream molecular pathways causing the phenotypic sex difference. If such “activational” effects of hormones do not completely explain the effect, then the investigator may test for “organizational” effects of perinatal testosterone. This is done by interfering with testosterone actions or exposing females to testosterone at that period of life. If the investigator finds an effect of hormone perinatally, which causes a sex difference later in life, the finding leads to identification of the receptors involved, their sites of action, and downstream molecular mechanisms. If both of these types of manipulations of gonadal hormones do not completely explain the sex difference, then the investigator may test for the effect of sex chromosomes in specific mouse models are appropriate (Four Core Genotypes and XY*). Even if there is no sex difference in the overt phenotype, there may exist sex differences in underlying mechanisms, which cancel each other out. This figure is adapted from (Becker et al., 2005).
Figure 2. Mouse models to study the role of gonadal hormones.
(A) A sex-biased trait is hypothesized to be due to E2 action in females. (B) This question is addressed by performing ovariectomy (OVX) in females to suppress ovarian E2 in a three group design: gonad-intact sham operated controls, OVX with vehicle treatment and OVX with E2 replacement. If OVX abolishes the sex difference in the trait (and makes the female like the male), then E2 replacement therapy should be performed to ascertain that E2 restores the phenotype of the intact female, supporting the concept that E2 contributes to the sex difference in the trait. (C) To determine which estrogen receptor (ER) mediates E2 effect; one can use selective ERα (PPT), ERβ (DPN) or the G protein-coupled ER (G1) agonists in OVX mice followed by mice with a knockout of the target ER, globally and in a tissue-specific manner.
Evidence that estrogens make females different from males does not necessarily imply that testicular secretions of males are not also important contributions to sex differences. For example, if gonadectomy makes the female similar to the gonad-intact male, testicular androgens might still influence the trait. A course of studies, similar to those outlined for estrogens in the last paragraph, would then be used to investigate the role of androgens acting in adulthood. These experiments lay the foundation for mechanistic studies to determine the cell types that are directly influenced by these hormones, and the molecular pathways that they influence.
Role of perinatal masculinization by testicular testosterone
If a sex difference is present before puberty or is not altered by gonadectomy, it can result from sexual differentiation by the pre- or postnatal testicular testosterone surge in males (Fig. 1 and 3). These “organizational” effects of testosterone can be studied by perinatal hormonal manipulations. As early as 1936, it was reported that neonatal castration in male rats reproduces the female potential for ovulation when male rats are transplanted with ovaries (Pfeiffer, 1936). Similarly, transplanting neonatal rat testes into a female (Harris, 1964) or injecting testosterone on the day of birth (Barraclough, 1961) produced a permanent failure of ovulation and luteinization in the adult (masculinization). In multiple subsequent studies of sexual differentiation of the male brain, investigators have used the model of transient prenatal or neonatal exposure to exogenous testosterone in female rodents to show that testosterone defeminizes and masculinizes the structure and function of the female hypothalamus (Arnold and Gorski, 1984; MacLusky and Naftolin, 1981; Morris et al., 2004; Negri-Cesi et al., 2008; Simerly, 2002; Wu et al., 2009).
Because the process of sexual differentiation of the brain and body is a widespread series of developmental events with functional significance for diverse behavioral and physiological responses, this model can also be used to assess the role of testicular testosterone in programming the sex differences in energy and glucose homeostasis that will remain in adults. To study the effect of testosterone in programming metabolism in female animals as a model of developmental masculinization, investigators have used both prenatal testosterone exposure in pregnant females and postnatal testosterone exposure in neonates (Alexanderson et al., 2007; Demissie et al., 2008; Eisner et al., 2003; Nilsson et al., 1998; Nohara et al., 2013a; Nohara et al., 2013c; Nohara et al., 2011a). It should be emphasized, however, that the development of adipose tissue and hypothalamic neurons controlling metabolism occur at somewhat different times in primates and rodents. In primates, including humans, development of adipose tissue and synaptogenesis of hypothalamic neurons controlling energy balance occurs during the second trimester of pregnancy (Ailhaud et al., 1992; Gesta et al., 2007; Koutcherov et al., 2002). In mice and rats, developmental plasticity of hypothalamic circuits controlling energy balance and peripheral adipose tissue development occur during the first two weeks of neonatal life (Ailhaud et al., 1992; Bouret et al., 2004; Gesta et al., 2007). Therefore, with regard to sexual differentiation of the hypothalamus and development of adipose tissue, the mouse first week of neonatal life parallels human fetal development during the second trimester of pregnancy (Mauvais-Jarvis, 2014). This later window of neonatal developmental plasticity in the mouse provides an experimental advantage that allows manipulation of the neonatal sex steroid milieu in the presence or absence of androgen receptor (AR) or/and estrogen receptors (ERs). However, to actually assess the masculinization, unexposed male littermates must be used as controls of androgenized females. Thus, neonatally androgenized female rodents develop several metabolic alterations consistent with masculinization. They exhibit masculinization of lean tissue mass including heart and skeletal muscle, kidney and bone (Nohara et al., 2013b). Neonatal testosterone also masculinizes adipose tissue distribution and morphology as well as serum adiponectin set point in females to an extent similar to that observed in littermate males (Nohara et al., 2013b). Interestingly, neonatal testosterone also masculinizes the hypothalamic melanocortin system by decreasing the expression of POMC and the intensity of neuronal projections from POMC neurons within the ARC which is associated with increased food intake in females as in littermate males (Nohara et al., 2011b). There are significant limitations to this model to study the sexual differentiation of energy homeostasis. First, the injection of testosterone in female rodent neonates is only an approximate model for masculinization in males because the dose and timing of testosterone injection do not reproduce the actual physiological testosterone surge of males. Second, using androgen treatment of females as a model for normal masculinization of the males has the potential problem that the sex chromosomes are not equivalent. Indeed, neonatally androgenized males have a different phenotype than their androgenized female littermates (Nohara et al., 2013a). This suggests interactions between perinatal testosterone and complements of sex-linked genes in sex differentiation of metabolic homeostasis. Finally, although some traits of metabolic programming by testosterone in females are typical of masculinization, others are inconsistent with masculinization (Mauvais-Jarvis, 2014).
Testosterone is a prohormone that is locally converted to 5α-dihydrotestosterone (DHT), the most potent ligand of the androgen receptor (AR), or 17β-estradiol (E2), which acts on estrogen receptors (ERs). For example in rodents, testosterone is transformed to E2 in the brain, where is acts on ERs to cause most of the masculinization in neural structure and behavior (Arnold and Gorski, 1984; MacLusky and Naftolin, 1981; Morris et al., 2004; Wu et al., 2009). To address the role of testosterone metabolites, four groups may be used, including rodents neonatally injected with vehicle, testosterone, DHT and E2. Using this experimental design in female mice, a study reported that the sexual differentiation of energy homeostasis involves an AR-dependent masculinization of hypothalamic POMC neurons and increase in energy intake (Nohara et al., 2011b).
The use of neonatal castration to study the effect of the neonatal testosterone surge in programming sex differences in adult males is complicated by the confounding effect of the loss of testosterone in adult males (Navarro et al., 2015). However, the transient neonatal S.C. injection of male pups with flutamide (AR antagonist) or tamoxifen (ER antagonist) to block the effect of the testosterone surge can be used to study the effect of AR or ER in programing sexual differences in metabolic homeostasis in adults. The study of molecular and cellular mechanisms of AR and ER programing of physiology are beyond the scope of this review but include effects of hormones on cell number (apoptosis), neuronal connectivity, synaptogenesis and axonal guidance (Simerly, 2002).
The evidence presented above suggests that the perinatal testosterone surge in males masculinizes the hypothalamus and the peripheral tissues in a way that permanently programs sex differences in metabolic homeostasis in adults. A major limitation in this area, however, is the absence of modern tools to manipulate the testosterone surge in males during the perinatal window, without relying on the neonatal castration in males or testosterone injection in females.
Is the sex difference due to the effects of sex chromosomes?
As described above, male-female differences that remain after removal of the gonads may represent long-lasting (organizational) effects of gonadal hormones during development, or genetic effects of XX vs. XY sex chromosome complement that act independently of gonadal hormones (Fig. 1 and 3). To tease apart the determinants of sex differences in metabolism, it is valuable to break sex into its component parts of gonadal (male vs. female) and genetic (XX vs. XY) effects using experimental models.
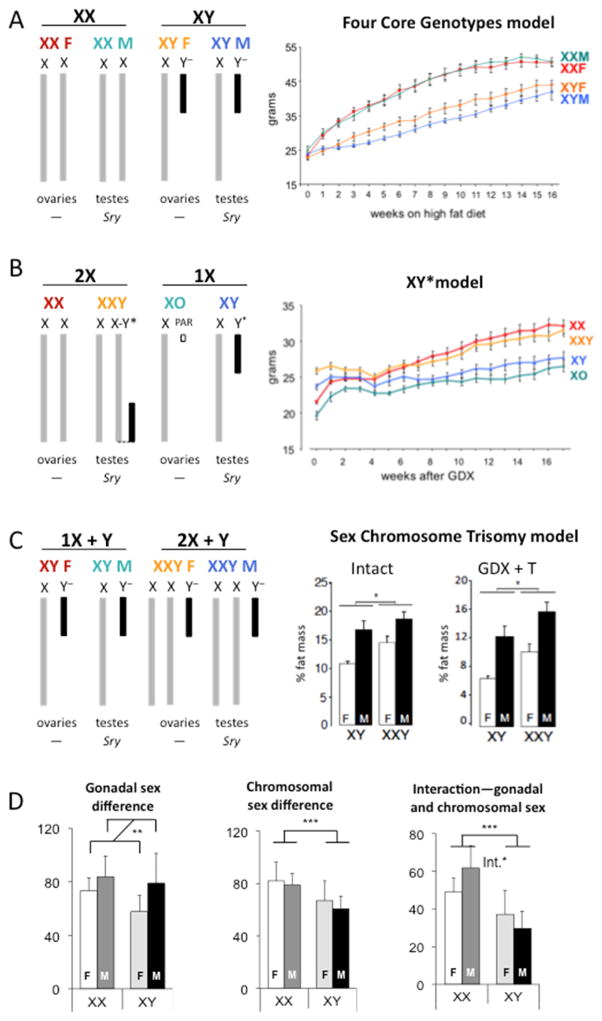
A mouse model that is valuable to distinguish the effects of gonads from sex chromosomes is the Four Core Genotypes (FCG) mouse model (Arnold and Chen, 2009; Burgoyne and Arnold, 2016). This model is available on a C57BL/6 inbred background, making results comparable to many of the models used in metabolic studies. In this model, the Sry gene that determines male gonad development has been de-coupled from the Y chromosome and transplanted as a transgene onto an autosome. Prenatal and adult androgen levels appear not to differ in XX and XY male mice that possess the autosomal Sry (Itoh et al., 2015) (Burgoyne and Arnold, 2016). With the FCG model, it is possible to develop mice with 4 combinations of gonads–sex chromosomes: XX mice with female gonads, XY mice with male gonads, XX mice with male gonads, and XY mice with female gonads (Fig. 4a). The FCG model has been applied to identify sex chromosome complement influence on metabolic traits such as obesity, food intake, hyperlipidemia, and hypertension (Bonthuis and Rissman, 2013; Chen et al., 2012; Chen et al., 2015; Ji et al., 2010; Link et al., 2015). Using obesity as an example, in FCG mice that were gonadectomized as adults to remove the acute effects of gonadal hormones, mice with XX chromosomes had greater body weight and adiposity than XY mice on both a chow and a high fat diet, regardless of the original gonadal type or presence of the Sry gene (Fig. 4a; Chen et al. 2012). The enhanced weight gain in XX mice was associated with increased food intake in XX mice during the light period of the diurnal cycle (Chen et al., 2012; Chen et al., 2015).
Figure 4. Mouse models to study the role of X and Y chromosomes.
(A) Left: The Four Core Genotypes model distinguishes effects that correlate with XX versus XY sex chromosome complement from effects that differ based on male vs. female gonads. Right: body weight gain on a high fiat diet is accelerated in XX compared to XY mice that were gonadectomized as adults. From (Chen et al., 2012). (B) Left: After the identification of differences between XX and XY mice, the XY* model is used to investigate the contribution of X versus Y chromosome copy number. Right: Gonads were removed from adult mice (time 0) and 4 weeks later the body weights converged, followed by increased weight gain in mice with two X chromosomes (XX and XXY) compared to those with one X chromosome (XO and XY). The presence of the Y chromosome does not affect body weight gain. From (Chen et al., 2012). (C) Left: The Sex Chromosome Trisomy model assesses differences related to one or two X chromosomes with a Y chromosome and different gonadal combinations. Right: Animals were examined with either intact gonads or after gonadectomy (GDX) and delivery of testosterone (T) to normalize levels across genotypes. In both conditions, presence of two X chromosomes led to increased percent body fat compared to a single X chromosome. From (Chen et al., 2013). (D). FCG studies lend themselves to analysis by 2-way ANOVA, with gonadal sex and sex chromosomes as main effects. These analyses can reveal (Left) a main effect of gonadal sex, (Middle) a main effect of chromosomal sex, or (Right) an interaction between gonadal and chromosomal sex.
If studies using the Four Core Genotypes model reveal differences between XX and XY mice, the role of X and Y chromosomes may be explored further using additional models (Burgoyne and Arnold, 2016). One of these models is the XY* mouse model. In the XY* model, the Y chromosome (Y*) has a normal Sry gene, but differs from wild-type Y chromosomes in having a duplication in a portion of the pseudoautosomal region (Burgoyne et al., 1998; Eicher et al., 1991). This duplication allows pairing with the X chromosome pseudoautosomal region in unusual ways during meiosis, leading to generation of sex chromosome complements that are nearly equivalent to XX, XY, XO and XX (Fig. 4b; Eicher, Hale et al. 1991; Burgoyne, Mahadevaiah, et al. 1998). (It should be noted that in the XY* model, XO mice also possess a small second chromosome that is nearly equivalent to a normal pseudoautosomal region, and that XXY mice possess a hybrid chromosome that contains X and Y* genetic material on a single chromosome (Burgoyne and Arnold, 2016)). A comparison of traits in these genotypes parses the effects of two doses of the X chromosome (XX and XXY) vs. one dose of X (XY and XO), and the presence (XY and XXY) vs. absence (XO and XX) of Y chromosome genes. When C57BL/6 XY* mice were gonadectomized as adults, body weight segregated into two groups, with mice having two doses of the X chromosome (XX and XXY) gaining more weight and fat mass than mice with a single X dose (XO and XY) (Fig. 4b; Chen, McClusky et al. 2012). The presence of the Y chromosome had no effect. Thus, the genetic determinant of higher body weight is two copies of the X chromosome.
Additional confirmation of the effects of sex chromosome dosage can be obtained with models such as the Sex Chromosome Trisomy model (Chen et al., 2013). This model allows the generation of eight genotypes: XX, XY, XXY, and XYY mice, each with either male or female gonads (Chen et al., 2013; Park et al., 2008). One use of this model is to emulate the XXY male genotype that occurs in Klinefelter syndrome, which has been associated with abdominal obesity, increased rates of type 2 diabetes, and metabolic syndrome (Bojesen et al., 2010; Jiang-Feng et al., 2012). The Trisomy model can be used to determine what role sex chromosome and hormone levels each play in determining metabolic traits (similar to the FCG model), and also whether number or dose of X and Y chromosomes has an effect when examined on the background of both female and male gonads. One drawback to this model is that the genotypes are not all robust on an inbred background, and studies are therefore performed on the outbred MF1 strain background. Nevertheless, in studies of body weight and adiposity, results with this model extended those observed in FCG and XY* mice. Two doses of the X chromosome led to higher body weight and adiposity than a single X chromosome dose, regardless of male or female gonads, and independent of testosterone levels (Fig. 4c; Chen, Williams-Burris et al. 2013).
Once it is established that there is a sex chromosomal effect on a metabolic trait of interest, subsequent steps will depend on the sex chromosome involved. In the example shown in Fig. 4, there is a consistent effect of X chromosome dose as a determinant of differences in body weight, weight gain on a high fat diet, and proportional fat mass. The X chromosome is special in that dosage is normalized between XX and XY cells for a majority of X genes through the process of X chromosome inactivation during development. This suggests that most genes on the X chromosome are unlikely to contribute to the effects of X chromosome dosage on sex differences and focuses attention onto specific subsets of X chromosome genes. Possible candidates include genes that escape inactivation and remain transcriptionally active on the ‘inactive’ X chromosome, as well as paternally imprinted genes on the X chromosome (Balaton and Brown, 2016; Lee and Bartolomei, 2013). In both cases, dosage of these specific genes would be higher in XX (and XXY) compared to XY (and XO) tissues, and could influence metabolic phenotypes. Indeed, a handful of X chromosome escapee genes have been shown to exhibit 40–60% higher expression levels in key metabolic tissues (liver, adipose tissue, skeletal muscle) of XX compared to XY mice (Chen et al., 2012; Link et al., 2015). On the other hand, if Y dosage influences a trait, key candidates for the effect are narrowed to a small number of Y chromosome genes that are expressed in the tissue of interest. To date, studies of sex chromosome effects have not been widely explored, but represent fertile ground for future elucidation of sex differences in metabolic traits.
Statistical considerations for the analyses of sex differences
As described in preceding sections, mouse models allow the identification of the components of sex that determine a specific trait. These may include gonadal sex, sex chromosome complement, acute effects or organizational effects of gonadal hormones, and interactions between these components. To identify the contribution of the various components, it is critical to design and analyze experiments with appropriate statistical considerations. A useful paradigm is illustrated by studies performed with the FCG model, where the study of four genotypes (XX and XY mice with male gonads and XX and XY mice with female gonads) lends itself to a 2 × 2 comparison via 2-way ANOVA (Arnold and Chen, 2009; Burgoyne and Arnold, 2016; Chen et al., 2012; Chen et al., 2015; Link et al., 2015; Link et al., 2017; Reue, 2017). If analysis of the four genotypes by ANOVA reveals that mice with male gonads (XX and XY) differ from mice with female gonads (XX and XY), the cause is gonadal sex (or more precisely, the presence vs. absence of the Sry gene) (Figure 4D). On the other hand, if a trait is influenced by sex chromosome complement, differences will be observed between XX and XY mice (Figure 4). The power to detect main effects of gonadal and chromosomal sex in a 2-way ANOVA is aided by the fact that two groups are combined for each analysis. For example, in a study with 5 mice of each of the four genotypes, the comparison of mice with male vs. female gonads would actually be a comparison of 10 mice with male and 10 mice with female gonads. In addition to the main effects, analysis of FCG studies via 2-way ANOVA allows detection of interactions between gonadal sex and sex chromosome genotype. An interaction is evident when an effect occurs between two groups only when another condition is also satisfied. An example would be when the trait studied in mice with male gonads has a greater value than in mice with female gonads, but only in the context of XX chromosomes (Figure 4D). A larger group size may be required to evaluate interactions, and would depend on the effect size. To assess the acute effects of gonadal hormones vs. the organizational effects of gonadal hormones, comparisons would be made between mice with and without gonadectomy with via 2-way ANOVA and with the same considerations discussed above.
Important considerations for the study of sex differences in preclinical studies of metabolism
Impact of the estrous cycle
In gonadally intact rodents, it has been suggested that males be compared to females on two specific days of their estrous cycle, which represent two ends of the continuum of hormone levels (Becker et al., 2005). It is possible that metabolic traits such as food intake, thermogenesis and locomotor activity may differ as a function of the day of the female reproductive cycle because these traits change by rapid alterations in neuronal firing, neuropeptide secretion and autonomic nervous activation. However, for other metabolic traits, the estrous cycle may be less relevant because these traits are the consequence of hormone-induced chronic alterations in gene and protein expression leading to progressive modification of tissue function (e.g., adipose tissue mass, lipid biology, insulin sensitivity or islet biology). In fact, a meta-analysis comparison of male and female mice, with no regard to the stage of the estrous cycle, established that variability in most traits was equivalent in females and males and that for most end points, it was unnecessary to stage the estrous cycle (Prendergast et al., 2014).
Furthermore, female rats can synchronize their estrous cycles if housed together as a result of chemosignals from pheromones (McClintock, 1984). However, if female mice are housed together at high density (5–6 per cage), which promotes stress, cycling is suppressed compared to mice housed at lower density (2 per cage) because of adrenal-mediated urinary metabolites (Champlin, 1971; Ma et al., 1998; Whitten, 1959). Therefore, housing at optimal density seems appropriate.
Impact of the vivarium environment in rodents
The abundance of exogenous sources of estrogens coming from normal rodent chow (soy phytoestrogens), rodent bedding (corncob), or cages and water bottles (Bisphenol-A) could affect estrogen-sensitive metabolic parameters in a sexually dimorphic manner (Thigpen et al., 2013). A non-soy low-phytoestrogen chow increases fetal serum E2 concentration resulting in a “fetal estrogenization syndrome” with obesity and hyperleptinemia in adults mice of both sexes compared to soy-based high-phytoestrogen chow. However, only males developed glucose intolerance on the non-soy chow, thus creating a diet-induced sex difference compared to mice on the soy-based chow (Ruhlen et al., 2008).
Perhaps the most critical challenge created by the environment in confounding the exploration of sex differences in biological traits, is the influence of the metagenome – the interaction between host and microbiome genes- on the experimental reproducibility of in vivo studies in rodents. Light/dark cycle schedules, type of rodent diet, pH and sterility of water, defined vivarium pathogens, all influence the metagenome in ways that can modify the phenotype of rodents. For example, the development of autoimmune type 1 diabetes in mice is characterized by a female predominance under standard vivarium conditions. However, under germ-free conditions, the incidence of T1D in males become similar to that of females suggesting that in this model the sex bias is microbiome dependent (Markle et al., 2013). The scope of metagenomic effects in rodent phenotypes and the principles to address them were recently defined (Stappenbeck and Virgin, 2016). However, the most important paradigm is certainly the direct comparison of littermate progeny of heterozygote matings that segregate alleles but maintain the same microbiome components.
Impact of stress in rodents
There are sex differences in the responses to acute stress in rodents that are mediated by different hormonal systems, with males being responsive to glucocorticoid increase and females more responsive to alterations of estrous cycle (Shors et al., 2001; Wood et al., 2001). Interestingly, the sex of the investigator is also a biological variable that needs to be seriously considered. A study of student volunteers reported that the sex of the investigator is associated with differences in pain responses. Thus, subjects tolerated pain better when tested by an experimenter of the opposite sex (Kallai et al., 2004). In addition, higher pain intensity was observed for subjects tested by female experimenters. These effects seem to occur in animals, but they are believed to occur and to be related not to competition or beliefs, as in humans, but to odors, sounds, and handling differences. For instance, exposure of mice and rats to male investigators induced a robust physiological stress response that resulted in stress-induced analgesia compared to exposure to female investigators (Sorge et al., 2014). So, in both humans and rodents, male sex of the investigator is associated with better tolerance to stress.
Assessment of sex differences in cell culture
The NIH has mandated researchers to consider sex as a biological variable in preclinical research to promote the study of cells from both sexes. Phenol red, a common pH indicator used in cell culture, is estrogenic. Accordingly, it is important to avoid its use when studying parameters that might be influenced by the presence of estrogens. Phenol red-free media are available. Charcoal-stripped serum is also used to avoid steroid hormones present in serum. When sex steroids are added, physiological concentrations of hormones (E2, T, DHT and P4) should be used, usually between 1–10nM. The use of pharmacological concentrations over 100nM risks finding effects that are not relevant to physiology. In addition, cells or cultured tissues isolated from ER and AR knockout mice should be cultured in the presence and absence of the ligand to validate the ligand-dependent and ligand-independent effect of the receptor deletion on the phenotype. For example, mice with global or β-cell-specific knockout of the AR exhibit a similar defect in glucose-stimulated insulin secretion compared to littermate controls (Dubois et al., 2016; Navarro et al., 2016). However, in cultured islets from these same mice, the insulin secretory defect is observed only when the control and AR-deficient islets are studied in the presence of physiological concentrations of testosterone (Navarro et al., 2016), not when the islets are cultured in absence of hormone (Dubois et al., 2016).
The major issue in studying sex differences in cultured cells is that the available research tools are lagging far behind. First, there are no genetically identical male and female cell lines available to date to study sex differences in vitro. Moreover, the prospect of establishing genetically comparable, immortalized cell lines from male and female humans is dim (Ritz et al., 2014; Shah et al., 2014). The use of primary cell cultures from inbred animals of the same genetic background is limited by the availability of tissue and most importantly, the impossibility to expand them. The development of clonal, immortalized cell lines from littermate inbred animals is not perfect but seems a logical first step in creating comparable male and female cell lines.
Second, even if cells from both sexes were available, the comparison of cultured male and female cells oversimplifies the question of sex because of the limitations of the in vitro environment. Although XX and XY cells differ because of cell autonomous factors, the sex of cells in vivo is more complicated (Wiseman and Pardue, 2001). Sex differences are dynamic and changeable properties of the body influenced by genetic sex, the organizational role of testosterone surge and the activational role of sex hormones at puberty. The resulting in vivo environments differ in multiple factors including hormones, metabolites, neural inputs, body composition and define two different male and female biological systems for cells in vivo (Fig. 5). For example, profiling of sex differences in serum metabolites revealed major sex differences in concentrations for over three quarters of the metabolites studied (Mittelstrass et al., 2011). This “sexome,” which is the sum of all sex-specific influences on cellular systems (Arnold and Lusis, 2012), produces phenotypic sex differences that are exclusive to the in vivo environment. Therefore, we need to study the systems biology of each sex globally in order to have an appreciation of the sex-specific aggregate behavior of cells (Fig. 5). When primary cells are isolated from the in vivo environment and cultured, the sex differences in the cells phenotype can come from sex chromosome effects or be caused by transient (e.g., gonadal hormone levels modifying gene expression) or permanent (epigenetic modifications induced by perinatal testosterone) sex differences present in the cells’ environments prior to harvest, which is carried over into the dish. To add to the complexity of cell culture, when cells are immortalized they become chromosomally unstable after multiple passages. In the ATCC collection, over a hundred male cell lines have lost their Y chromosome (Park et al., 2006; Shah et al., 2014).
Figure 5. Male and female biological systems.
Sex differences in vivo result from the sum of all sex-specific influences on cellular systems including hormones, metabolites, neural inputs, etc. They define two different male and female biological systems or “sexome”. When primary cells are isolated and cultured, the sex differences in the cells’ phenotype can come from sex chromosome effects or be caused by transient (e.g., gonadal hormone levels modifying gene expression) or permanent (epigenetic modifications induced by perinatal testosterone) sex differences present in the cells’ environments prior to harvest, which are carried over into the dish.
Conclusion and future directions
The study of animals and cells of both sexes is essential to catalyze scientific discoveries that will open avenues for sex-based treatments of metabolic disease. Several obstacles currently hinder progress in the field, and should be addressed. First, the science of sex differences in biology and disease is more complex and sophisticated than the research tools that are available to study it. This is particularly apparent for the study of developmental programming of sex differences and the availability of comparable cell lines from both sexes. Novel research tools in this area are urgently needed. Second, and most importantly, the potential for innovative research in this area requires significant efforts to improve familiarity with sex differences research among investigators as well as grant and manuscript reviewers. It is currently stylish to demand that high impact science address “mechanisms,” and studies of sex differences have been labeled by some as “descriptive.” Our perspective is that sex differences are at the core of the mechanism for biological traits and disease, and that failure to understand both sexes is also a failure to fully understand the mechanisms of interest. We believe that the incorporation of appropriately designed studies on sex differences in metabolism and other fields will accelerate discovery and enhance our ability to treat disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) (DK074970, DK107444), the American Diabetes Association (7-13-BS-101) and the Price Goldsmith-Endowed Chair at Tulane University Health Sciences Center to F.M.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (NCD-RiskC) NRFC. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measuement studies ith 19.2 million participants. Lancet. 2016a;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (NCD-RiskC), N.R.F.C. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016b doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Alexanderson C, Eriksson E, Stener-Victorin E, Lystig T, Gabrielsson B, Lonn M, Holmang A. Postnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: comparisons with estradiol and dihydrotestosterone. Endocrinology. 2007;148:5369–5376. doi: 10.1210/en.2007-0305. [DOI] [PubMed] [Google Scholar]
- Appiah D, Winters SJ, Hornung CA. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care. 2014;37:725–733. doi: 10.2337/dc13-1986. [DOI] [PubMed] [Google Scholar]
- Arnold AP. A general theory of sexual differentiation. J Neurosci Res. 2017;95:291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Lusis AJ. Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinology. 2012;153:2551–2555. doi: 10.1210/en.2011-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery B, Jorgensen CB, Madison V, Greve T. Morphological development and sex of bovine in vitro-fertilized embryos. Mol Reprod Dev. 1992;32:265–270. doi: 10.1002/mrd.1080320312. [DOI] [PubMed] [Google Scholar]
- Balaton BP, Brown CJ. Escape Artists of the X Chromosome. Trends Genet. 2016;32:348–359. doi: 10.1016/j.tig.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen A, Host C, Gravholt CH. Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod. 2010;16:396–401. doi: 10.1093/molehr/gaq016. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Rissman EF. Neural growth hormone implicated in body weight sex differences. Endocrinology. 2013;154:3826–3835. doi: 10.1210/en.2013-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ. 2016;7:68. doi: 10.1186/s13293-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Ojarikre OA, Turner JM. Evidence that postnatal growth retardation in XO mice is due to haploinsufficiency for a non-PAR X gene. Cytogenet Genome Res. 2002;99:252–256. doi: 10.1159/000071601. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350:253–260. doi: 10.1098/rstb.1995.0159. discussion 260–251. [DOI] [PubMed] [Google Scholar]
- Champlin AK. Suppression of oestrus in grouped mice: the effects of various densities and the possible nature of the stimulus. J Reprod Fertil. 1971;27:233–241. doi: 10.1530/jrf.0.0270233. [DOI] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang L, Loh DH, Colwell CS, Tache Y, Reue K, Arnold AP. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm Behav. 2015;75:55–63. doi: 10.1016/j.yhbeh.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Williams-Burris SM, McClusky R, Ngun TC, Ghahramani N, Barseghyan H, Reue K, Vilain E, Arnold AP. The Sex Chromosome Trisomy mouse model of XXY and XYY: metabolism and motor performance. Biol Sex Differ. 2013;4:15. doi: 10.1186/2042-6410-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe B, Bouret SG. Development of the hypothalamic melanocortin system. Front Endocrinol (Lausanne) 2013;4:38. doi: 10.3389/fendo.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danska JS. Sex matters for mechanism. Sci Transl Med. 2014;6:258fs240. doi: 10.1126/scitranslmed.3009859. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295:E262–268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois V, Laurent MR, Jardi F, Antonio L, Lemaire K, Goyvaerts L, Deldicque L, Carmeliet G, Decallonne B, Vanderschueren D, Claessens F. Androgen Deficiency Exacerbates High-Fat Diet-Induced Metabolic Alterations in Male Mice. Endocrinology. 2016;157:648–665. doi: 10.1210/en.2015-1713. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT, Washburn LL. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003;11:279–286. doi: 10.1038/oby.2003.42. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Forest MG, De Peretti E, Bertrand J. Hypothalamic-pituitary-gonadal relationships in man from birth to puberty. Clin Endocrinol (Oxf) 1976;5:551–569. doi: 10.1111/j.1365-2265.1976.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Geisler JG, Zawalich W, Zawalich K, Lakey JR, Stukenbrok H, Milici AJ, Soeller WC. Estrogen can prevent or reverse obesity and diabetes in mice expressing human islet amyloid polypeptide. Diabetes. 2002;51:2158–2169. doi: 10.2337/diabetes.51.7.2158. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Harris GW. Sex Hormones, Brain Development and Brain Function. Endocrinology. 1964;75:627–648. doi: 10.1210/endo-75-4-627. [DOI] [PubMed] [Google Scholar]
- Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51:1907–1912. doi: 10.2337/diabetes.51.6.1907. [DOI] [PubMed] [Google Scholar]
- Holdcroft A. Integrating the dimensions of sex and gender into basic life sciences research: methodologic and ethical issues. Gend Med. 2007;4(Suppl B):S64–74. doi: 10.1016/s1550-8579(07)80048-9. [DOI] [PubMed] [Google Scholar]
- Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17beta-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Arnold AP. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol Sex Differ. 2015;6:18. doi: 10.1186/s13293-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O’Neill R, Arnold AP. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8:69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, Butler PC. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang-Feng M, Hong-Li X, Xue-Yan W, Min N, Shuang-Yu L, Hong-Ding X, Liang-Ming L. Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril. 2012;98:1331–1335. doi: 10.1016/j.fertnstert.2012.07.1122. [DOI] [PubMed] [Google Scholar]
- Kallai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain. 2004;112:142–147. doi: 10.1016/j.pain.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24(Suppl):S317–320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- Kennedy GC. The development with age of hypothalamic restraint upon the appetite of the rat. J Endocrinol. 1957;16:9–17. doi: 10.1677/joe.0.0160009. [DOI] [PubMed] [Google Scholar]
- Kilic G, Alvarez-Mercado AI, Zarrouki B, Opland D, Liew CW, Alonso LC, Myers MG, Jr, Jonas JC, Poitout V, Kulkarni RN, Mauvais-Jarvis F. The islet estrogen receptor-alpha is induced by hyperglycemia and protects against oxidative stress-induced insulin-deficient diabetes. PLoS One. 2014;9:e87941. doi: 10.1371/journal.pone.0087941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C, Mauvais-Jarvis F. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab. 2014;3:177–190. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I. Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acad Sci U S A. 2015;112:5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutcherov Y, Mai JK, Ashwell KW, Paxinos G. Organization of human hypothalamus in fetal development. J Comp Neurol. 2002;446:301–324. doi: 10.1002/cne.10175. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav. 2009;97:146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, Arnold AP, Reue K. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol. 2015;35:1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JC, Hasin-Brumshtein Y, Cantor RM, Chen X, Arnold AP, Lusis AJ, Reue K. Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression. BMC Genomics. 2017;18:89. doi: 10.1186/s12864-017-3484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovre D, Mauvais-Jarvis F. Trends in Prevalence of the Metabolic Syndrome. JAMA. 2015;314:950. doi: 10.1001/jama.2015.8625. [DOI] [PubMed] [Google Scholar]
- Ma W, Miao Z, Novotny MV. Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: the lee-boot effect revisited. Biol Reprod. 1998;59:1317–1320. doi: 10.1095/biolreprod59.6.1317. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L, Gallego R, Saha AK, Kalsbeek A, Fliers E, Bisschop PH, Dieguez C, Nogueiras R, Rahmouni K, Tena-Sempere M, Lopez M. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Developmental androgenization programs metabolic dysfunction in adult mice: Clinical implications. Adipocyte. 2014;3:151–154. doi: 10.4161/adip.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Elucidating sex and gender differences in diabetes: a necessary step toward personalized medicine. J Diabetes Complications. 2015a;29:162–163. doi: 10.1016/j.jdiacomp.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015b;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Androgen-deprivation therapy and pancreatic beta-cell dysfunction in men. J Diabetes Complications. 2016 doi: 10.1016/j.jdiacomp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock MK. Estrous synchrony: modulation of ovarian cycle length by female pheromones. Physiol Behav. 1984;32:701–705. doi: 10.1016/0031-9384(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Power KL. Variation in maternal care and individual differences in play, exploration, and grooming of juvenile Norway rat offspring. Dev Psychobiol. 1992;25:165–182. doi: 10.1002/dev.420250303. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–835. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23:713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F. Extranuclear Actions of the Androgen Receptor Enhance Glucose-Stimulated Insulin Secretion in the Male. Cell Metab. 2016;23:837–851. doi: 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Pravettoni A, Casati L, Conti L, Celotti F. Sexual differentiation of the rodent hypothalamus: hormonal and environmental influences. J Steroid Biochem Mol Biol. 2008;109:294–299. doi: 10.1016/j.jsbmb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Niklasson M, Eriksson E, Bjorntorp P, Holmang A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J Clin Invest. 1998;101:74–78. doi: 10.1172/JCI1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Liu S, Meyers MS, Waget A, Ferron M, Karsenty G, Burcelin R, Mauvais-Jarvis F. Developmental androgen excess disrupts reproduction and energy homeostasis in adult male mice. J Endocrinol. 2013a;219:259–268. doi: 10.1530/JOE-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, Karsenty G, Burcelin R, Mauvais-Jarvis F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab. 2013b doi: 10.1152/ajpendo.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, Karsenty G, Burcelin R, Mauvais-Jarvis F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab. 2013c;304:E1321–1330. doi: 10.1152/ajpendo.00620.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Munzberg H, Mauvais-Jarvis F. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011a;152:1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Munzberg H, Mauvais-Jarvis F. Early-Life Exposure to Testosterone Programs the Hypothalamic Melanocortin System. Endocrinology. 2011b doi: 10.1210/en.2010-1288. NOT-OD-15-102 Consideration of Sex as a Biological Variable in NIH-funded Research - ( http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html) [DOI] [PMC free article] [PubMed]
- Ouyang P, Wenger NK, Taylor D, Rich-Edwards JW, Steiner M, Shaw LJ, Berga SL, Miller VM, Merz NB. Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. doi: 10.1186/s13293-016-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Burns-Cusato M, Dominguez-Salazar E, Riggan A, Shetty S, Arnold AP, Rissman EF. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav. 2008;7:609–617. doi: 10.1111/j.1601-183X.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Jeong SY, Kim HJ. Y chromosome loss and other genomic alterations in hepatocellular carcinoma cell lines analyzed by CGH and CGH array. Cancer Genet Cytogenet. 2006;166:56–64. doi: 10.1016/j.cancergencyto.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Pergament E, Fiddler M, Cho N, Johnson D, Holmgren WJ. Sexual differentiation and preimplantation cell growth. Hum Reprod. 1994;9:1730–1732. doi: 10.1093/oxfordjournals.humrep.a138783. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C. Sexual differences in the hypophyses and their determination by the gonads. Am J Anat. 1936;58:195. [Google Scholar]
- Plum L, Kluge R, Giesen K, Altmuller J, Ortlepp JR, Joost HG. Type 2 diabetes-like hyperglycemia in a backcross model of NZO and SJL mice: characterization of a susceptibility locus on chromosome 4 and its relation with obesity. Diabetes. 2000;49:1590–1596. doi: 10.2337/diabetes.49.9.1590. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Puah JA, Bailey CJ. Insulinotropic effect of ovarian steroid hormones in streptozotocin diabetic female mice. Horm Metab Res. 1985;17:216–218. doi: 10.1055/s-2007-1013496. [DOI] [PubMed] [Google Scholar]
- Ray PF, Conaghan J, Winston RM, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J Reprod Fertil. 1995;104:165–171. doi: 10.1530/jrf.0.1040165. [DOI] [PubMed] [Google Scholar]
- Reue K. Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SS, Reiches M, Shattuck-Heidorn H, LaBonte ML, Consoli T. Opinion: Focus on preclinical sex differences will not address women’s and men’s health disparities. Proc Natl Acad Sci U S A. 2015;112:13419–13420. doi: 10.1073/pnas.1516958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz SA, Antle DM, Cote J, Deroy K, Fraleigh N, Messing K, Parent L, St-Pierre J, Vaillancourt C, Mergler D. First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J. 2014;28:4–13. doi: 10.1096/fj.13-233395. [DOI] [PubMed] [Google Scholar]
- Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, Welshons WV, vom Saal FS. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome“ and obesity in CD-1 mice. Environ Health Perspect. 2008;116:322–328. doi: 10.1289/ehp.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, He Y, Yang Y, Zhu L, Wang C, Xu P, Hinton AO, Yan X, Zhao J, Fukuda M, Tong Q, Clegg DJ, Xu Y. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci Rep. 2016;6:23459. doi: 10.1038/srep23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol. 2014;306:C3–18. doi: 10.1152/ajpcell.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab. 2007;293:E316–326. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Virgin HW. Accounting for reciprocal host-microbiome interactions in experimental science. Nature. 2016;534:191–199. doi: 10.1038/nature18285. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C, Schwarz JM, Clayton JA, de Vries GJ, Sullivan C. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol Sex Differ. 2016;7:13. doi: 10.1186/s13293-016-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Kissling GE, Locklear J, Caviness GF, Whiteside T, Belcher SM, Brown NM, Collins BJ, Lih FB, Tomer KB, Padilla-Banks E, Camacho L, Adsit FG, Grant M. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. J Am Assoc Lab Anim Sci. 2013;52:130–141. [PMC free article] [PubMed] [Google Scholar]
- Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Occurrence of anoestrus in mice caged in groups. J Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- Wiseman T, Pardue M. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-{alpha} stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Yadav BR, King WA, Betteridge KJ. Sex-related differences in developmental rates of bovine embryos produced and cultured in vitro. Mol Reprod Dev. 1992;31:249–252. doi: 10.1002/mrd.1080310404. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]