Abstract
The development of catalytic enantioselective C(sp3)–H metal insertion reactions has been a significant challenge. Moderate success has recently been achieved via Pd-catalyzed desymmetrization of prochiral C–H bonds located on two different carbon centers. Herein, we report the discovery of chiral acetyl-protected aminoethyl quinoline (APAQ) ligands that enables Pd(II)-catalyzed enantioselective arylation of prochiral methylene C–H bonds on the same carbon center. The feasibility of performing asymmetric Pd insertion into ubiquitous β-methylene C–H bonds of aliphatic amides offers an alternative disconnection for constructing β-chiral centers. Systematic tuning of the ligand structure reveals that a six-membered instead of a five-membered chelation of these types of ligands with the Pd(II) is essential for accelerating the C(sp3)–H activation thereby achieving enantioselectivity.
Main Text
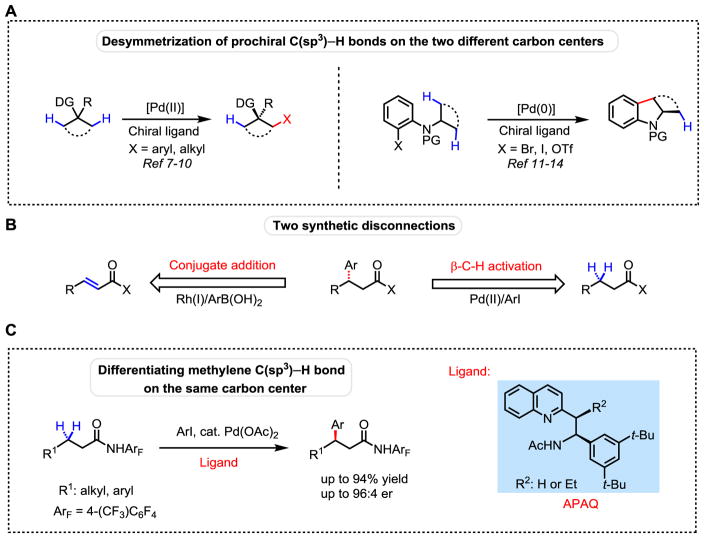
Enantioselective functionalizations of prochiral C–H bonds can potentially lead to a broad range of asymmetric reactions for preparing chiral compounds. Despite extensive efforts, the scope and efficiency of enantioselective C(sp3)–H activation reactions are far from being adequate for broad applications in asymmetric synthesis (1). Enantioselective carbene and nitrene insertions into C(sp3)–H bonds have been demonstrated in both diastereoselective and enantioselective fashion (2–6). However, asymmetric C(sp3)–H activation reactions via metal insertion are largely limited to the desymmetrization of C–H bonds located on two different carbon centers. For example, desymmetrizations of cyclopropyl and cyclobutyl C–H bonds have been achieved with Pd(II) catalysts and chiral mono-protected amino acid ligands (7–10). Desymmetrization of prochiral C–H bonds has also been achieved through a Pd(0)-catalyzed intramolecular arylation as demonstrated in a series of pioneering studies (11–14) (Fig. 1A). However, an efficient chiral metal catalyst capable of enantioselective insertion into ubiquitous methylene C–H bonds residing on the same carbon center has not been developed thus far. An effort to achieve such a process using a bidentate 8-aminoquinoline directing group and chiral phosphoric amide has afforded varied enantiomeric ratios (er) (ranging from 74:26 to 91:9) with benzyl C–H bonds and poor er (63:37) with alkyl C–H bonds (15). Recently, a transient chiral directing group has also been shown to perform enantioselective C–H arylation of benzylic C–H bonds (16).
Fig. 1. Enantioselective methylene C–H activation reactions.
(A) Desymmetrization of prochiral C(sp3)–H bonds on the two different carbon centers. (B) Two synthetic disconnections. (C) Differentiating methylene C(sp3)–H bond on the same carbon center. DG, directing group; PG, protecting group; OTf, trifluoromethanesulfonate; Ar, aryl group; Ac, acetyl group; Et, ethyl group; Bu, butyl group.
While solutions for achieving site selectivity with each C–H bond in a given molecule remain elusive, selective activation of a single C–H bond at a strategic site with a particular distal relationship to an existing functional group could provide a broadly useful synthetic disconnection. When considering retrosynthetic disconnections for the asymmetric synthesis of β-functionalized chiral carboxylic acids or amides, one immediately considers α,β-unsaturated esters or amides as building blocks which can be transformed to the desired products in the forward sense using state of the art conjugate addition reactions. Notably, Rh(I)-catalyzed asymmetric conjugate addition of α,β-unsaturated ketones with aryl boronic acids has afforded an elegant method for the preparation of chiral β-arylated compounds (17, 18). We therefore envision that enantioselective arylation of methylene C–H bonds at the β-position of amides through Pd(II) insertion could provide an alternative disconnection to these highly valuable synthons starting from saturated aliphatic acids (Fig. 1B). In our early efforts, we adopted a chiral auxiliary approach to gain insight into stereoselective Pd insertion into β-C(sp3)–H bonds (19). However, development of an enantioselective version of these diastereoselective β-C–H iodination and acetoxylation reactions has not been successful due to the lack of an appropriate ligand which can match the strongly coordinating oxazoline directing group (20). Employing a weakly coordinating amide directing group in combination with chiral mono-protected amino acid ligands (MPAA) has led to desymmetrization of methyl, cyclopropyl and cyclobutyl C–H bonds (Fig. 1A) at two different carbon centers (8, 9). Unfortunately, MPAA ligands have proven ineffective in promoting palladium insertion into β-methylene C–H bonds.
Herein we report the discovery of chiral acetyl-protected aminoethyl quinoline ligands (APAQ) that enable Pd(II)-catalyzed enantioselective arylation of β-methylene C–H bonds of aliphatic amides with er reaching up to 96:4 and yield as high as 94% (Fig. 1C). A wide range of simple aliphatic amides as well as aryl iodide coupling partners are compatible with this reaction. The design of these new chiral ligands merge the key structural motifs of our previous quinoline and acetyl protected amino acid ligands that are known to promote C(sp3)–H activation (21, 22). Strikingly, the adoption of a six-membered chelation of the APAQ ligand with the Pd(II) is essential for accelerating the C(sp3)–H activation thereby controlling the stereoselectivity. In contrast, the acetyl-protected aminomethyl quinoline coordinating with Pd(II) via five-membered chelation is completely inactive in this reaction.
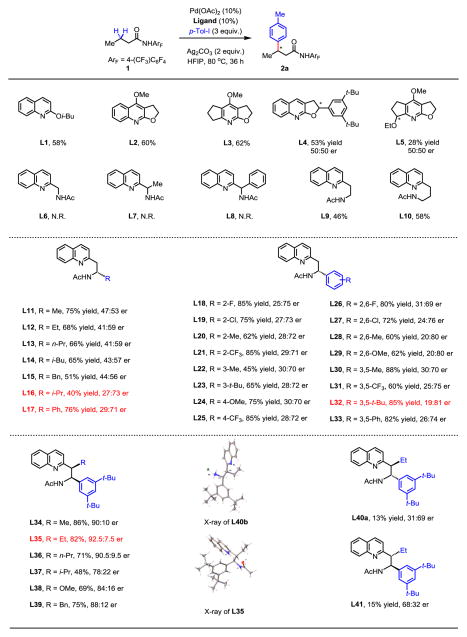
Guided by our overarching goal of developing ligand-accelerated enantioselective C–H activation of weakly coordinating substrates, we set out to use the electron-deficient amide substrate 1 and evaluate the effects of chiral ligands on the extensively studied C–H arylation reaction (23–25). Following our previous finding that quinoline and pyridine ligands can accelerate C(sp3)–H activation (26) (Fig. 2, L1-3), we prepared a number of corresponding chiral ligands including L4, L5 and examined their activity under standard reaction conditions. Unfortunately, these monodentate chiral ligands do not exert significant influence on the stereochemistry of the Pd insertion step. Considering the effectiveness of bidentate mono-protected amino acid ligands (MPAA) in controlling the stereochemistry of Pd-catalyzed desymmetrization of prochiral cyclopropyl and cyclobutyl C–H bond on two different carbon centers, we began to develop bidentate ligands incorporating structural motifs from both quinoline and MPAA ligands. The crucial role of the NHAc moiety of MPAA ligands in the C–H cleavage step, identified by experimental and computational studies (27), prompted us to develop acetyl-protected aminomethyl quinoline ligands which incorporate this coordinating moiety. Disappointingly, such ligands L6-8 resulted in a complete loss of reactivity. We reasoned that the five-membered bidentate chelation with Pd(II) could result in the formation of a stable, but inactive palladium complex tetra-coordinated with two ligands. As such, we prepared acetyl-protected aminoethyl quinoline (APAQ) and aminopropyl quinoline ligands that will coordinate with Pd(II) via six- and seven-membered chelate structures, respectively (L9, L10), both of which should have significantly reduced binding constants compared to the corresponding five-membered chelate (L6-8, Fig. 2). Remarkably, such subtle modification restored the reactivity with L9 and L10, thus offering a novel bidentate ligand scaffold for further development.
Fig. 2. Ligand development for enantioselective methylene C–H arylation.
The yields were determined by 1H NMR analysis of the crude product using CH2Br2 as an internal standard. Enantiomeric ratios (er) were determined by chiral high-performance liquid chromatography. The absolute configurations of L13, L21, L35 and L40b were determined by X-ray crystallography (see supplementary material). HFIP, hexafluoro-2-propanol; Me, methyl group; Pr, propyl group; Bn, benzyl group; Ph, phenyl group.
Although aminopropyl quinoline L10 is more reactive than aminoethyl quinoline L9, we chose to focus on the latter scaffold due to its synthetic accessibility. A series of chiral acetyl-protected aminoethyl quinoline ligands were prepared from 2-methylquinoline and optically pure sulfinyl imines using Ellman’s highly efficient asymmetric imine addition reaction (28). We initially found that ligand L11 containing an α-methyl group at the chiral center enhanced the reactivity significantly (75% yield), albeit giving poor enantioselectivity (47:53 er). The α-methyl group was then replaced with various alkyl groups and only the sterically bulky isopropyl group was found to give significantly improved er reaching 27:73, but with diminished yield (L16). While further tuning of the alkyl substitution proved less promising, the result obtained with the α-phenyl substitution in L17 provided us with an encouraging lead for ligand optimization (76% yield, 29:71 er). With L17 in hand, we surveyed various protecting groups on the amino group (see Table S1). Replacing the methyl group in the acetyl protecting group by more hindered alkyls or phenyls decreased the yields significantly. Other types of protecting groups such as carbamates and sulfonyls are completely inactive. We thus prepared a number of APAQ ligands (L18-33) with a range of steric and electronic variation on the α-phenyl ring. We found the steric effect is predominant as indicated by the drastically improved yield and enantioselectivity obtained with ligand L32 bearing the sterically hindered 3,5-di-tert-butylphenyl group (85% yield, 19:81 er). At this point of optimization, we decided to introduce a second chiral center at the benzylic position, hoping to further improve the enantioselectivity. Since the origin of the stereoselectivity is believed to derive from creating a less hindered face on the square planar palladium complex (7–10), we focused on the variations of syn-APAQ ligands in which both substituents will point upwards or downwards upon chelating with Pd(II). The introduction of a methyl group at the benzylic position (L34) afforded a significant improvement in enantioselectivity (90:10 er) while maintaining the high yield. A slightly more bulky ethyl group (L35) further improved the enantioselectivity to 92.5:7.5 er. Further increasing steric hindrance at the benzylic position decreased both yield and enantioselectivity (L36-39). To obtain insight into the stereochemical model of this unprecedented enantioselective palladium insertion process, we also tested the anti-APAQ ligands (L40a, L41). While both yield and enantioselectivity dropped significantly with these two anti-ligands, the reversal of chiral induction by altering the absolute configuration at the α-position suggests that the chiral center adjacent to the amino group dictates enantioselection (see Table S2).
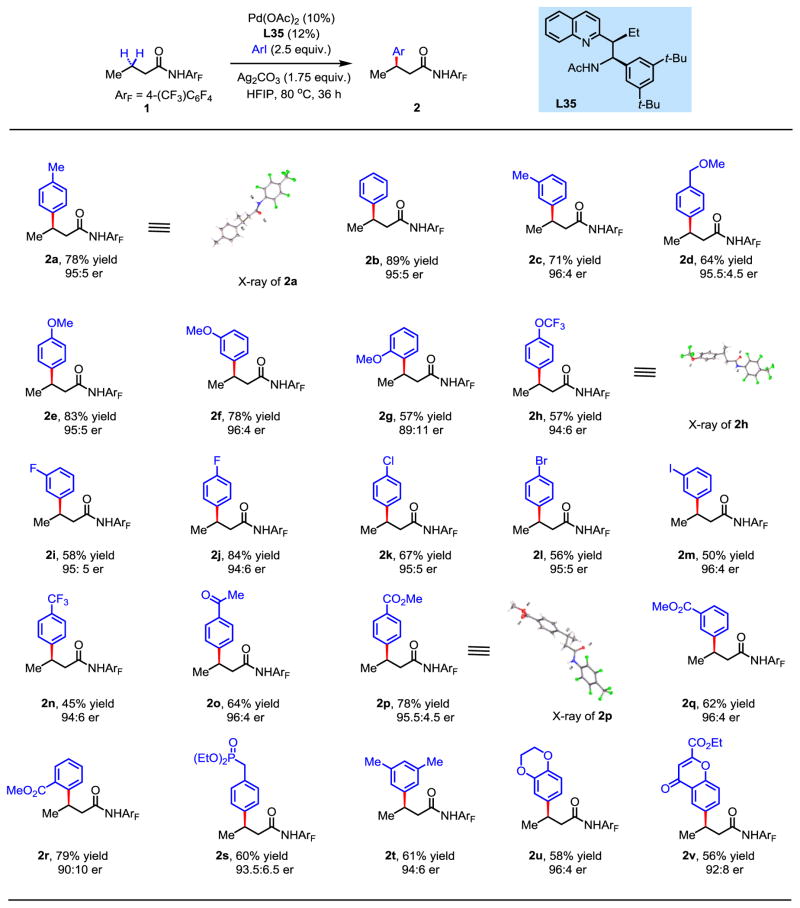
With the optimal ligand L35 in hand, we further optimized the reaction conditions for the arylation of 2a and improved the enantioselectivity to 95:5 er (see Table S3, entry 21). We next surveyed the scope of aryl iodides for this enantioselective β-C–H arylation (Fig. 3). Simple phenyl iodide and electron-rich aryl iodides containing methyl and methoxy groups afforded excellent enantioselectivity (2a-f) with the exception of o-methoxyphenyl iodide (2g, 89:11 er). The absolute configuration of the arylated product 2a was determined to be (R) by X-ray crystallographic analysis, which is consistent with a stereochemical model based on steric repulsion. Electron-deficient aryl iodides bearing trifluoromethoxy, fluoro, chloro, bromo, and iodo substituents were also compatible, providing consistently high enantioselectivity (2h-m), although the yield dropped to 45% with trifluoromethyl substitution (2n). Other electron-withdrawing functional groups including ketones, ester and phosphonates are also compatible, affording the desired enantioselectivity and good yields (2o-s). Disubstituted aryl iodides also proved to be suitable coupling partners (2t-v).
Fig. 3. Scope of coupling partners in enantioselective C–H arylation.
Isolated yield of purified compounds. The absolute configuration was determined by X-ray crystallography.
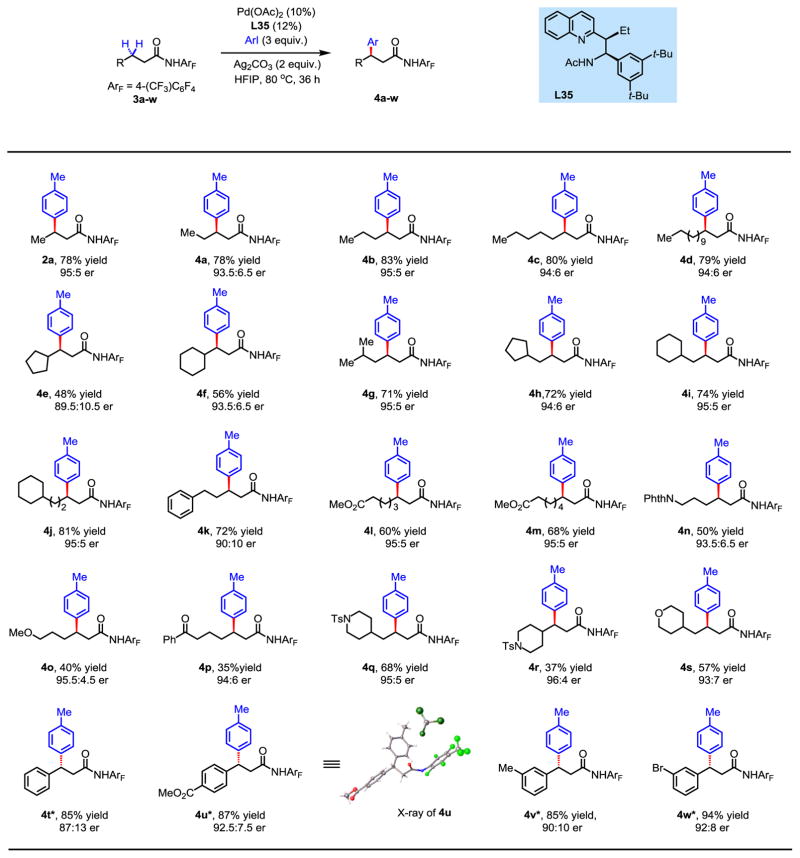
We were pleased to find that this protocol for enantioselective arylation of methylene C–H bonds was also applicable to other aliphatic amides (Fig. 4). Aliphatic amides with various chain lengths were well tolerated with excellent enantioselectivity and high yields (4a-d). Substrates containing sterically hindered alkyl groups at the β-position (cyclopentyl, clyclohexyl) provided good enantioselectivity, but gave lower yields (4e, 4f). Isopropyl, cyclopentyl, cyclohexyl and cyclohexylmethyl at the γ-positions are well tolerated, affording satisfactory yields and enantioselectivity (4g-j). Phenyl, ester, amino, ether and ketone functionalities at δ-and ε-positions consistently afforded high enantioselectivity (4k-p). However, lower yields were obtained with the ether and ketone substrates (4o, 4p). Piperidine at the γ-position afforded good yield and high enantioselectivity (4q), whereas the presence of piperidine at the β-position gave lower yield (4r). The presence of tetrahydropyran motif at the γ-position is also well tolerated, affording synthetically useful yield and enantioselectivity (4s). Interestingly, arylation of benzylic C–H with 3t using ligand L35 provided poor yield and enantioselectivity (38% yield, 68:32 er). Switching to ligand L32 improved both yield and enantioselectivity significantly (4t). β-phenyl groups containing both electron-withdrawing and -donating groups were also compatible with this reaction (4u-w), thus demonstrating that this ligand scaffold is also applicable to enantioselective activation of benzylic C–H bonds.
Fig. 4. Enantioselective methylene C–H arylation of aliphatic amides.
Isolated yield of purified compound. The absolute configuration was determined by X-ray. *Compounds 4t–w were obtained using 1.5 equiv. Ag2CO3, 2.5 equiv. aryl iodide and L32 as ligand. Phth, phthalimido group.
In summary, a chiral bidentate acetyl protected aminoethyl quinoline ligand scaffold is found to enable enantioselective arylation of β-methylene C–H bonds through palladium catalysis. The feasibility of such asymmetric palladium insertion opens a new avenue for developing a wide range of synthetically useful enantioselective C–H activation reactions.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01GM084019) for their financial support. We thank the Shanghai Institute of Organic Chemistry, Zhejiang Medicine Co., and Pharmaron for a postdoctoral fellowship (G.C.) and the Deutsche Forschungsgemeinschaft (DFG) for a research fellowship (M.S.A.). We acknowledge earlier computational studies that enhanced our understanding of catalyst development, conducted in collaboration with Jamal Musaev and Ken Houk within the NSF CCI Center for Selective C–H Functionalization (CHE-1205646). J.-Q. Y. conceived the concept. G. C. and W. G. developed the chiral ligands and optimized the reaction conditions. Z. Z., M. S. A., Y.-Q. C. and T. L. optimized the chiral ligands and surveyed the substrate scope. J.-Q. Y. directed the project. Metrical parameters for the structures of L13, L21, L40b, L35, 2a, 2h, 2p and 4u are available free of charge from the Cambridge Crystallographic Data Centre under reference number CCDC-14522020, CCDC-14522021, CCDC-14522023, CCDC-14522022, CCDC-14522016, CCDC-14522017, CCDC-14522018 and CCDC-14522019, respectively.
Footnotes
References and Notes
- 1.Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem Soc Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]
- 2.Doyle MP, Duffy R, Ratnikov M, Zhou L. Catalytic carbene insertion into C–H bonds. Chem Rev. 2010;110:704–724. doi: 10.1021/cr900239n. [DOI] [PubMed] [Google Scholar]
- 3.Reddy RP, Davies HML. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org Lett. 2006;8:5013–5016. doi: 10.1021/ol061742l. [DOI] [PubMed] [Google Scholar]
- 4.Liang C, Robert-Peillard F, Fruit C, Muller P, Dodd RH, Dauban P. Efficient diastereoselective intermolecular rhodium-catalyzed C–H amination. Angew Chem Int Ed. 2006;45:4641–4644. doi: 10.1002/anie.200601248. [DOI] [PubMed] [Google Scholar]
- 5.Zalatan DN, Du Bois J. A chiral rhodium carboxamidate catalyst for enantioselective C–H amination. J Am Chem Soc. 2008;130:9220–9221. doi: 10.1021/ja8031955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milczek E, Boudet N, Blakey S. Enantioselective C–H amination using cationic ruthenium (II)–pybox catalysts. Angew Chem Int Ed. 2008;47:6825–6828. doi: 10.1002/anie.200801445. [DOI] [PubMed] [Google Scholar]
- 7.Shi BF, Maugel N, Zhang YH, Yu JQ. PdII-catalyzed enantioselective activation of C(sp2)–H and C(sp3)–H bonds using monoprotected amino acids as chiral ligands. Angew Chem Int Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- 8.Wasa M, Engle KM, Lin DW, Yoo EJ, Yu JQ. Pd(II)-catalyzed enantioselective C–H activation of cyclopropanes. J Am Chem Soc. 2011;133:19598–19601. doi: 10.1021/ja207607s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao KJ, Lin DW, Miura M, Zhu RY, Gong W, Wasa M, Yu JQ. Palladium(II)-catalyzed enantioselective C(sp3)–H activation using a chiral hydroxamic acid ligand. J Am Chem Soc. 2014;136:8138–8142. doi: 10.1021/ja504196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KSL, Fu HY, Yu JQ. Palladium(II)-catalyzed highly enantioselective C–H arylation of cyclopropylmethylamines. J Am Chem Soc. 2015;137:2042–2046. doi: 10.1021/ja512529e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi M, Katayev D, Besnard C, Kündig EP. Fused indolines by palladium-catalyzed asymmetric C–C coupling involving an unactivated methylene group. Angew Chem Int Ed. 2011;50:7438–7441. doi: 10.1002/anie.201102639. [DOI] [PubMed] [Google Scholar]
- 12.Anas S, Cordi A, Kagan HB. Enantioselective synthesis of 2-methyl indolines by palladium catalysed asymmetric C(sp3)–H activation/cyclisation. Chem Comm. 2011;47:11483–11485. doi: 10.1039/c1cc14292e. [DOI] [PubMed] [Google Scholar]
- 13.Martin N, Pierre C, Davi M, Jazzar R, Baudoin O. Diastereo- and enantioselective intramolecular C(sp3)–H arylation for the synthesis of fused cyclopentanes. Chem Eur J. 2012;18:4480–4484. doi: 10.1002/chem.201200018. [DOI] [PubMed] [Google Scholar]
- 14.Saget T, Lemouzy S, Cramer N. Chiral monodentate phosphines and bulky carboxylic acids: cooperative effects in palladium-catalyzed enantioselective C(sp3)–H functionalization. Angew Chem Int Ed. 2012;51:2238–2242. doi: 10.1002/anie.201108511. [DOI] [PubMed] [Google Scholar]
- 15.Yan SB, Zhang S, Duan WL. Palladium-catalyzed asymmetric arylation of C(sp3)–H bonds of aliphatic amides: controlling enantioselectivity using chiral phosphoric amides/acids. Org Lett. 2015;17:2458–2461. doi: 10.1021/acs.orglett.5b00968. [DOI] [PubMed] [Google Scholar]
- 16.Zhang FL, Hong K, Li TJ, Park H, Yu JQ. Functionalization of C(sp3)–H bonds using a transient directing group. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthon G, Hayashi T. Rhodium- and Palladium-Catalyzed Asymmetric Conjugate Additions. In: Cordova A, editor. Catalytic Asymmetric Conjugate Reactions. Wiley; 2010. pp. 1–67. [Google Scholar]
- 18.Paquin JF, Defieber C, Stephenson CRJ, Carreira EM. Asymmetric synthesis of 3,3-diarylpropanals with chiral diene–rhodium catalysts. J Am Chem Soc. 2005;127:10850–10851. doi: 10.1021/ja053270w. [DOI] [PubMed] [Google Scholar]
- 19.Giri R, Chen X, Yu JQ. Palladium-catalyzed asymmetric iodination of unactivated C–H bonds under mild conditions. Angew Chem Int Ed. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 20.Engle KM, Yu JQ. Developing ligands for palladium(II)-catalyzed C–H functionalization: intimate dialogue between ligand and substrate. J Org Chem. 2013;78:8927–8955. doi: 10.1021/jo400159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasa M, Chan KSL, Zhang XG, He J, Miura M, Yu JQ. Ligand-enabled methylene C(sp3)–H bond activation with a Pd(II) catalyst. J Am Chem Soc. 2012;134:18570–18572. doi: 10.1021/ja309325e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KSL, Wasa M, Chu L, Laforteza BN, Miura M, Yu JQ. Ligand-enabled cross-coupling of C(sp3)–H bonds with arylboron reagents via Pd(II)/Pd(0) catalysis. Nat Chem. 2014;6:146–150. doi: 10.1038/nchem.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaitsev VG, Shabashov D, Daugulis O. Highly regioselective arylation of sp3 C–H bonds catalyzed by palladium acetate. J Am Chem Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 24.Reddy BVS, Reddy LR, Corey EJ. Novel acetoxylation and C–C coupling reactions at unactivated positions in α-amino acid derivatives. Org Lett. 2006;8:3391–3394. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- 25.Feng YQ, Chen G. Total synthesis of celogentin C by stereoselective C–H activation. Angew Chem Int Ed. 2010;49:958–961. doi: 10.1002/anie.200905134. [DOI] [PubMed] [Google Scholar]
- 26.He J, Jiang H, Takise R, Zhu R-Y, Chen G, Dai H-X, Murali Dhar TG, Shi J, Zhang H, Cheng PTW, Yu J-Q. Ligand-promoted borylation of C(sp3)–H bonds with Pd(II) catalysts. Angew Chem Int Ed. 2016;55:785–789. doi: 10.1002/anie.201509996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng GJ, Yang YF, Liu P, Chen P, Sun TY, Li G, Zhang X, Houk KN, Yu JQ, Wu YD. Role of N-acyl amino acid ligands in Pd(II)-catalyzed remote C–H activation of tethered arenes. J Am Chem Soc. 2014;136:894–897. doi: 10.1021/ja411683n. [DOI] [PubMed] [Google Scholar]
- 28.Robak MT, Herbage MA, Ellman JA. Synthesis and applications of tert-butanesulfinamide. Chem Rev. 2010;110:3600–3740. doi: 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.