Abstract
Body condition is a gauge of the energy stores of an animal, and though it has important implications for fitness, survival, competition, and disease, it is difficult to measure directly. Instead, body condition is frequently estimated as a body condition index (BCI) using length and mass measurements. A desirable BCI should accurately reflect true body condition and be unbiased with respect to size (i.e., mean BCI estimates should not change across different length or mass ranges), and choosing the most-appropriate BCI is not straightforward. We evaluated 11 different BCIs in 248 Burmese pythons (Python bivittatus), organisms that, like other snakes, exhibit simple body plans well characterized by length and mass. We found that the length-mass relationship in Burmese pythons is positively allometric, where mass increases rapidly with respect to length, and this allowed us to explore the effects of allometry on BCI verification. We employed three alternative measures of ‘true’ body condition: percent fat, scaled fat, and residual fat. The latter two measures mostly accommodated allometry in true body condition, but percent fat did not. Our inferences of the best-performing BCIs depended heavily on our measure of true body condition, with most BCIs falling into one of two groups. The first group contained most BCIs based on ratios, and these were associated with percent fat and body length (i.e., were biased). The second group contained the scaled mass index and most of the BCIs based on linear regressions, and these were associated with both scaled and residual fat but not body length (i.e., were unbiased). Our results show that potential differences in measures of true body condition should be explored in BCI verification studies, particularly in organisms undergoing allometric growth. Furthermore, the caveats of each BCI and similarities to other BCIs are important to consider when determining which BCI is appropriate for any particular taxon.
Introduction
As a measure of the relative energy stores of an animal [1], body condition has both theoretical and practical importance because of its strong associations with reproductive capacity [2, 3], survivorship [3–5], competition [6, 7], and disease [8–10]. Despite this importance, body condition is generally unfeasible to measure directly because direct measurement requires destruction of the whole specimen [11–14]. Instead, biologists frequently estimate body condition using a body condition index (BCI) calculated from mass and length variables obtained from non-invasive measurements (reviewed in [14]; see [15] for alternative means to estimate body condition).
There are many BCIs based on length and mass, and most can be categorized into two groups: ratio BCIs and regression BCIs (Table 1). The first proposed BCIs were ratio BCIs, and these are still commonly used today (e.g., the body mass index for humans is the Quételet index, or mass/length^2; [16, 17]). Ratio BCIs are intuitive and are comparable among individuals from different statistical populations, but they are plagued with bias (described below; [1, 18]). Regression-based BCIs are also commonly used; the residuals from a regression of log-transformed mass on log-transformed length (i.e., the observed mass minus the expected mass) are the BCI. These BCIs, where a positive value indicates a fat animal and a negative value indicates a skinny animal, are also intuitive, but they must satisfy a number of assumptions, including: the data are linear, the variance is homoscedastic, and the residuals are normally distributed [1, 19]. Furthermore, Type I regressions (e.g., ordinary least squares or OLS regressions, which are commonly employed as BCIs) assume that body length as the independent variable is known without error so that variation around the best-fit line is attributable only to variation in mass as the dependent variable, but there are a number of potential sources of error in body-length data [19]. Also, and unlike the ratio BCIs, estimates from regression BCIs cannot be compared among individuals from different statistical populations because the regression slopes among populations may be different [18, 19]. Not surprisingly, choosing which BCI to use is not straightforward, and different studies have championed different BCIs as most appropriate [13, 18, 20–23].
Table 1. Summary of body condition indices (BCIs) used in this study.
When available, the names were derived from the literature. Otherwise, we gave arbitrary, descriptive names to unnamed BCIs to facilitate communication.
| Description | Abbreviation | Name | Category | Citations |
|---|---|---|---|---|
| Body mass divided by body length | M/L | Ratio index | Ratio | [14] |
| Body mass divided by body length squared | M/L^2 | Quételet index | Ratio | [17] |
| Body mass divided by body length cubed | M/L^3 | Fulton’s index | Ratio | [24, 25] |
| Body mass divided by predicted body mass from SMA regression | M/prM | Relative index | Ratio | [26] |
| Log-transformed body mass divided by log-transformed body length | logM/logL | Log ratio index | Ratio | [14] |
| Log-transformed body mass divided by log-transformed predicted body mass from SMA regression | logM/log(prM) | Log relative index | Ratio | [1] |
| Residuals from OLS linear regression of log-transformed body mass on log-transformed body length | OLSres | OLS residual index | Regression (Type I) |
[18] |
| Residuals from MA linear regression of log-transformed body mass on log-transformed body length | MAres | MA residual index | Regression (Type II) |
[13, 19] |
| Residuals from SMA linear regression of log-transformed body mass on log-transformed body length | SMAres | SMA residual index | Regression (Type II) |
[13, 19] |
| Residuals from SMA linear regression of body mass on body length cubed | res(M~L^3) | Cubed regression index | Regression (Type II) |
[22] |
| Scaled Mass Index | SMI | Scaled mass index (SMI) | Allometric | [27] |
OLS = Ordinary least squares
MA = Major axis
SMA = Standardized major axis
An ideal BCI should be accurate (i.e., correlated with true body condition) and unbiased with respect to size (i.e., not correlated with body mass or a linear length measurement; [14]). The requirement that the BCI is accurate is straightforward because body condition is what the BCI is estimating. The requirement that the BCI is unbiased with respect to size is important for hypothesis testing, because a lack of correlation with size allows a researcher to compare BCI estimates across individuals of different size ranges [11, 14]. Generally speaking, ratio BCIs are correlated with size but by definition residual BCIs are not [1, 11].
Many animal species undergo allometric rather than isometric growth, meaning that their shape changes over their lifespan as their mass increases or decreases in proportion to length, and allometric changes are another potential challenge to BCI inference [19, 28]. One BCI was specifically proposed as a solution to the allometry problem–the scaled mass index (SMI; [27]). The SMI accommodates allometric changes using the Thorpe-Lleonart (TL) scaling model:
| (1) |
where is the predicted value of Y (mass) for individual i after correcting for the scaling relationship between X (length) and Y (mass); Xi and Yi are the observed values of X (length) and Y (mass) for individual i; X0 is the arithmetic mean of X (length) for the study population (this value is arbitrary and can be any value of X observed in the study population); and bSMA is the slope of a standardized (reduced) major axis (SMA) regression of log-transformed mass on log-transformed length for the study population [27, 29]. This approach eliminates all allometric effects on size [29], has few assumptions, and is comparable among populations, but how well the SMI correlates with true body condition is variable among datasets [20–22].
Here we explore the performance of BCIs in a large constricting snake species, the Burmese python (Python bivittatus). Snakes are perhaps ideal animals to explore BCI performance because their body plan is simple; they do not have appendages or other additional features that may change in shape and confound BCI inference. Like other squamates, pythons store fat in discrete fat bodies in their coelomic cavity, and the fat bodies can be removed and weighed during necropsy (i.e., wet-fat mass; [30]). In viperid and colubrid snakes, both the wet and dry weights for whole bodies and the wet and dry weights for fat bodies are highly correlated, suggesting that the proportional mass of water, organic matter, and inorganic matter in both whole bodies and fat bodies in snakes is constant [31].
This consistency in wet and dry weights of fat mass makes wet-fat mass available to use in a measure of ‘true’ body condition to evaluate BCIs in snakes and likely other squamates. Wet-fat mass has been used in a measure of true body condition to evaluate BCIs [32, 33] and body condition scores (i.e., numerical values that are associated with specific body-condition categories [e.g., shoulders are ‘v-shaped’]; [33, 34]) in squamates and in other taxa. Nonetheless, there are factors that may affect how well measures using wet-fat mass approximate true body condition. For example, fat may be stored elsewhere in addition to the fat bodies (e.g., the liver; [32]). Also, other tissues–most notably muscle–contribute to body condition [14], and fat mass ignores the contribution of these other tissues. These caveats likely reduce the accuracy of wet-fat mass as an approximation of true body condition, but we posit that BCI evaluation using such an approximation is justifiable in an animal species that is otherwise too large to evaluate by traditional methods (i.e., drying and grinding the specimen prior to lipid extraction; [11]).
Dry- and wet-fat mass have been used in a variety of ways to approximate true body condition and verify BCIs. Common measures include both total fat mass and percent fat (total fat mass / total body mass; [11, 13, 18, 22, 27, 35]). Fat mass and percent fat do not accommodate allometric changes in true body condition; if an animal exhibits allometry in true body condition, then these measures will be biased with respect to animal size. Measures of true body condition that accommodate allometry include scaled fat, where fat mass is scaled to length using the TL scaling model described above [27], and residual fat, where fat mass is regressed on length to calculate residuals [22]. Neither scaled fat nor residual fat are expected to change with body size [29, 36].
We identified 11 BCIs from the literature (Table 1) and assessed their performance using wet-fat masses of Burmese pythons collected in Florida as part of invasive-species removal efforts. We characterized the allometric relationship between mass and length for these snakes, considered percent fat, scaled fat, and residual fat as alternative measures of true body condition, tested these for an association with snake length (i.e., allometry), and used them to assess BCI accuracy.
Methods
We performed necropsies on Burmese pythons that were collected in southern Florida during 2004–2014 as part of ongoing invasive-species management activities. The snakes were humanely euthanized via captive bolt, kept on ice, and either necropsied within 24 hours of euthanasia or frozen and later thawed on ice for necropsy. No Institutional Animal Care and Use Committee approval was necessary because the invasive pythons were euthanized as part of management–and not research–activities, but methods of safe and humane euthanasia were developed in consultation with the National Park Service Wildlife Health Team. Similarly, these necropsies were not performed explicitly to evaluate BCIs, but we nonetheless collected the necessary data. We used measurements of total body mass (g) and snout-vent length (SVL; cm) as body-mass and length metrics for our BCIs. We used SVL instead of total length because SVL is more tightly associated with mass than is total length in snakes (because tail length varies; [37]). During necropsy we removed all visible fat from the coelomic cavity and weighed it (wet-fat mass; g). We recorded sex (male or female) and for a subset of individuals we recorded whether the snake was necropsied while fresh or frozen and later thawed. We excluded pythons that were not immediately put on ice or frozen after death (e.g., we excluded pythons found dead on roads). We performed all subsequent analyses in R v.3.3.3 [38] using the car [39], corrplot [40], cowplot [41], extrafont [42], fBasics [43], ggplot2 [44], lmodel2 [45], lmtest [46], moments [47], and smatr [48] packages.
We wanted to know whether differences in sex or in our specimen-handling procedures (i.e., storing on ice vs. freezing/thawing prior to necropsy) are associated with differences in the relationship between wet-fat mass and SVL in our dataset because if they are, these groups should be treated separately in downstream analyses. We used likelihood ratio tests for common slopes [49] to test the null hypotheses that the slopes of SMA regressions of log-transformed wet-fat mass on log-transformed SVL are equal between sexes and between frozen and fresh specimens. We had complete specimen-handling information for 73 specimens, so we constrained the specimen-handling test to this smaller dataset. We consider p ≤ 0.05 for these and similar tests to be statistically significant but acknowledge that applying a p-value threshold for significance is arbitrary [50].
We calculated percent fat, scaled fat, and residual fat for each snake as alternative measures of true body condition. Percent fat was wet-fat mass divided by total body mass. We calculated scaled fat using measurements of wet-fat mass and SVL and the TL scaling model. We calculated residual fat by taking the residuals from a SMA regression of log-transformed wet-fat mass on log-transformed SVL. Because we observed an influence of sex on the relationship between wet-fat mass and SVL (see Results), we calculated scaled fat and residual fat for each of these groups separately and conducted all downstream analyses separately by sex.
We characterized the relationship between body length and body mass, fat mass, percent fat, scaled fat, and residual fat. We used a SMA regression of log-transformed mass on log-transformed SVL and of log-transformed fat mass on log transformed SVL to infer the regression slopes (i.e., the slopes of the allometric lines for total mass and fat mass, respectively) and used likelihood ratio tests to test the null hypotheses that the slopes between sexes are equal. An ideal measure of true body condition is not associated with body length, so we estimated the slopes and r2 values from OLS regressions of percent fat, scaled fat, and residual fat on log-transformed SVL; if there is no relationship between the body-condition measure and body length, then the r2 value will be zero and the slope will be non-significant. We used OLS regressions to characterize the relationship between the true body condition measures and length because in this context any variation around the best-fit line is attributable only to variation in the dependent variable as the values for length in both the dependent and independent variables are equal.
We calculated each of the 11 BCIs (Table 1) for each python. The residual BCIs have three basic assumptions: 1) the data are linear; 2) the variance is constant (i.e., homoscedastic); and 3) the frequency distribution of residuals is normal. We tested for linearity using Ramsey’s RESET test [51] and for non-constant variance using the Breusch-Pagan test [52]. We tested for normality using the Shapiro-Wilk test [53]. For the residual BCIs for which we rejected normality, we tested for skewedness using the D'Agostino test [54] and for kurtosis using the Anscombe-Glynn test [55].
An optimal BCI closely approximates true body condition and is unbiased with respect to body size, and we characterized this relationship in several ways. First, we estimated the Kendall rank correlation coefficient (τ; [56]) to test for a correlation between each BCI and percent fat, scaled fat, and residual fat as measures of true body condition (i.e., accuracy) and SVL as a measure of size (i.e., bias). Second, and similarly, we regressed each BCI against percent fat, scaled fat, residual fat, and log-transformed SVL for each sex and estimated the proportion variation in each of the response variables that can be explained by SVL (r2).
We wanted to know to what extent any given BCI could be substituted for another, and so we created a correlation matrix to explore how similar each of the BCIs are to each other with our dataset. For each sex separately, we generated pairwise Kendall rank correlation coefficients between inferred BCI values.
The BCIs most strongly correlated with percent fat were biased with respect to length (see Results), so we explored the extent of the bias. More specifically, we wanted to know whether these BCIs exhibited size bias among pythons that differ in SVL by ≤ 0.5 m in our dataset. We partitioned both female and male datasets into two subsets: pythons ≤ 0.25 m of the mean SVL and pythons ≥ 0.25 m of the mean SVL. For each sex and using the BCIs identified as best-fitting by r2 values as described above, we used Mann-Whitney U tests [57] to test the hypothesis that BCI values from each of the two size categories are pulled from the same distribution. A non-significant p-value for these tests would suggest that BCI estimates of pythons that differ by ≤ 0.5 m SVL in our dataset are comparable. SVL measurements for Burmese pythons collected in southern Florida range approximately 0.5–5.0 m for females and 0.5–3.5 m for males (Table 2), and we believe that 0.5 m is the smallest possible SVL size category that may allow sufficient sample sizes for future hypothesis testing (e.g., are Burmese pythons getting skinnier because of a lack of available prey?).
Table 2. Summary of snout-vent length (SVL), total mass, wet-fat mass, percent fat, scaled fat, and residual fat data from 248 Burmese pythons collected in southern Florida during 2004–2014.
Values are expressed as: mean (total range). Females in this dataset are generally longer, heavier, and fatter than males.
| Sex | N | SVL (cm) | Total Mass (g) | Wet-fat Mass (g) | Percent Fat | Scaled Fat | Residual Fat |
|---|---|---|---|---|---|---|---|
| Female | 114 | 235 (70–482) | 11,852 (186–75,500) | 1226 (5.0–8406) | 0.0824 (0.00652–0.173) | 772 (55.2–3076) | 0.0 (-2.43–1.59) |
| Male | 134 | 214 (76–347) | 7683 (260–32,600) | 571 (4.0–4374) | 0.0645 (0.00827–0.143) | 451 (38.8–1023) | 0.0 (-2.32–0.943) |
Results
We obtained records of total mass, wet-fat mass, SVL, and sex for 248 Burmese pythons that were immediately placed on ice or frozen after death. Of these, 137 were male and 109 were female, and males were usually smaller and had less fat than females (Table 2). We rejected the null hypothesis that differences in sex do not affect the relationship between wet-fat mass and SVL (p ≤ 0.05), and so we separated males and females in all downstream analyses. In contrast, we could not reject the null hypothesis that our specimen-handling procedures (i.e., whether the animals had been frozen prior to necropsy) do not affect the relationship between wet-fat mass and SVL (p = 0.28), so we ignored specimen-handling information in downstream analyses.
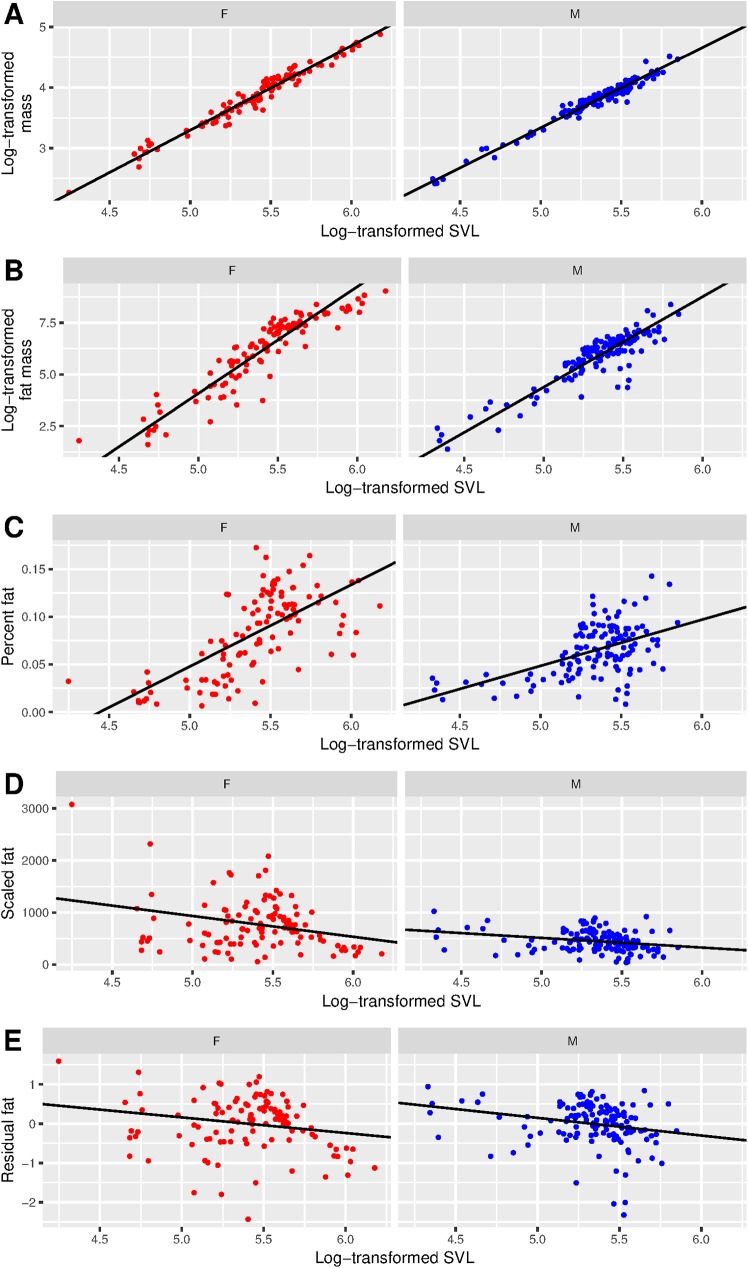
Females exhibited more positive allometry than males in both total mass and fat mass, and fat mass was more positively allometric than total mass in both sexes (Table 3, Fig 1A and 1B). The slope of the SMA regression (i.e., the allometric line) for total mass was 3.21 [95% CI: 3.10–3.33] for females and 3.04 [95% CI: 2.94–3.14] for males (Fig 1A). The 95% CIs for these slope estimates slightly overlap between the two sexes, but we rejected the hypothesis that the slopes are equal (p ≤ 0.05; i.e., male and female pythons exhibit different allometric relationships between length and total mass). The slope of the SMA regression for fat mass was 5.16 [95% CI: 4.81–5.55] for females and 4.37 [95% CI: 4.06–4.72] for males (Fig 1B). We also rejected the hypothesis that these slopes are equal (p ≤ 0.05; i.e., male and female pythons also exhibit different allometric relationships between length and fat mass; Table 3).
Table 3. Relationships between body length and mass, fat, and true body condition measures for male and female Burmese pythons.
We estimated the probability that the slope is not zero (i.e., p ≤ 0.05) and calculated the r2 values from SMA regressions of log-transformed body mass and log-transformed wet-fat mass on log-transformed snout-vent length (SVL) and from OLS regressions of percent fat, scaled fat, and residual fat on log-transformed SVL. Ideally, measures of true body condition will have no relationship with body length (i.e., percent fat, scaled fat, and residual fat should have non-significant regression slopes and r2 values of zero), but each of them do. These results are visualized in Fig 1.
| Female | Male | |||
|---|---|---|---|---|
| Variable | Slope | r2 | Slope | r2 |
| Body mass | ** | 0.96 | ** | 0.96 |
| Fat mass | ** | 0.85 | ** | 0.81 |
| Percent fat | ** | 0.45 | ** | 0.26 |
| Scaled fat | * | 0.078 | * | 0.068 |
| Residual fat | * | 0.038 | * | 0.051 |
* p ≤ 0.05
**p ≤ 0.001
Fig 1. Relationships between snout-vent length (SVL) and mass, fat, and true body condition measures for male and female Burmese pythons.
The SMA regression of log-transformed body mass on log-transformed snout-vent length (A) and of log-transformed fat mass on log-transformed SVL (B) shows the allometric relationship among these variables. As measures of true body condition, OLS regressions of percent fat on log-transformed SVL (C), scaled fat on log-transformed SVL (D), and residual fat on log-transformed SVL (E) should have zero slopes, but they do not. Significance for the regression slopes and r2 values can be found in Table 3.
Each of our measures of true body condition was associated with size (Table 3; Fig 1C–1E). Percent fat exhibited the strongest relationship with SVL, having a significant positive slope (p ≤ 0.001) and r2 values that suggest > 40% of the variation in percent fat is explained by SVL. Scaled fat exhibited a slight but significant negative relationship with SVL (p ≤ 0.05), and the r2 values suggest that approximately 7% of the variation in scaled fat is explained by SVL. Residual fat was similar, exhibiting a slightly negative but still significant relationship with SVL (p ≤ 0.05), and the r2 values suggest that 4–5% of the variation in residual fat is explained by SVL.
The testable assumptions of the regression-based BCIs were satisfactorily met in many cases, but not all (Table 4). We could not reject linearity or homoscedasticity for regressions of log-transformed mass on log-transformed length, and though we rejected normality for these regressions in females, we could not reject deviations due skewedness or kurtosis, suggesting that deviations from normality are minimal (i.e., assumptions for the OLS, MA, and SMA regressions were generally met in both sexes). Though we could not reject linearity, we strongly rejected both homoscedasticity and normality for the regression of mass on length cubed.
Table 4. Tests of assumptions of the regression-based body condition indices (BCIs).
We used several tests to test the hypotheses that: 1) the data are linear; 2) the variance is constant (i.e., homoscedastic); and 3) the frequency distribution of residuals is normal. For clarity, only p-values are reported. Most assumptions are met for regressions of log-transformed mass on log-transformed length, but regressions of mass on length cubed exhibited neither homoscedasticity nor normality. Abbreviations for each of the BCIs are provided in Table 1.
| BCI | Regression | Ramsey’s RESET Test (linearity) | Breusch-Pagan Test (homoscedasticity) | Shapiro-Wilk Test (normality) | d’Agostino Test (skewness) | Anscombe-Glynn Test (kurtosis) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | ||
| OLSres | OLS (logM~logL) | — | — | — | — | * | — | — | n/a | — | n/a |
| MAres | MA (logM~logL) | * | — | — | n/a | — | n/a | ||||
| SMAres | SMA (logM~logL) | * | — | — | n/a | — | n/a | ||||
| res(M~L^3) | SMA (M~L^3) |
— | — | ** | ** | ** | ** | ** | ** | ** | ** |
— p > 0.05
* p ≤ 0.05
**p ≤ 0.001
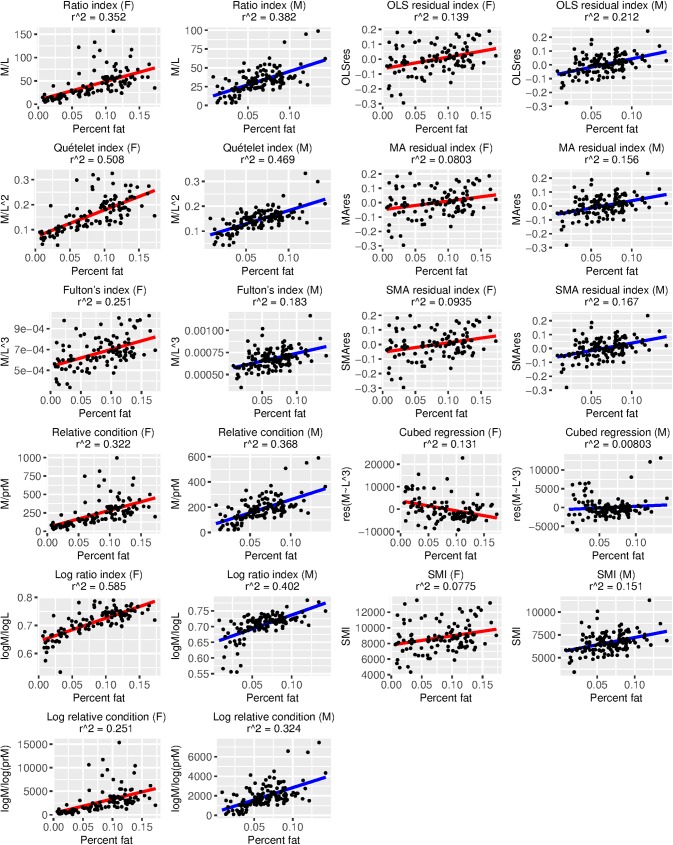
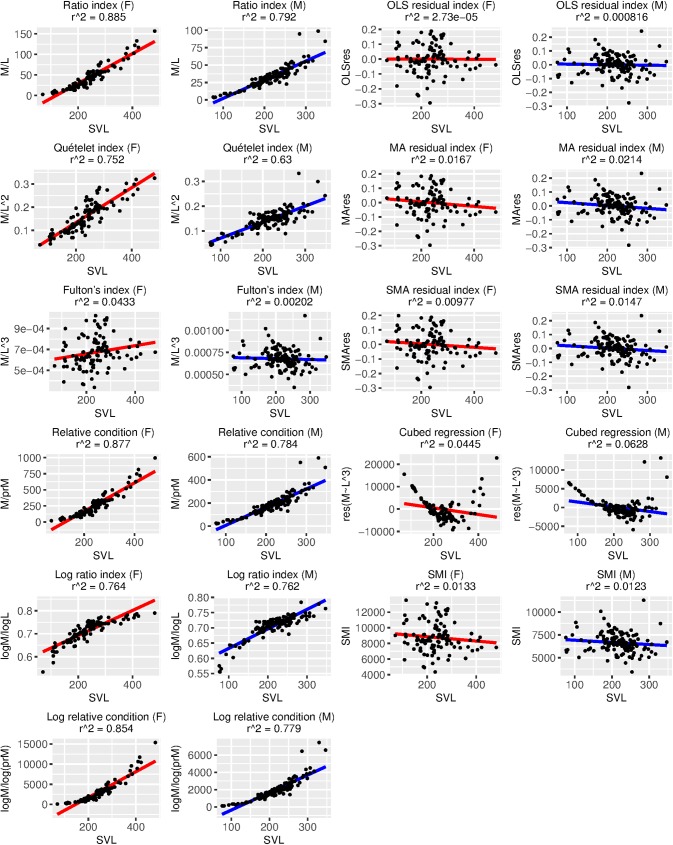
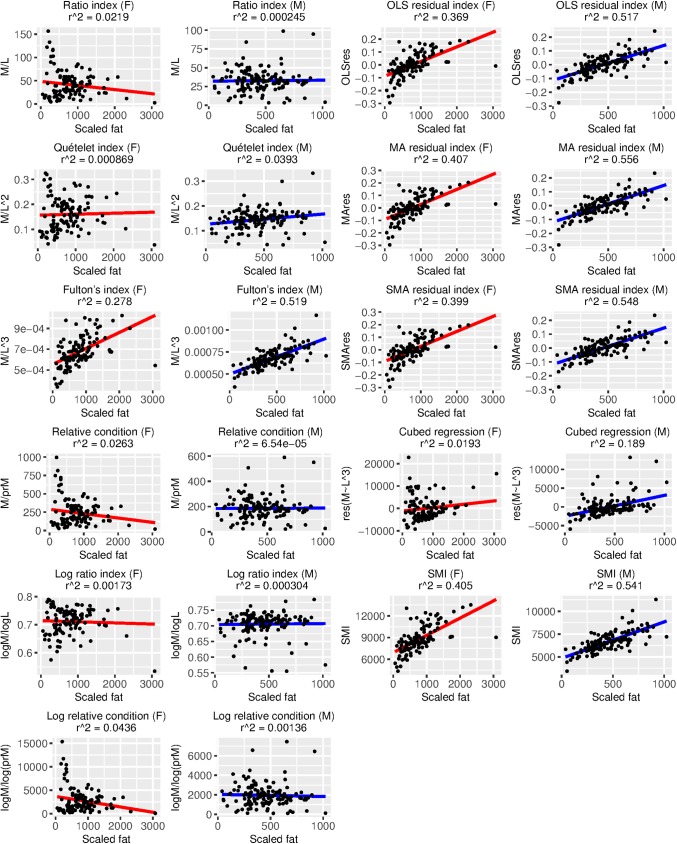
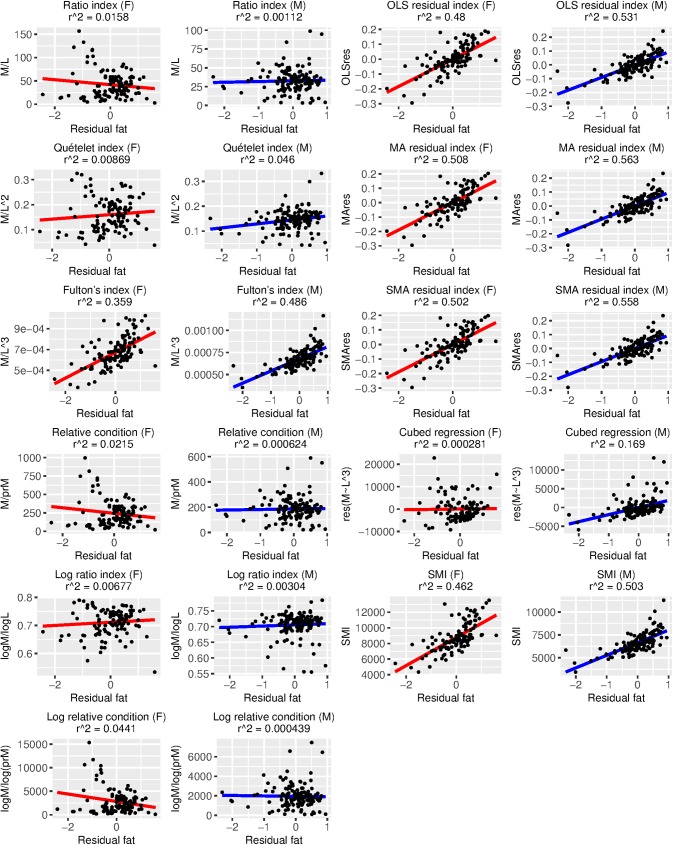
BCI performance was variable and depended heavily on which measure of true body condition we employed (Table 5, Figs 2–5). Using percent fat as a measure of true body condition, the log ratio index in females (τ = 0.56, Table 5; r2 = 0.59, Fig 2) and the Quételet index in males (τ = 0.46, Table 5; r2 = 0.47, Fig 2) were among the best-performing, but each of these was more strongly associated with SVL than percent fat (log ratio index in females: τ = 0.76, Table 5; r2 = 0.76, Fig 5; Quételet index in males: τ = 0.76, Table 5; r2 = 0.55, Fig 5). The best-performing BCIs using scaled fat and residual fat as measures of true body condition contrasted with the results using percent fat. For example, the MA residual index had the highest r2 values (0.41–0.56; Figs 3 and 4) and τ values (0.54–0.56; Table 5) in both sexes for scaled fat and residual fat. Notably, only ~2% of the variation in the MA residual index is associated with SVL (Fig 5), but though this relationship is small, it is significant in males (τ = -0.14, p ≤ 0.05; Table 5).
Table 5. Kendall’s τ correlation coefficients between body condition indices (BCIs) and percent fat, scaled fat, residual fat, and snout-vent length (SVL).
A desirable BCI is strongly correlated with ‘true’ body condition (i.e., percent fat, scaled fat, or residual fat) but is not correlated with size (i.e., SVL). Values with the greatest correlation coefficient for each column are italicized. Generally speaking, BCIs that are strongly correlated with percent fat are also strongly correlated with SVL in our dataset. Abbreviations for each of the BCIs are provided in Table 1.
| BCI | Percent fat | Scaled fat | Residual fat | SVL | ||||
|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | |
| M/L | 0.56** | 0.41** | 0.03 | -0.02 | 0.03 | -0.02 | 0.83** | 0.77** |
| M/L^2 | 0.57** | 0.46** | 0.11 | 0.15* | 0.11 | 0.15* | 0.71** | 0.55** |
| M/L^3 | 0.35** | 0.29** | 0.45** | 0.54** | 0.45** | 0.54** | 0.21* | -0.09 |
| M/prM | 0.56** | 0.41* | 0.03 | -0.02 | 0.03 | -0.02 | 0.83** | 0.78** |
| logM/logL | 0.56** | 0.44** | 0.07 | 0.08 | 0.07 | 0.08 | 0.76** | 0.65** |
| logM/log(prM) | 0.54** | 0.39* | 0.00 | -0.06 | 0.00 | -0.06 | 0.86** | 0.82** |
| OLSres | 0.25** | 0.30** | 0.52** | 0.54** | 0.52** | 0.54** | 0.03 | -0.07 |
| MAres | 0.19* | 0.25** | 0.54** | 0.56** | 0.54** | 0.56** | -0.06 | -0.1* |
| SMAres | 0.20* | 0.26** | 0.54** | 0.55** | 0.54** | 0.55** | -0.04 | -0.13* |
| res(M~L^3) | -0.24** | 0.01 | 0.08 | 0.33** | 0.08 | 0.32** | -0.40** | -0.28** |
| SMI | 0.20* | 0.26** | 0.54** | 0.55** | 0.54** | 0.55** | -0.04 | -0.13* |
* p≤0.05
**p≤0.001
Fig 2. Relationships between 11 body condition indices (BCIs) and percent fat, a measure of true body condition, for each sex.
Variation in each BCI that can be explained by percent fat is provided as an r2 value. Ideally, most variation in a BCI should be attributable to variation in true body condition, and there should be a positive linear relationship between the BCI and true body condition. Descriptions of each BCI are provided in Table 1.
Fig 5. Relationships between 11 body condition indices (BCIs) and snout-vent length (SVL) for each sex.
Variation in each BCI that can be explained by SVL is provided as an r2 value. An ideal BCI has no relationship (i.e., zero regression slope and zero r2 value) with SVL. Descriptions of each BCI are provided in Table 1.
Fig 3. Relationships between 11 body condition indices (BCIs) and scaled fat, a measure of true body condition, for each sex.
Variation in each BCI that can be explained by scaled fat is provided as an r2 value. Ideally, most variation in a BCI should be attributable to variation in true body condition, and there should be a positive linear relationship between the BCI and true body condition. Descriptions of each BCI are provided in Table 1.
Fig 4. Relationships between 11 body condition indices (BCIs) and residual fat, a measure of true body condition, for each sex.
Variation in each BCI that can be explained by residual fat is provided as an r2 value. Ideally, most variation in a BCI should be attributable to variation in true body condition, and there should be a positive linear relationship between the BCI and true body condition. Descriptions of each BCI are provided in Table 1.
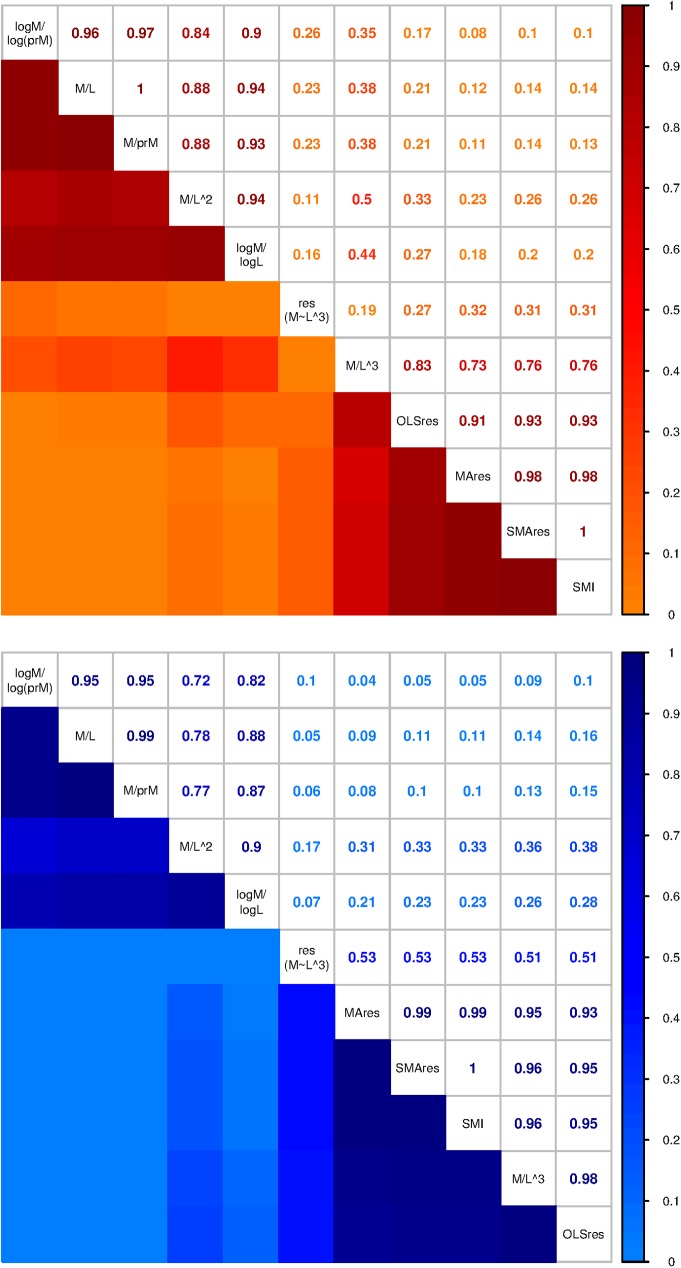
The correlations among BCIs revealed that most BCIs fall out into one of two groups, where a group is characterized by pairwise Kendall rank correlation coefficients of τ > 0.7 (Fig 6). One group contains all the ratio indices except the Fulton index. The other group contains the Fulton index, the SMI, and all the regression indices except the cubed regression. Notably, the MA residual index, SMA residual index, and the SMI were very strongly correlated to each other in both sexes (τ ≥ 0.98). The cubed regression was dissimilar to all others (τ = 0.05–0.53).
Fig 6. Correlations (Kendall’s τ) among 11 body condition indices (BCIs) for each sex.
Most BCIs fall into two groups, and certain BCIs are very similar to others (e.g., the MA residual index, the SMA residual index, and the SMI are all strongly correlated to each other). Descriptions of each BCI are provided in Table 1.
We tested the extent of the length bias for the log ratio index for females and the Quételet index for males because these had the highest r2 values in a regression on percent fat (Fig 2). After creating a length category ≥ 0.25 m and another ≤ 0.25 m than the mean SVL for each sex, we rejected the hypothesis that the two length categories exhibit the same BCIs in females (W = 43, p ≤ 0.05) but not males (W = 333, p = 0.14). In other words, the log ratio index for females exhibits significant length bias in 0.5 m length categories in our dataset, but the Quételet index for males does not.
Discussion
We evaluated 11 BCIs using percent fat, scaled fat, and residual fat as alternative measures of true body condition in Burmese pythons. In our dataset, females exhibit a more positive allometric relationship between mass and length and between fat mass and length than do males, and fat mass is strongly associated with length in both sexes. None of our three measures of true body condition completely removed this effect of size, though the effects of size were minor for scaled fat and residual fat. Our inferences of the best-performing BCIs heavily depended on our measure of true body condition. BCIs exhibiting strong associations with percent fat often exhibited even stronger relationships with SVL. These BCIs included most of the ratio indices (ratio index, Quételet index, relative condition index, log ratio index, and log relative condition index). Conversely, BCIs with strong associations with scaled fat and residual fat exhibited weak or no relationships with SVL. These BCIs included most of the regression indices (OLS residual index, MA residual index, and SMA residual index), the Fulton index, and the SMI.
It is unclear to what extent BCI evaluation in other taxa may be sensitive to the chosen measure of true body condition. In both sexes of house mice, for example, the best-performing BCI was the same when evaluated against both percent fat and residual fat (that best-performing BCI was a multiple regression containing skeletal measurements and is not otherwise comparable to our study; [22]). In an evaluation of the OLS residual index in several species (e.g., chipmunks, meadow voles, starlings, and watersnakes), the correlations with percent fat and with scaled fat were sometimes similar and sometimes different [27]. In chipmunks, for example, the coefficient dropped by more than half from 0.3 (scaled fat) to 0.12 (percent fat), but in watersnakes it remained relatively stable increasing slightly from 0.49 (scaled fat) to 0.54 (percent fat; [27]). This latter result is particularly interesting in the context of our study because it is also a snake with a simple body plan, but that species exhibits an opposite trend in association between the OLS residual index and percent fat and between the OLS residual index and scaled fat because in Burmese pythons, the OLS residual index is more strongly associated with scaled fat than percent fat (Table 5). This difference may be a result of differences in allometry between Burmese pythons and watersnakes. We are not aware of any other studies that compared BCI performance when using percent fat, scaled fat, residual fat, or other transformations of fat mass as measures of true body condition, and additional work is necessary to demonstrate whether BCI evaluation in other taxa is sensitive to interpretation of true body condition.
We consider scaled fat and residual fat to be preferable approximations of true body condition because these measures exhibit minimal association with length. Though minimal, they were unfortunately not free from a size bias. The source of the bias may be an artefact of our dataset, as the residuals from the SMA regression of log-transformed fat mass on log-transformed SVL (these residuals are residual fat, a measure of true body condition, and this regression slope is included in the TL scaling model to calculate scaled fat, which is another measure of true body condition) are leptokurtic in males (6.17, p ≤ 0.001) and negatively skewed in both females (-0.71, p ≤ 0.05) and males (-1.4, p ≤ 0.001). Burmese pythons, particularly males, seem to have a more restrictive upper than lower limit on true body condition (i.e., pythons can be more excessively skinny than excessively fat), and this deviance from a normal distribution is somewhat problematic for calculations of both scaled fat and residual fat because it violates an assumption of the regression analysis.
We consider the SMI, the MA residual index, and the SMA residual index to be the best-performing BCI’s for our dataset because they exhibited a strong association with both scaled fat and residual fat but not SVL. It is difficult to compare BCI performance in Burmese pythons to other taxa because most studies used either fat mass or percent fat (when using fat and not protein, etc., for validation) as a measure of true body condition. Nevertheless, there are some BCI verification data that use scaled fat, and the associations between BCIs and true body condition are widely variable among taxa, so it is no surprise that our results fall within the set of these previous observations. For example, we observed correlation coefficients of 0.54–0.55 between the SMI and scaled fat, and these were as low as -0.151 in deer mice and 0.164 in meadow voles and as high as 0.758 in watersnakes and 0.841 in starlings [27]. The r2 values for residual fat and the optimal BCI in house mice (the aforementioned multiple regressions of skeletal measurements that are not comparable to our study) were 0.39 in females and 0.19 in males, whereas we observed r2 values for residual fat and the MA residual index of 0.41 in females and 0.56 in males (Fig 4). We found the log ratio index, the Quételet index, and similar BCIs to have size biases that render them inappropriate for use in pythons.
The strong correlations within groups of BCIs are encouraging, because this suggests that–at least in some cases–certain BCIs are interchangeable with others. The SMI, MA residual index, and SMA residual index provided nearly equivalent results in our dataset, as did the ratio index, the relative index, and the log-relative index (Fig 6). The estimated r2 values of regressions of the OLS residual BCI on the SMI for five rodent species, starlings, and watersnake ranged 0.636–0.963, with the highest r2 value observed in watersnakes [27]. Snakes, with their simple body plans, may exhibit higher correlations among BCIs than other species with legs and wings, and further study is necessary to parse which factors contribute to the strength of correlations among different BCIs.
Though we are not the first to use wet-fat mass to evaluate BCIs (e.g., [3, 32, 33]), it is not common, and some associated limitations warrant discussion. First, fat may be stored elsewhere than in the fat bodies (e.g., the liver; [32]), and our measure of fat mass may be a biased approximation of actual fat mass if accumulation in these other areas does not consistently vary with fat accumulation in the fat bodies. Second, we ignore the contribution of other tissue types to body condition, particularly muscle. Muscle (i.e., protein) is an important component of body condition [14], and the proportions of muscle and fat in Burmese pythons may not covary. Nonetheless, we believe that fat mass is a meaningful biological indicator of body condition in Burmese pythons. Similar to observations in other snakes [58–61], fat may directly influence fitness in Burmese pythons: adults have large fat stores just prior to the breeding season, and these fat stores facilitate a drop in feeding rates as the animals enter their breeding season and actively search for mates (when we observe a Burmese python in Florida without prey remains in its gastrointestinal tract, it is almost always a reproductively capable adult during the breeding season; Falk unpublished). After breeding, females lay eggs and stay with them until they hatch, and the total duration of the reproductive season is enough to deplete the fat stores of these brooding females [62]. Thus, large fat stores may allow more opportunities for both sexes to find mates and may facilitate greater clutch success for females. Note that this additional need for energy reserves by females (i.e., laying and brooding a clutch) is a potential explanation for the higher proportions of fat and more positive length/mass allometry in females vs. males (Table 2; Fig 1).
This reproduction-related caveat brings another potential limitation to using fat mass to evaluate BCIs in Burmese pythons: the total mass of tissues besides fat (and other tissues commonly associated with body condition) in individual Burmese pythons may change according to reproductive and feeding cycles, which in turn may affect the observed proportion of fat. The size and mass of reproductive organs change throughout the year in adult Burmese pythons (Falk unpublished), as do the size and mass of digestion-related organs of all life stages during periods of fasting and eating [63]. This variation affects both dry- and wet-fat mass measurements, and it may introduce noise into our dataset as the proportional mass of these tissues change inversely with the proportion of fat (e.g., if the masses of the follicles and oviducts increase while the remaining tissues remain the same, the proportion of fat mass relative to total mass will decrease), but it is variation and not an introduction of a systematic bias that would allow us to reject our conclusions. Furthermore, these effects as related to digestion may be small; only approximately 10% of Burmese pythons necropsied in Florida have prey in their stomach, when the increase in organ mass may be greatest, and the majority of the digested material is only feathers and hair (i.e., the added mass from feeding and digestion is probably small; [64]).
Finally, we do not know for certain that the proportions of water, organic matter, and inorganic matter remain constant as both whole-body and fat-body masses change in Burmese pythons. Consistency in dry and wet masses has been demonstrated in the other snake species tested [31], and though the pattern has not yet been evaluated in Burmese pythons, there is no evidence to suggest that Burmese pythons would not be the same. To reiterate, we are confident that wet fat is a satisfactory means to approximate true body condition in the context of BCI verification with our dataset, but additional studies that incorporate the dried weights of fat and other tissues in Burmese pythons would be an improvement.
Given the low number of BCI-verification studies relative to the number of studies that use un-verified BCIs to answer ecological questions, employing wet-fat mass as we have here may improve body-condition inference generally. Traditional methods of BCI verification involve drying the specimen in an oven, grinding it, and then extracting and quantifying each of the remaining components (e.g., fat, organic matter, inorganic matter; [14]). While feasible for a limited number of small-sized animals, this approach becomes much less practical for larger animals. This association between body size and feasibility has resulted in a bias in the BCI-verification literature towards small-bodied subjects including arthropods, rodents, songbirds, etc. (e.g., [13, 18, 20–22]). Incorporating alternative approaches to BCI evaluation, including using wet-fat mass as we did here, may result in a larger number of species for which we have verified BCIs, which in turn may result in a better understanding of how BCIs can be appropriately applied to ecological questions.
Acknowledgments
We thank the many individuals, especially the participants in Everglades National Park’s Authorized Agent Program, who found and captured the pythons used in this study. Christopher Somers, Jillian Josimovich, and one anonymous reviewer made many helpful suggestions that improved the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Data Availability
The data are available on the USGS ScienceBase Catalog (https://doi.org/10.5066/F7ST7NCG).
Funding Statement
Research for this project was funded by Everglades National Park, the USGS Greater Everglades Priority Ecosystem Science Program (http://access.usgs.gov/about.html), and the USGS Invasive Species Science Program (https://www2.usgs.gov/ecosystems/invasive_species/). These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hayes J, Shonkwiler J. Morphometric indicators of body condition: Worthwhile or wishful thinking? In: Speakman JR, editor. Body composition analysis of animals: A handbook of non-destructive methods. Cambridge, UK: Cambridge University Press; 2001. p. 8–38. [Google Scholar]
- 2.Guinet C, Roux JP, Bonnet M, Mison V. Effect of body size, body mass, and body condition on reproduction of female South African fur seals (Arctocephalus pusillus) in Namibia. Canadian Journal of Zoology. 1998;76(8):1418–24. [Google Scholar]
- 3.Naulleau G, Bonnet X. Body condition threshold for breeding in a viviparous snake. Oecologia. 1996;107(3):301–6. doi: 10.1007/BF00328446 [DOI] [PubMed] [Google Scholar]
- 4.Burton NH, Rehfisch MM, Clark NA, Dodd SG. Impacts of sudden winter habitat loss on the body condition and survival of redshank Tringa totanus. Journal of Applied Ecology. 2006;43(3):464–73. [Google Scholar]
- 5.Reading C. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia. 2007;151(1):125–31. doi: 10.1007/s00442-006-0558-1 [DOI] [PubMed] [Google Scholar]
- 6.Reichert M, Gerhardt H. The role of body size on the outcome, escalation and duration of contests in the grey treefrog, Hyla versicolor. Animal Behaviour. 2011;82(6):1357–66. [Google Scholar]
- 7.Steen DA, McClure CJ, Smith LL, Halstead BJ, Dodd C, Sutton WB, et al. The effect of coachwhip presence on body size of North American racers suggests competition between these sympatric snakes. Journal of Zoology. 2013;289(2):86–93. [Google Scholar]
- 8.Dawson RD, Bortolotti GR. Effects of hematozoan parasites on condition and return rates of American kestrels. The Auk. 2000;117(2):373–80. [Google Scholar]
- 9.Møller A, Christe P, Erritzøe J, Mavarez J. Condition, disease and immune defence. Oikos. 1998;83(2):301–6. [Google Scholar]
- 10.Neff B, Cargnelli L. Relationships between condition factors, parasite load and paternity in bluegill sunfish, Lepomis macrochirus. Environmental Biology of Fishes. 2004;71(3):297–304. [Google Scholar]
- 11.Weatherhead PJ, Brown GP. Measurement versus estimation of condition in snakes. Canadian Journal of Zoology. 1996;74(9):1617–21. [Google Scholar]
- 12.Reynolds D, Kunz T. Standard methods for destructive body composition analysis In: Speakman JR, editor. Body composition analysis of animals: A handbook of non-destructive methods. Cambridge, UK: Cambridge University Press; 2001. p. 39–55. [Google Scholar]
- 13.Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of mass-size residuals: Validating body condition indices. Ecology. 2005;86(1):155–63. [Google Scholar]
- 14.Stevenson R, Woods WA. Condition indices for conservation: New uses for evolving tools. Integrative and Comparative Biology. 2006;46(6):1169–90. doi: 10.1093/icb/icl052 [DOI] [PubMed] [Google Scholar]
- 15.Wilder SM, Raubenheimer D, Simpson SJ. Moving beyond body condition indices as an estimate of fitness in ecological and evolutionary studies. Functional Ecology. 2016;30:108–15. [Google Scholar]
- 16.Jelliffe DB, Jelliffe E. Underappreciated pioneers. Quetelet: Man and index. American Journal of Clinical Nutrition. 1979;32:2519–21. [DOI] [PubMed] [Google Scholar]
- 17.Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. International Journal of Obesity. 1984;9(2):147–53. [PubMed] [Google Scholar]
- 18.Jakob EM, Marshall SD, Uetz GW. Estimating fitness: A comparison of body condition indices. Oikos. 1996;77(1):61–7. [Google Scholar]
- 19.Green AJ. Mass/length residuals: Measures of body condition or generators of spurious results? Ecology. 2001;82(5):1473–83. [Google Scholar]
- 20.Peig J, Green AJ. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Functional Ecology. 2010;24(6):1323–32. [Google Scholar]
- 21.Kelly CD, Tawes BR, Worthington AM. Evaluating indices of body condition in two cricket species. Ecology and Evolution. 2014;4(23):4476–87. doi: 10.1002/ece3.1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labocha MK, Schutz H, Hayes JP. Which body condition index is best? Oikos. 2014;123(1):111–9. [Google Scholar]
- 23.Cox RM, Calsbeek R. Survival of the fattest? Indices of body condition do not predict viability in the brown anole (Anolis sagrei). Functional Ecology. 2015;29(3):404–13. [Google Scholar]
- 24.Ricker WE. Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada. 1975;191:1–382. [Google Scholar]
- 25.Nash RD, Valencia AH, Geffen AJ. The origin of Fulton’s condition factor—setting the record straight. Fisheries. 2006;31(5):236–8. [Google Scholar]
- 26.Le Cren E. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). The Journal of Animal Ecology. 1951;20(2):201–19. [Google Scholar]
- 27.Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos. 2009;118(12):1883–91. [Google Scholar]
- 28.Kotiaho JS. Estimating fitness: Comparison of body condition indices revisited. Oikos. 1999;87(2):399–402. [Google Scholar]
- 29.Lleonart J, Salat J, Torres GJ. Removing allometric effects of body size in morphological analysis. Journal of Theoretical Biology. 2000;205(1):85–93. doi: 10.1006/jtbi.2000.2043 [DOI] [PubMed] [Google Scholar]
- 30.Jacobson ER. Infectious diseases and pathology of reptiles: Color atlas and text Boca Raton, Florida: CRC Press; 2007. [Google Scholar]
- 31.Cale WG Jr, Gibbons JW. Relationships between body size, size of the fat bodies, and total lipid content in the canebrake rattlesnake (Crotalus horridus) and the black racer (Coluber constrictor). Herpetologica. 1972;28(1):51–3. [Google Scholar]
- 32.Bonnet X, Naulleau G. Estimation of body reserves in living snakes using a body condition index (BCI). Scientifica Herpetologica. 1995;3(13):237–40. [Google Scholar]
- 33.Sutton SG, Bult TP, Haedrich RL. Relationships among fat weight, body weight, water weight, and condition factors in wild Atlantic salmon parr. Transactions of the American Fisheries Society. 2000;129(2):527–38. [Google Scholar]
- 34.Teixeira A, Delfa R, Colomer-Rocher F. Relationships between fat depots and body condition score or tail fatness in the Rasa Aragonesa breed. Animal Production. 1989;49(02):275–80. [Google Scholar]
- 35.Schamber JL, Esler D, Flint PL. Evaluating the validity of using unverified indices of body condition. Journal of Avian Biology. 2009;40(1):49–56. [Google Scholar]
- 36.Reist JD. An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Canadian Journal of Zoology. 1985;63(6):1429–39. [Google Scholar]
- 37.Feldman A, Meiri S. Length–mass allometry in snakes. Biological Journal of the Linnean Society. 2013;108(1):161–72. [Google Scholar]
- 38.Team RC. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 39.Fox J, Weisberg S. An R companion to applied regression Second ed. Thousand Oaks, California: Sage; 2011. [Google Scholar]
- 40.Wei T, Simko V. Corrplot: Visualization of a correlation matrix. R package version 0.77. https://CRAN.R-project.org/package=corrplot. 2016.
- 41.Wilke CO. Cowplot: Streamlined plot theme and plot annotations for 'ggplot2'. R package version 0.7.0. https://CRAN.R-project.org/package=cowplot. 2016.
- 42.Chang W. Extrafont: Tools for using fonts. R package version 0.17. https://CRAN.R-project.org/package=extrafont. 2014.
- 43.Wuertz D, Setz T, Chalabi Y. fBasics: Rmetrics—markets and basic statistics. R package version 3011.87. https://CRAN.R-project.org/package=fBasics. 2014.
- 44.Wickham H. ggplot2: Elegant graphics for data analysis New York: Springer-Verlag; 2009. [Google Scholar]
- 45.Legendre P. Lmodel2: Model II Regression. R package version 1.7–2. Available at: http://CRAN.R-project.org/package=lmodel2; 2014.
- 46.Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2(3):7–10. [Google Scholar]
- 47.Komsta L, Novomestky F. Moments: Moments, cumulants, skewness, kurtosis and related tests. R package version 0.14. https://CRAN.R-project.org/package=moments. 2015.
- 48.Warton DI, Duursma RA, Falster DS, Taskinen S. smatr 3: an R package for estimation and inference about allometric lines. Methods in Ecology and Evolution. 2012;3(2):257–9. [Google Scholar]
- 49.Warton DI, Weber NC. Common slope tests for bivariate errors-in-variables models. Biometrical Journal. 2002;44(2):161–74. [Google Scholar]
- 50.Wasserstein RL, Lazar NA. The ASA's statement on p-values: Context, process, and purpose. The American Statistician. 2016;70(2):129–33. [Google Scholar]
- 51.Ramsey JB. Tests for specification errors in classical linear least-squares regression analysis. Journal of the Royal Statistical Society Series B (Methodological). 1969;31(2):350–71. [Google Scholar]
- 52.Breusch TS, Pagan AR. A simple test for heteroscedasticity and random coefficient variation. Econometrica. 1979;47(5):1287–94. [Google Scholar]
- 53.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52(3–4):591–611. [Google Scholar]
- 54.D'Agostino RB. Transformation to normality of the null distribution of g1. Biometrika. 1970;57(3):679–81. [Google Scholar]
- 55.Anscombe FJ, Glynn WJ. Distribution of the kurtosis statistic b2 for normal samples. Biometrika. 1983;70(1):227–34. [Google Scholar]
- 56.Kendall MG. A new measure of rank correlation. Biometrika. 1938;30(1/2):81–93. [Google Scholar]
- 57.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. The Annals of Mathematical Statistics. 1947;18(1):50–60. [Google Scholar]
- 58.Madsen T, Shine R. Determinants of reproductive output in female water pythons (Liasis fuscus: Pythonidae). Herpetologica. 1996;52(2):146–59. [Google Scholar]
- 59.Madsen T, Shine R. Energy versus risk: Costs of reproduction in free‐ranging pythons in tropical Australia. Austral Ecology. 2000;25(6):670–5. [Google Scholar]
- 60.Plummer MV. Annual variation in stored lipids and reproduction in green snakes (Opheodrys aestivus). Copeia. 1983;1983(3):741–5. [Google Scholar]
- 61.Shine R, Mason RT. Do a male garter snake's energy stores limit his reproductive effort? Canadian Journal of Zoology. 2005;83(10):1265–70. [Google Scholar]
- 62.Van Mierop L, Barnard SM. Further observations on thermoregulation in the brooding female Python molurus bivittatus (Serpentes: Boidae). Copeia. 1978;1978(4):615–21. [Google Scholar]
- 63.Secor SM. Digestive physiology of the Burmese python: Broad regulation of integrated performance. Journal of Experimental Biology. 2008;211(24):3767–74. [DOI] [PubMed] [Google Scholar]
- 64.Falk BG, Reed RN. Challenges to a molecular approach to prey identification in the Burmese python, Python molurus bivittatus. PeerJ. 2015;3:e1445 doi: 10.7717/peerj.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on the USGS ScienceBase Catalog (https://doi.org/10.5066/F7ST7NCG).