Abstract
Pain is a common complication of herpes zoster (HZ) infection which results from reactivation of a latent varicella zoster virus (VZV). A third of HZ patients’ progress to a chronic pain state known as post herpetic neuralgia (PHN), and about a quarter of these patients’ have orofacial pain. The mechanisms controlling the pain responses are not understood. Studies suggest central pathways involving the thalamus could control pain related to HZ, and studies in our lab suggest vesicular GABA transporter (VGAT) in the lateral thalamus influences orofacial pain. We hypothesized that thalamic VGAT functions, in part, to reduce pain, particularly orofacial pain, associated with VZV. To address this hypothesis VZV was injected into the whisker pad. Affective and motivational aspects of pain were measured using the Place Escape/Avoidance Paradigm. Thalamic neuronal activity was modulated after injecting an adeno-associated virus (AAV) expressing an engineered acetylcholine Gi-protein coupled receptor. This receptor inhibits neuronal firing when bound by clozapine-n-oxide (CNO). VGAT expression was attenuated in the thalamus by injecting an AAV construct that expressed a VGAT silencing shRNA. VZV induced nociception was significantly decreased after administering CNO in male rats. Nociception significantly increased concomitant with increased thalamic c-fos expression after attenuating thalamic VGAT expression. These data establish that the lateral thalamus (posterior, ventral posteromedial, ventral posterolateral and/or reticular thalamic nucleus) controls VZV induced nociception in the orofacial region, and that GABA in this region appears to reduce the response to VZV induced nociception possibly by gating facial pain input.
Keywords: VGAT, GABA, pain, shingles, face, orofacial, thalamus
Introduction
Infection with the Herpesvirus Varicella zoster virus (VZV) causes chickenpox, a self-limiting childhood disease that is followed by a latent state. Reactivation of VZV results in the clinically distinct disease herpes zoster (HZ), commonly termed “shingles” (Johnson et al., 2010). The trigeminal ganglia has the highest viral load (Mahalingam et al., 1990) and cadaver studies suggest about 80% of the population harbors VZV in the trigeminal ganglia (Pevenstein et al., 1999). Moreover, a significant fraction of HZ cases (up to 56%) have facial involvement (Ragozzino et al., 1982; Pavan-Langston, 1995). A form of HZ known as herpes zoster ophthalmicus (HZO) may develop in ocular tissues resulting in ocular pain and loss of visual acuity; HZO occurs in about 50,000 individuals annually (Pavan-Langston, 2000). Zoster-associated pain occurs during the acute phase, while chronic pain lasting longer than 90 days develops in about 20–30% of HZ sufferers (Mitchell et al., 2003; Dubinsky et al., 2004; Colon, 2009; Fashner and Bell, 2011; Kinchington and Goins, 2011). This chronic condition is termed post-herpetic neuralgia (PHN). In approximately 30% of PHN patients, pain can last for more than one year (Kawai et al., 2014). HZ incidence increases with age, with most HZ sufferers being 60 years of age or older. Given that the population over 60 years of age is predicted to double in the next 50 years (Kawai et al., 2014), HZ and the associated pain are increasingly important problems in the future. Although vaccines for both varicella and HZ are available, the HZ vaccine prevents only about half of HZ and reduces “clinical illness” (including PHN) by about two-thirds (Harpaz et al., 2008). Thus, even in a fully vaccinated population (which is far from being achieved) zoster associated pain, HZ and PHN will remain common.
A rat model of PHN was first detailed by Fleetwood Walker’s group, where footpad inoculation of VZV induced long term hyperalgesia and allodynia mirroring the chronic allodynia seen in PHN patients (Fleetwood-Walker et al., 1999; Dalziel et al., 2004). In this model the rat exhibits pain behaviors after a primary infection rather than reactivation, currently no animal model of reactivation is known. The response profile of VZV induced pain was similar to that seen in humans; for example, antivirals, opioids or NSAIDS were not highly effective treatments (Garry et al., 2005; Hasnie et al., 2007). Even with current treatment there remains a need for more effective therapy, since up to half of PHN patients gain little to no relief from any current therapy (Beal et al., 2012; Derry et al., 2013). Mechanisms for VZV induced pain are not yet resolved, but VZV may not need to replicate completely to induce a response (Surman et al., 1990; Hempenstall et al., 2005; Guedon et al., 2015). VZV gene expression is required for pain (Guedon et al 2014) and the VZV transcriptional regulator IE62 has been postulated to alter host gene expression programs leading to pain induction (Kinchington and Goins, 2011).
The thalamus has a role in orofacial pain responses (Bezdudnaya and Keller, 2008). A facial HZ patient with PHN was found to have reduced thalamic activity on a PET scan (Iadarola et al., 1995). In humans, MRI studies have shown that reduced GABA activity in the thalamus was associated with pain involving the trigeminal pathway (Henderson et al., 2013). Lesion of the lateral thalamic region, including the reticular thalamic nucleus, can heighten pain responses (Saade et al., 1999). Interestingly, all neurons in the reticular thalamic region express GABA with only a miniscule population of GABA positive cells in the adjacent ventroposterior nuclei (Barbaresi et al., 1986). Neurons in the ventroposterior thalamic nucleus send collaterals to the adjacent reticular thalamic nucleus and GABA positive neurons in the reticular thalamic nucleus send axons back to the ventroposterior thalamic nucleus “gating” pain signals passing through the thalamus to the cortex (Vahle-Hinz et al., 1994; Lam and Sherman, 2011). The trigeminal nuclei project to the posterior thalamic nucleus and GABA neurons in the zona incerta affect thalamic activity (Masri et al., 2009; Chang et al., 2012). To extend these studies our lab has shown that vesicular GABA transporter (VGAT) expression was elevated in the lateral thalamic region, including the reticular thalamic nucleus, when the nociceptive response was decreased (Umorin et al., 2016). Although it has been hypothesized that central pain pathways have a role in HZ pain the genes and pathways involved have not been established. In this study we hypothesized that VGAT expression in the lateral thalamic region will affect HZ associated pain in the orofacial region of the rat. Most VGAT is expressed in the reticular thalamic nucleus of the lateral thalamic region (Lein et al., 2007). Accordingly, we evaluated nociception by measuring an escape and/or avoidance behavior after injecting the whisker pad with VZV and determined the response after modulating lateral thalamic neuronal activity and VGAT expression.
Experimental Procedures
This study was approved by the Baylor College of Dentistry Institutional Animal Care and Use Committee. Male (300–350 grams) Sprague-Dawley rats from Envigo (Indianapolis, IN) were kept on a 14:10 light/dark cycle. The rats were given food and water ad libitum. After a 4 day acclimation period experiments were carried out in accordance with the NIH regulations on animal use. Two experiments using two different batches of rats were completed, in Experiment #1 twenty-two male rats were injected with 100 μl of a high concentration of VZV, the parent Oka strain (pOka), at >1000 plaque forming units (pfu)/μl (provided by Dr. Kinchington). In Experiment #2 thirty-four male rats were injected with a lower concentration of VZV from a different preparation (650 pfu/μl).
Surgery
Adult male Sprague-Dawley rats were anesthetized with 2% isoflurane and an air flow of 2 liter per minute. Aseptic stereotaxic (David Kopf Instruments, Tujunga, CA, Model 1460–61) injection of virus was performed with the needle tip (Hamilton #7002KH Neuros syringe, Reno, NV) at coordinates 3.6 mm posterior of Bregma, 3.0 mm from midline at a depth of 6.0 mm. A Stoelting stereotaxic syringe pump system was used to infuse 0.250 μl of 2–8 ×1012 pfu/ml AAV8 or 1 ×1013 pfu/ml AAV5 or AAV9 at a rate of 20 nanoliters per minute. In the vehicle (no virus) group 0.250 μl of vehicle (350 nM NaCl, 5% D-Sorbitol in PBS) was infused. After infusion the needle was left in place for 5 minutes and then removed. In Experiment #1 twenty-two male rats were infused bilaterally with AAV8 expressing a neuronal silencing construct Syn-hM4D(Gi)-mcherry (Gene Therapy Center Vector Core, University of North Carolina at Chapel Hill) or vehicle. Expression of the receptor was driven by the neuronal synapsin-1 promoter (Syn), which drove expression in most neurons. The hM4D(Gi) gene was an engineered acetylcholine Gi-protein coupled receptor that inhibits neuronal firing when bound by clozapine-n-oxide (CNO) (Rogan and Roth, 2011). Upon CNO binding the receptor stimulates calcium release, ERK1/2 activation, inhibits forskolin-induced cAMP formation and potentially GIRK activation, thereby causing hyperpolarization and inhibition of basal action potential firing (Rogan and Roth, 2011). To affect change in neuronal activity, the modified acetylcholine Gi protein-coupled receptor was expressed primarily in the posterior, ventral posteromedial and ventral posterolateral thalamic nuclei of Sprague-Dawley rats (Fig. 1A), and activated by binding CNO (Alexander et al., 2009). In Experiment #2 the right thalamus of thirty-four male rats was injected with AAV9 containing either a verified VGAT shRNA construct (5′-CACCGCATCATCGTGTTCAGCTACACTCGAGTGTAGCTGAACACGATGATGCTTTTT-3′) driven by the U6 promoter with a mCherry tag or AAV5 containing a scrambled shRNA construct (5′-AGGATCCAGTACTGCTTACGATACGGTTCAAGAGACCGTATCGTAAGCAGTACTTTTTTT-3′) driven by the U6 promoter containing a GFP tag (Vector Biolabs, Philadelphia, PA). Sites of injection were verified by identification of cells positive for the immunofluorescent tag. Animals were given a 5 mg/kg dose of nalbuphine I.M. (Hospira, Lake Forest, IL) after surgery and allowed to recover 7 days.
Figure 1. Lateral thalamic neurons modulate orofacial VZV induced nociception in male Sprague Dawley rats (Experiment #1).
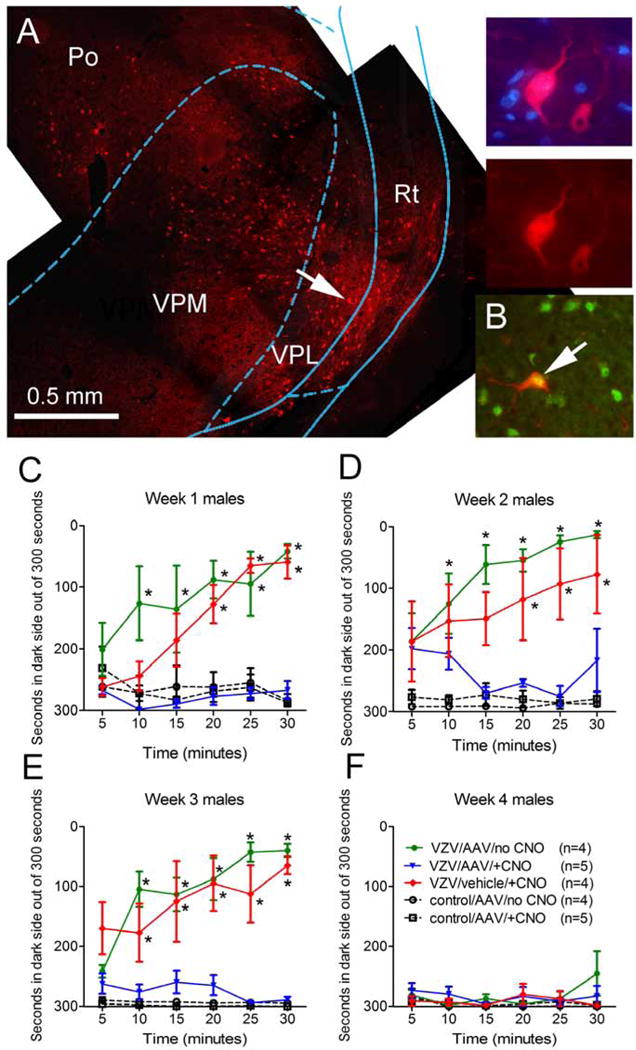
Panel A is a representative fluorescent image of the lateral thalamic region after injection of AAV8 containing an Syn-hM4D(Gi) construct which co-expresses mcherry (red). The right three panels are a magnified image of the cell indicated by an arrow in panel A. Bar equals 500 μm. Po= posterior thalamic nuclei, VPM= ventral posteromedial thalamic nuclei, VPL= ventral posterolateral thalamic nuclei and Rt= reticular thalamic nuclei. In panel B an arrow points to a NeuN positive cell (green) infected with AAV8 construct Syn-hM4D(Gi) mcherry (red) within the thalamic region. In panels C–F, nociception was measured in each rat using the Place Escape/Avoidance Paradigm (PEAP). Two weeks before nociception was measured the thalamus was injected with AAV8-Syn-hM4D(Gi) or vehicle bilaterally. PEAP was completed one (C) two (D) three (E) or four weeks (F) after injecting the left whisker pad with VZV (pOka strain) (>1000 pfu/μl) or equivalent uninfected control MeWo cells. Thirty minutes before PEAP testing the rats were intraperitoneally injected with a 1 mg/kg dose of clozapine-n-oxide (CNO) or 0.5 ml of saline. ANOVA and Bonferroni post-hoc testing was performed and an asterisk indicates a significant difference (p<0.05) between the VZV/AAV/no CNO and the VZV/vehicle/+CNO group versus the control and VZV/AAV/+CNO groups. The predetermined number of animals in each group is shown in parenthesis in panel B.
Place Escape/Avoidance Paradigm testing in male rats
One week after thalamic injections were complete the rats were anesthetized briefly with 2% isoflurane using a 2 liter per minute flow of air and the left whisker pad was injected with 100 μl of MeWo cells (human melanoma fibroblast cell line) infected with VZV (n=20) or control MeWo cells lacking virus (n=14) (Guedon et al., 2014). The rats were ambulatory after 5 minutes following injection. Seven days following whisker pad injection rats were evaluated for nociception by individuals blinded to the groups using the Place Escape/Avoidance Paradigm (PEAP) test as detailed by the Fuchs lab (LaBuda and Fuchs, 2000). Rats were placed in a 30 cm × 30 cm × 30 cm acrylic box where half the box was covered in black cloth. The PEAP test is based on the assumption that an aversive stimulus results in an escape and/or avoidance behavior (LaBuda and Fuchs, 2000). Rodents, being nocturnal, prefer the dark side of the chamber. Animals in the chamber were then poked with a 60 gram Von Frey filament every 15 seconds, applying stimulus to the injected side when rats are on the dark side of the chamber, and to the un-injected side when on the light side of the chamber. Stimuli were applied to the region below the eye and caudal to the whisker pad, a region innervated by the second branch of the trigeminal ganglia (Leiser and Moxon, 2006). The time spent on the dark side of the box was recorded in 5 minute bins during the total testing time of 30 minutes. The 30 minute test was performed weekly. In Experiments #1 the rats received an intraperitoneal injection of 1 mg/kg CNO dissolved in 0.9% saline or an injection of 0.9% saline in a 0.5 ml volume 30 minutes before testing. Testing was completed for 4 weeks in Experiment #1 and for two weeks in experiment number #2.
Tissue collection
In Experiment #2, rats were sacrificed three weeks after VZV injection by exposure to CO2 for 90 seconds followed by decapitation. The brain was extracted within five minutes of decapitation using a rongeur, placed on dry ice and cut on a Zivic brain slicer (Zivic, Pittsburgh, PA). Sections were cut 2 mm thick and the sections between Bregma −3 to −5 were placed on glass slides and kept on dry ice. Lateral thalamic tissue was collected with punches 2 mm in diameter centered around the injection site, punches included the posterior thalamic nucleus, the ventral posteromedial and ventral posterolateral thalamic nuclei and a portion of the reticular thalamic nucleus. Tissue was stored in liquid nitrogen until RNA or protein extraction. Three of the 17 MeWo samples were lost in this isolation procedure.
Real time PCR
Fourteen samples from the 20 VZV injected rats and 8 samples from the 17 MeWo injected rats in Experiment #2 were randomly selected for RNA analysis. In Experiment #2 RNA extraction was performed using the RNA Lipid Tissue Kit from Qiagen (Valencia, CA) and RNA concentration was determined on a Nanodrop2000. A one-step reverse transcription PCR reaction was performed on BioRAD C1000 Thermal Cycler using the SYBR-Green 1-Step RT-PCR kit and primers from Qiagen (VGAT primer catalog # QT00378413, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primer catalog # QT00199633). The thermal protocol was 30 min @ 50 °C for the reverse transcription reaction, 15 min @ 95 °C for DNA pol activation and 40× (15 s @ 94 °C melting, 30 s @ 56 °C annealing, 30 s @ 72 °C extension). A melting curve was obtained thereafter for quality assurance. Sample amount was adjusted according to total RNA concentration to obtain 20 ng of total RNA per well in the final reaction mix. All reactions were run in triplicate. PCR runs that did not exhibit a proper amplification profile were discarded. For each sample, the threshold Ct value for GAPDH was subtracted from the Ct of value for VGAT to give a ΔCt. The mean ΔCt from the right thalamus was subtracted from the left thalamus to give a ΔΔCt. To get the fold change the −ΔΔCt was raised to the second power (2−ΔΔCt). Values for decreased expression were calculated by using the formula (−1/fold change).
Immunofluorescent Staining
Six samples from the 20 VZV injected rats and 6 samples from the 17 MeWo injected rats from Experiment #2 were randomly selected for immunofluorescent staining. After anesthetization with 100 mg/kg ketamine and 10 mg/kg xylazine the animals were first perfused with 9% sucrose and then second with 4% paraformaldehyde in 1× PBS, pH 7.4. Fixed tissues were stored in 25% sucrose, frozen, cryo-sectioned and the 20 μm sections placed on Histobond slides (VWR international, Radnor, PA). The tissue was then blocked with a PBS solution containing 5% normal goat serum and 0.3% Triton-X 100 for 2 hours at room temperature. The slides were incubated in primary antibody overnight at 4°C. Primary antibodies consisted of the mouse monoclonal NeuN antibody at a 1:250 dilution (Millipore, Billerica, MA), 1:200 dilution of the rabbit polyclonal c-fos antibody (Cell Signaling, 96F), 1:250 dilution of the rabbit polyclonal GFAP antibody (Millipore, GA5) or the rabbit polyclonal VGAT antibody (AB5062P, Millipore) at a 1:200 dilution. Primary antibodies were diluted in PBS containing 5% BSA and 0.3% Triton X-100. After rinsing three times in PBS with 0.3% Triton X-100 for a total of 45 min, slides were placed for 2 hours in secondary antibody. Secondary antibodies (1:500 dilution) included goat anti-mouse 488, goat anti-mouse 568, goat anti-rabbit 488 or goat anti-rabbit 568 (Invitrogen, Carlsbad, CA). After rinsing the slides three times in PBS for a total of 45 min, the slides were mounted with Fluoromount-G mounting medium containing Hoechst 33342 stain (Electron Microscopy Sciences, Hatfield, PA). The fluorescent signal was imaged using a Nikon fluorescent microscope and NIS-Elements imaging software and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA).
Cell counting
Representative cell counts were completed on 20 μm coronal sections. Every other section was selected for staining. Typically three sections were counted for each animal. The injection site was the center point from which sections were selected. On each section two randomly selected fields near the tip of the injection site was counted. The background was subtracted from the image and a fluorescent signal associated with a cell nucleus was counted as a positive cell. The number of AAV transduced cells was counted within a 0.2 mm2 field adjacent to the injection site. The number of c-fos positive cells was counted within a 0.5 mm2 field. Counts were completed within the ventral thalamic nuclei including the posterior, ventral posteromedial, ventral posterolateral and reticular thalamic nuclei. Cell counts from each section were then averaged for each animal. Values were given as a mean and SEM representing an average of the three values for the three animals for each treatment group.
Statistics
PEAP data was analyzed by two-way ANOVA with repeated measures (multiple time bins; 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25 minutes, 30 minutes) the independent variables were virus (VZV, AAV, control) and drug (no CNO, CNO) and the dependent variable was the PEAP values. PCR data was analyzed by two-way ANOVA and the independent variables were virus and drug (VZV and shRNA) and the dependent variable was the fold change in gene expression. Statistical and Bonferroni post-hoc tests were completed on Prism 5.04, GraphPad Software, La Jolla, CA. The cell count data was analyzed by a Mann-Whitney test. Each animal was treated as a single data point. A value two standard deviations from the mean of that treatment group was not included in the analysis. Data analyzed by ANOVA had normal distributions and equal variances. Significance was indicated when p<0.05. All values are given as the mean and SEM.
Results
Activation of Gi construct reduced the nociceptive response
Thalamic AAV infection of the Syn-hM4D(Gi)-mcherry construct resulted in cells expressing the fluorescent virus tag. These fluorescent cells were located in the posterior, ventral posteromedial, ventral posterolateral and reticular thalamic nuclei (Fig. 1A). Many of the cells infected with virus were neurons as indicated by the staining of axons (Fig. 1A, arrow, high magnification images) and co-localization with the neuronal marker NeuN (Fig. 1B). No co-localization was observed between virus infected cells and the glial marker GFAP (data not shown).
In rats injected with VZV a significant nociceptive response was observed over three weeks (Fig. 1C–E) as compared to control (i.e., MeWo cells). The main effect was significant in weeks one F(4, 90)=17.0, P<0.0001, two F(4, 90)=14.6, P<0.0001 and three F(4, 90)=36.9, P<0.0001 but not in week four F(4, 90)=1.17, P=0.38 (Fig. 1F). However, activation of the neuronal inhibitor hM4D(Gi) by CNO administration significantly reduced the nociceptive response after VZV injection as compared to control rats in weeks one (Fig. 1C), two (Fig. 1D) and three (Fig. 1E) but not in week four (Fig. 1F). A significant interaction between virus (VZV) and drug (CNO) was observed in weeks one F(4, 90)=4.1, P<0.0001, two F(4, 90)=3.2, P<0.0001 and three F(4, 90)=2.6, P=0.001 but not in week four F(4, 90)=1.14, P=0.32.
Thalamic neurons were transduced with VGAT shRNA
Thalamic neurons were transduced with shRNA after injecting the ventral posteromedial thalamic nucleus with AAV. Following AAV injection into the thalamus the whisker pad of these rats were injected with either VZV or control (i.e., MeWo cells). The shRNA construct was observed in cells of the posterior nucleus, ventral posteromedial, ventral posterolateral and reticular thalamic nuclei (Fig. 2A, B). Cells were positive for the neuronal marker NeuN and expressed the fluorescent tag for shRNA production (Fig. 2C–F). Cell counts of these fluorescent cells were then completed. In rats given a VZV injection the number of NeuN positive cells in a 0.2 mm2 field was 40 ± 6 and of these cells 12 ± 3 co-localized with the VGAT shRNA marker. In rats injected with control 44 ± 2 cells were NeuN positive and 14 ± 2 cells co-localized with the VGAT shRNA marker. For the control shRNA construct 15 ± 3 cells were positive out of the 34 ± 5 NeuN positive cells in VZV injected rats and in control rats 11 ± 2 cells were positive for the control shRNA out of the 32 ± 6 NeuN positive cells. VZV injection had no significant effect on the number of cells infected with the VGAT or control shRNA construct (n=3). Of note, the VGAT shRNA construct co-localized to VGAT expressing cells in the reticular thalamic nucleus (Fig. 3A–C, arrows).
Figure 2. Neurons in the lateral thalamic region were transduced with shRNA expression vectors (Experiment #2).
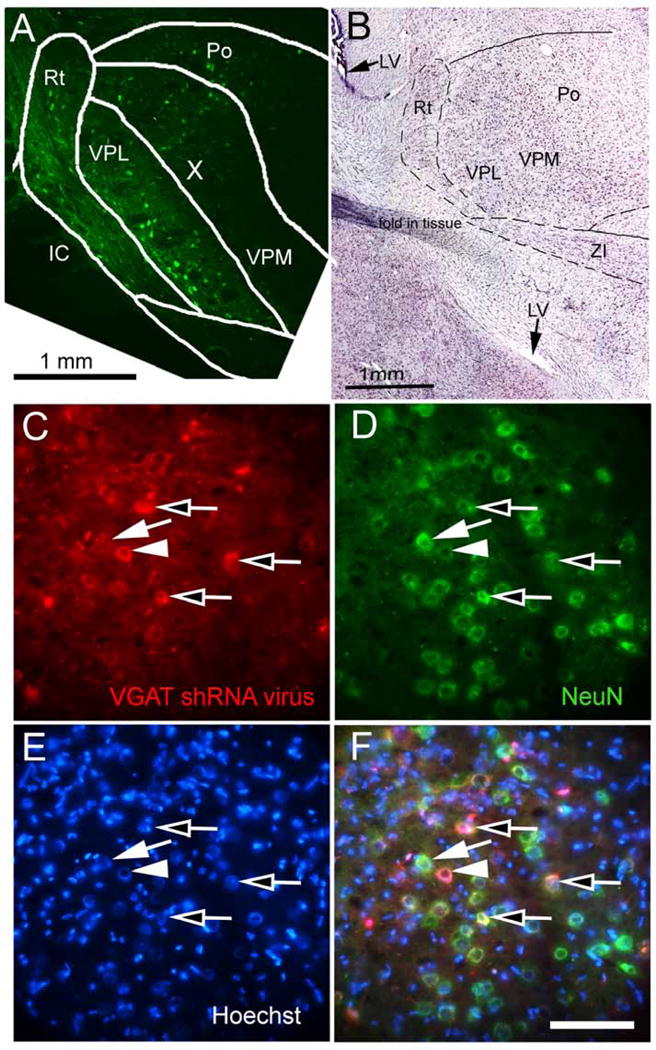
Representative low magnification image of the lateral thalamic region from a male rat three weeks after injecting AAV5 containing a scrambled shRNA-GFP construct (green) (Panel A). Panel B is a composite image of an adjacent Nissl stained section. Po= posterior thalamic nuclei, VPM= c, VPL= ventral posterolateral thalamic nuclei, ZI= zona incerta, IC= internal capsule and Rt= reticular thalamic nuclei, LV= lateral ventricle (arrow). In panels C-F the images were taken near the border of the ventroposterior nucleus and the reticular thalamic nucleus three weeks after injecting AAV9 containing the VGAT shRNA construct. This particular animal received a VZV injection in the whisker pad contralateral to the thalamic infusion. In panel C the image shows AAV9 transduced cells in red that are indicated by an arrow head and open arrows. A cell staining for only AAV9 is shown in panel C and is indicated by an arrow head (note: no corresponding green signal in panel D). The cell indicated by an arrow in panel C does not express the AAV9 marker mCherry. In panel D NeuN positive neurons are shown in green and are indicated by an arrow and open arrows. A cell staining for only NeuN is shown in panel D and is indicated by an arrow (note: no corresponding red signal in panel C). A cell not staining for NeuN is indicated by an arrowhead in panel D. Hoechst stain is shown in blue, panel E. AAV9 transduced neurons co-localize with the neuronal NeuN (panel F, yellow, open arrows). Bar equals 50 μm.
Figure 3. VGAT positive cells in the reticular thalamic nuclei were transduced with the shRNA constructs (Experiment #2).
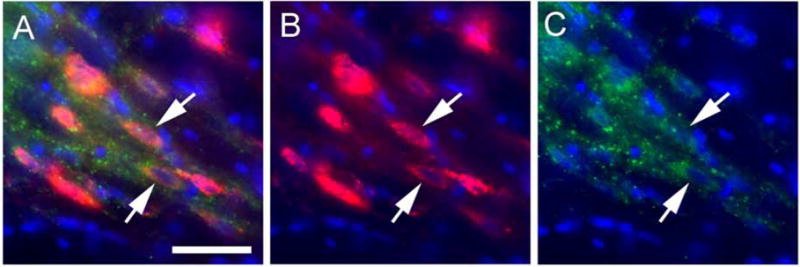
Panels A and B show a high magnification image of AAV9 transduced cells in the reticular thalamic nucleus three weeks after injecting AAV9 containing the VGAT shRNA-mCherry construct (red). Panels A and C show immunofluorescent staining for VGAT (green). AAV9 positive cells co-localized with VGAT in panel A (arrows). Blue is nuclear Hoechst stain. Bar equals 20 μm.
Attenuation of VGAT expression with VGAT shRNA altered the VZV induced nociceptive response
A significant >4 fold reduction in VGAT expression F(1, 17)=21.9, P=0.0002 was observed in the thalamus after infection of VGAT shRNA (Fig. 4A). VZV injection did not significantly affect VGAT expression and no interaction between VZV injection and shRNA treatment was detected F(1, 17)=0.01, P=0.9. After reducing VGAT expression (i.e., VGAT shRNA treatment) there was an increase in the VZV nociceptive response versus rats that received control shRNA in weeks one F(3, 150)=5.9, P=0.002 and two F(3, 150)=15.8, P<0.0001 (Fig. 4B, C) strongly supporting the conclusion that VZV induced pain involves thalamic VGAT. A significant interaction between treatment and time was observed in weeks one F(3, 150)=7.1, P<0.001 and two F(3, 30)=11.7, P<0.0001.
Figure 4. Transduction of the VGAT shRNA construct reduced VGAT transcript and increased the VZV induced nociceptive response (Experiment #2).
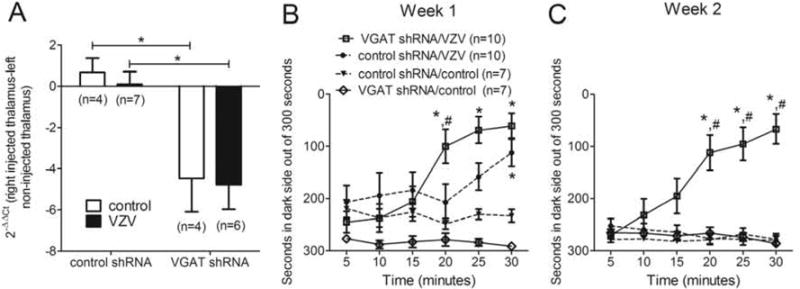
In panel A the expression of VGAT significantly decreased three weeks after treatment with VGAT shRNA. On the graph the ΔCt from the right thalamus (contralateral to VZV injection) was subtracted from the left thalamus (ipsilateral to VZV injection) to give a ΔΔCt. To get the fold change the −ΔΔCt was raised to the second power (2−ΔΔCt). In this experiment RT-PCR for VGAT expression was completed on thalamic punches after transducing a VGAT shRNA or a control shRNA construct. The whisker pads of these animals were also injected with either VZV or control two weeks before quantitation of VGAT transcript. An asterisk indicates a significant difference of p<0.05. In panels B and C male rats were injected with AAV containing the scrambled shRNA or VGAT shRNA construct and tested using PEAP after whisker pad injection of VZV (650 pfu/μl). The PEAP test was performed once a week for two weeks starting 7 days after VZV or control (i.e., MeWo cell) injection. Values are the average time, in seconds, that each group of animals spent on the dark side of the testing chamber out of a 5 minute bin (i.e., 300 seconds). Panel B shows the data for week one and panel C shows the data for week two. The hash sign indicates a significant difference (p<0.05) between the control shRNA/VZV and the VGAT shRNA/VZV group. The asterisk indicates a significant difference between the VZV and control treated groups, that is, the control shRNA/control versus control shRNA/VZV and VGAT shRNA/control versus VGAT shRNA/VZV group. The predetermined number of animals in each group is shown in parenthesis.
The males in Experiment #2 were injected with (100 μl volume × 650 pfu/μl)=65,000 pfu/whisker pad and responded for one week (Fig. 4B) and the male rats in Experiment #1 responded for three weeks (Fig. 1B–D) but were injected with over 100,000 pfu/whisker pad. The higher dose of VZV induced a longer response consistent with the idea that there was a dose response.
A correlate of cellular activity was measured in the thalamus by counting c-fos positive cells in the ventral posterior nuclei after treatment with control shRNA (Fig. 5A–C) and VGAT shRNA (Fig. 5D–F). VZV injection increased the number of c-fos positive cells P<0.05 (n=3) (Fig. 5G). In rats treated with VGAT shRNA the number of c-fos positive cells significantly increased in the ventral posteromedial nuclei P<0.05 (n=3) (Fig. 5G).
Figure 5. Thalamic c-fos expression increased after administration of VGAT shRNA (Experiment #2).
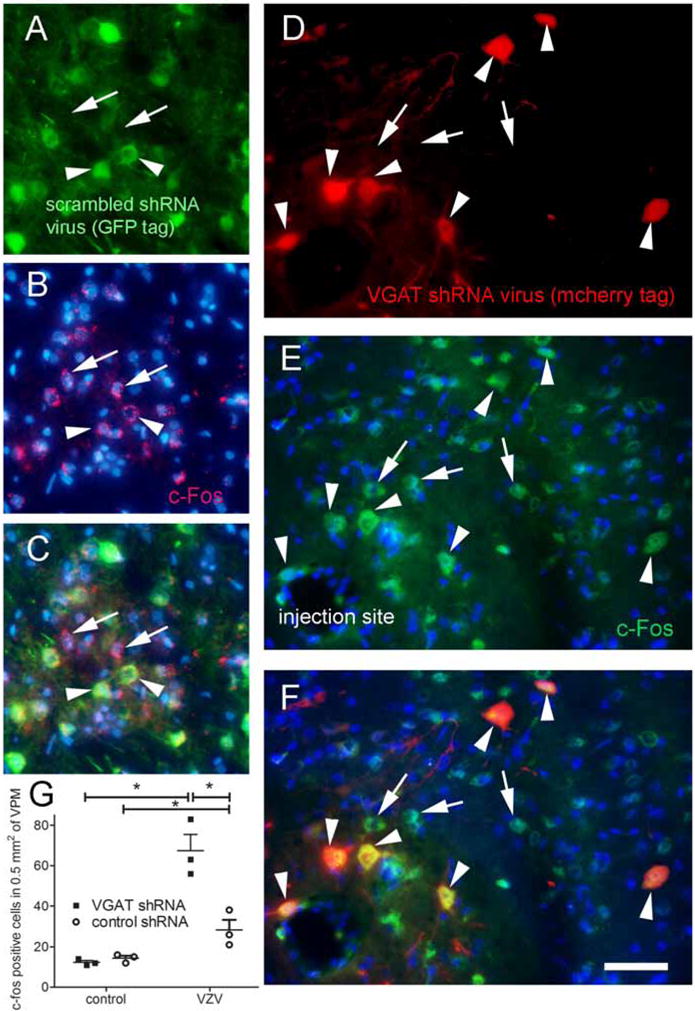
In these experiments AAV was injected, then VZV was injected one week after AAV and then one week after VZV injection the nociceptive measurements were started. Nociceptive measurements were carried out for two weeks after VZV injection. Animals were sacrificed immediately following the tests in week two for c-fos quantitation. Panel A shows cells in the ventral posteromedial thalamic nucleus three weeks after being transduced with the AAV5 virus containing the control shRNA construct expressing GFP (arrowheads, green). Panel D shows cells in the thalamus three weeks after being transduced with AAV9 containing the VGAT shRNA construct expressing mCherry (arrowheads, red). Panels B and E shows c-fos positive cells (arrows and arrowheads, red). In panels C and F c-fos positive cells co-localize with shRNA expressing cells (yellow, arrowheads). In this rat VZV was injected into the contralateral whisker pad. Blue is nuclear Hoechst stain. Bar equals 50 μm. Panel G is a histogram for cell counts of c-fos positive cells in the ventral posteromedial thalamic nucleus after three weeks after infusion of AAV containing shRNA constructs and after injection of VZV or control. An asterisk indicates a significant difference of p<0.05. There were three animals in each treatment group.
Discussion
Pain resulting from VZV infection is common, debilitating, can last for years and be frequently refractory to any treatment (Kawai et al., 2014). The central mechanisms for chronic pain associated with VZV are unknown. In this study we looked at pain related to orofacial VZV infection. Studies from our lab suggested thalamic expression of VGAT modulates pain in the orofacial region (Umorin et al., 2016) but it was unclear if VGAT would alter orofacial nociception or if VGAT could affect VZV induced nociception. To address this question neurons were transduced with constructs to reduce neuronal activity or VGAT expression. The result of VGAT knock-down increasing activity was in contrast to Gi stimulation decreasing activity. In addition, activation of the engineered Gi coupled receptor reduced the behavioral response to VZV infection in the whisker pad. Because this engineered receptor has been shown to reduce neuronal activity (Armbruster et al., 2007) these results suggest inhibition of neuronal activity in the lateral thalamus reduced VZV induced nociception. Alternatively, Gi stimulation attenuated an inhibitory neuronal pathway(s), increasing the behavioral response when testing the non-infected site.
Most VGAT in the lateral thalamic region is expressed in the reticular thalamic nucleus (Lein et al., 2007). Because GABA positive neurons in the reticular thalamic region send collaterals to the ventroposterior thalamic nucleus (Barbaresi et al., 1986; Lam and Sherman, 2011) it is possible that inhibition of VGAT in GABA positive neurons of the reticular thalamic region reduced inhibitory signaling to the ventroposterior thalamic nucleus. This attenuation of the inhibitory pathway impinging upon the excitatory neurons within the ventroposterior thalamic nucleus (Storm-Mathisen et al., 1983) would result in increased cellular activity (c-fos staining) and an increase in the behavioral response, as was observed in this study. Together the results suggest that the lateral thalamus functions in controlling VZV pain in the face and that GABA in the thalamus inhibits the VZV pain response.
The excitatory glutamatergic cells are present in the posteromedial/posterolateral thalamic nuclei (VGLUT2 positive) while most of the inhibitory neurons (VGAT positive) are in the reticular thalamic nuclei (Lein et al., 2007). Injection of the virus into the ventral posteromedial thalamic nuclei resulted in transduction of many cells in the ventral posteromedial/posterolateral thalamic nuclei and fewer cells in the reticular thalamic nuclei. It is possible that the disproportionate transduction of excitatory neurons resulted in greater attenuation of the excitatory neurons versus inhibitory neurons resulting in a reduced behavioral response. VGAT miRNA was also injected into the posteromedial region but only affected VGAT expressing cells in the reticular nucleus thus, resulting in attenuated of only inhibitory cells that then increased the behavioral response.
Knock-down of VGAT expression would attenuate GABAergic inhibition. Reduction of GABAergic inhibition would be expected to result in an increased nociceptive response after VZV injection, consistent with results from Experiment #2. Of note is the fact that VZV did not increase the behavioral response in week two in Experiment #2, although VZV and VGAT shRNA treatment together increased the behavioral response. We explain this phenomenon as VZV treatment sensitizing the pain pathway but at a level below the threshold detectable by the PEAP assay. But in the group treated with VGAT shRNA concomitant with VZV injection the nociceptive response increased to levels detectable by our assay. This effect of VGAT shRNA treatment would potentially be due to inhibition of GABA signaling. Note the reduction in VGAT was over 4 fold after infection with VGAT shRNA, the response could have been much greater in specific sub-nuclei but the tissue plug used for analysis was relatively large and included posterior, ventroposterior and reticular thalamic nuclei.
After injecting the whisker pad with VZV the behavioral response decreased over time, consistent with results from paw injection (Fleetwood-Walker et al., 1999). The decreased response is likely to result from reduced inflammation and/or viral silencing. In support of this idea VZV results in inflammation (Guedon et al., 2015) and this inflammation has been shown to decrease over time after an initial herpes virus infection (St Leger and Hendricks, 2011). Also, VZV genes have been hypothesized to alter neuronal function and result in pain (Kinchington and Goins, 2011). Over time VZV becomes silent though a mechanism that is still unclear (Kennedy et al., 2015). Silencing of the virus in neurons would result in reduced VZV gene expression and be hypothesized to lead to a decrease in the nociceptive response as observed in this study.
Importantly, upon sectioning the brain no fluorescent signal was observed in the somatosensory cortex, a region projecting to the thalamus, supporting the idea that virus did not transport from the injection site to effect other brain nuclei resulting in our observed response but caution must be taken as the AAV5 and AAV6 serotype can result in axonal transport (Aschauer et al., 2013; Low et al., 2013; Salegio et al., 2013).
In these studies cells were transduced with serotypes AAV2/5, 2/8 and 2/9. These hybrid serotypes contain the chromosome from AAV2 that is then packaged with proteins making up a capsid shell from either AAV5, AAV8 or AAV9. It is the capsid that results in virus attachment to a cell and dictates transduction specificity. In previous work neurons in the thalamus of the mouse were efficiently transduced with AAV2/8 and AAV2/9 serotypes but few astrocytes or oligodendrocytes were transduced (Cearley and Wolfe, 2006). AAV2/5 efficiently transduced neurons in the brain of rats, but few transduced astrocytes were detected (Burger et al., 2004). Consistent with the previous reports most transduced cells in this study were neurons. Furthermore, the efficiency of transfection was not significantly different between the serotypes but some non-neuronal cells were transduced. Because astrocytes have a role in neuropathic pain (Grace et al., 2014) transduction of glial cells could have contributed to the altered behavioral effects observed after modulating Gi activity.
Inhibiting VGAT expression in the lateral thalamic area increased c-fos staining in the ventral posteromedial nuclei suggesting increased cellular activity in this region. Rats receiving whisker pad injection of VZV also increased the number c-fos positive cells in comparison to the control suggesting that VZV exposure results in increased cell activity. Although mechanical stimulation (injection/infusion) could elevate c-fos expression in these studies we did not include a non-injected group. All animals in these studies received the same mechanical stimulation equalizing the effect of mechanical stimulation on c-fos expression in all groups. These studies also did not study c-fos expression over time and it is likely c-fos expression was greater after the initial VZV injection and diminished over time.
Conclusions
Affective and motivational aspects of pain resulting from VZV infection of the face can be reduced by modulating neuronal activity in the thalamus, which suggests that affective and motivational aspects pain signals from the face are transmitted by the lateral thalamus. This is consistent with studies demonstrating noxious orofacial input from the trigeminal nucleus caudalis (Vc) are conveyed to the ventral posteromedial thalamic nuclei (VPM) (Sessle, 1999). The reduction of thalamic VGAT increased nociception suggesting that GABA signaling in the lateral thalamic region gates or inhibits VZV facial pain; potentially through GABA positive interneurons in the reticular thalamic region (Lam and Sherman, 2011).
Highlights.
VZV injected into the whisker pad induces orofacial pain in rats
Reduced neuronal activity in the thalamus altered orofacial pain
GABA signaling in the thalamus gates or inhibits VZV induced facial pain
Acknowledgments
The authors wish to thank Zaki Razvi, Xiaoju Zou, Priscilla Gillaspie-Hooks, Connie Tillberg and Gerald Hill for their excellent technical assistance. This study was supported by NIDCR grant DE022129 (PRKramer) and a grant from the Virginia Kaufman Endowment Fund administered by the Clinical Science and Translational Institute of Pittsburgh (PRKinchington). PRKinchington also acknowledges support from NINDS NS064022, NEI core grant EY08098 and Research to prevent Blindness Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest with this work.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc of the Natl Acad of Sciences. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res. 1986;382:305–326. doi: 10.1016/0006-8993(86)91340-5. [DOI] [PubMed] [Google Scholar]
- Beal B, Moeller-Bertram T, Schilling JM, Wallace MS. Gabapentin for once-daily treatment of post-herpetic neuralgia: a review. Clin Interv Aging. 2012;7:249–255. doi: 10.2147/CIA.S23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Keller A. Laterodorsal nucleus of the thalamus: A processor of somatosensory inputs. J Comp Neurol. 2008;507:1979–1989. doi: 10.1002/cne.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chang Z, Okamoto K, Bereiter DA. Differential ascending projections of temporomandibular joint-responsive brainstem neurons to periaqueductal gray and posterior thalamus of male and female rats. Neuroscience. 2012;203:230–243. doi: 10.1016/j.neuroscience.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon Y. Patient education and self-advocacy: questions and responses on pain management. J Pain Palliat Care Pharmacother. 2009;23:51–61. doi: 10.1080/15360280902728336. [DOI] [PubMed] [Google Scholar]
- Dalziel RG, Bingham S, Sutton D, Grant D, Champion JM, Dennis SA, Quinn JP, Bountra C, Mark MA. Allodynia in rats infected with varicella zoster virus–a small animal model for post-herpetic neuralgia. Brain Res Brain Res Rev. 2004;46:234–242. doi: 10.1016/j.brainresrev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Derry S, Sven-Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;2:CD007393. doi: 10.1002/14651858.CD007393.pub3. [DOI] [PubMed] [Google Scholar]
- Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–965. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2011;83:1432–1437. [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, Nash AA, Dalziel RG. Behavioural changes in the rat following infection with varicella-zoster virus. J Gen Virol. 1999;80(Pt 9):2433–2436. doi: 10.1099/0022-1317-80-9-2433. [DOI] [PubMed] [Google Scholar]
- Garry EM, Delaney A, Anderson HA, Sirinathsinghji EC, Clapp RH, Martin WJ, Kinchington PR, Krah DL, Abbadie C, Fleetwood-Walker SM. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118:97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedon JM, Yee MB, Zhang M, Harvey SA, Goins WF, Kinchington PR. Neuronal changes induced by Varicella Zoster Virus in a rat model of postherpetic neuralgia. Virology. 2015;482:167–180. doi: 10.1016/j.virol.2015.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedon JM, Zhang M, Glorioso JC, Goins WF, Kinchington PR. Relief of pain induced by varicella-zoster virus in a rat model of post-herpetic neuralgia using a herpes simplex virus vector expressing enkephalin. Gene Ther. 2014;21:694–702. doi: 10.1038/gt.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. quiz CE32–34. [PubMed] [Google Scholar]
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington PR, Dickenson AH, Pheby T, Rice AS. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Peck CC, Petersen ET, Rae CD, Youssef AM, Reeves JM, Wilcox SL, Akhter R, Murray GM, Gustin SM. Chronic Pain: Lost Inhibition? The Journal of Neuroscience. 2013;33:7574–7582. doi: 10.1523/JNEUROSCI.0174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. doi: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96:1581–1602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington PR, Goins WF. Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models. J Neurovirol. 2011;17:590–599. doi: 10.1007/s13365-011-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the thalamic input to the thalamic reticular nucleus. J Neurosci. 2011;31:6791–6799. doi: 10.1523/JNEUROSCI.3073-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Moxon KA. Relationship between physiological response type (RA and SA) and vibrissal receptive field of neurons within the rat trigeminal ganglion. J Neurophysiol. 2006;95:3129–3145. doi: 10.1152/jn.00157.2005. [DOI] [PubMed] [Google Scholar]
- Low K, Aebischer P, Schneider BL. Direct and retrograde transduction of nigral neurons with AAV6, 8, and 9 and intraneuronal persistence of viral particles. Hum Gene Ther. 2013;24:613–629. doi: 10.1089/hum.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Wolf W, Dueland AN, Cohrs R, Vafai A, Gilden D. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. J Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BM, Bloom DC, Cohrs RJ, Gilden DH, Kennedy PG. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J Neurovirol. 2003;9:194–204. doi: 10.1080/13550280390194000. [DOI] [PubMed] [Google Scholar]
- Pavan-Langston D. Herpes zoster ophthalmicus. Neurology. 1995;45:S50–51. doi: 10.1212/wnl.45.12_suppl_8.s50. [DOI] [PubMed] [Google Scholar]
- Pavan-Langston D. Varicella-Zoster Virus: virology and clinical management. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade NE, Kafrouni AI, Saab CY, Atweh SF, Jabbur SJ. Chronic thalamotomy increases pain-related behavior in rats. Pain. 1999;83:401–409. doi: 10.1016/S0304-3959(99)00123-2. [DOI] [PubMed] [Google Scholar]
- Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, Beyer J, Forsayeth J, Bankiewicz KS. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20:348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci. 1999;26(Suppl 3):S7–11. doi: 10.1017/s0317167100000135. [DOI] [PubMed] [Google Scholar]
- St Leger AJ, Hendricks RL. CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation. J Neurovirol. 2011;17:528–534. doi: 10.1007/s13365-011-0062-1. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Surman OS, Flynn T, Schooley RT, Baer L, Parker S, Hirsch MS, Davis LG. A double-blind, placebo-controlled study of oral acyclovir in postherpetic neuralgia. Psychosomatics. 1990;31:287–292. doi: 10.1016/s0033-3182(90)72166-4. [DOI] [PubMed] [Google Scholar]
- Umorin M, Stinson C, Bellinger LL, Kramer PR. Genes in the GABA Pathway Increase in the Lateral Thalamus of Sprague-Dawley Rats During the Proestrus/Estrus Phase. J Cell Physiol. 2016;231:1057–1064. doi: 10.1002/jcp.25198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahle-Hinz C, Hicks TP, Gottschaldt KM. Amino acids modify thalamo-cortical response transformation expressed by neurons of the ventrobasal complex. Brain Res. 1994;637:139–155. doi: 10.1016/0006-8993(94)91227-0. [DOI] [PubMed] [Google Scholar]


